Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5877
Peer-review started: November 2, 2014
First decision: December 2, 2014
Revised: January 17, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: May 21, 2015
Processing time: 198 Days and 20.3 Hours
AIM: To investigate the roles of nuclear factor (NF)-κB and angiotensin II receptor type 1 (AT1R) in the pathogenesis of non-alcoholic fatty liver disease (NAFLD).
METHODS: Forty-two healthy adult male Sprague-Dawley rats were randomly divided into three groups: the control group (normal diet), the model group, and the intervention group (10 wk of a high-fat diet feeding, followed by an intraperitoneal injection of PDTC); 6 rats in each group were sacrificed at 6, 10, and 14 wk. After sacrifice, liver tissue was taken, paraffin sections of liver tissue specimens were prepared, hematoxylin and eosin (HE) staining was performed, and pathological changes in liver tissue (i.e., liver fibrosis) were observed by light microscopy. NF-κB expression in liver tissue was detected by immunohistochemistry, and the expression of AT1R in the liver tissue was detected by reverse transcription-polymerase chain reaction (RT-PCR). The data are expressed as mean ± SD. A two-sample t test was used to compare the control group and the model group at different time points, paired t tests were used to compare the differences between the intervention group and the model group, and analysis of variance was used to compare the model group with the control group. Homogeneity of variance was analyzed with single factor analysis of variance. H variance analysis was used to compare the variance. P < 0.05 was considered statistically significant.
RESULTS: The NAFLD model was successful after 6 wk and 10 wk. Liver fibrosis was found in four rats in the model group, but in only one rat in the intervention group at 14 wk. Liver steatosis, inflammation, and fibrosis were gradually increased throughout the model. In the intervention group, the body mass, rat liver index, serum lipid, and transaminase levels were not increased compared to the model group. In the model group, the degree of liver steatosis was increased at 6, 10, and 14 wk, and was significantly higher than in the control group (P < 0.01). In the model group, different degrees of liver cell necrosis were visible and small leaves, punctated inflammation, focal necrosis, and obvious ballooning degeneration were observed. Partial necrosis and confluent necrosis were observed. In the model group, liver inflammatory activity scores at 6, 10, and 14 wk were higher than in the control group (P < 0.01). Active inflammation in liver tissue in the intervention group was lower than in the model group (P < 0.05). HE staining showed liver fibrosis only at 14 wk in 4/6 rats in the model group and in 1/6 rats in the intervention group. NF-κB positive cells were stained yellow or ensemble yellow, and NF-κB was localized in the cytoplasm and/or nucleus. The model group showed NF-κB activation at 6, 10, and 14 wk in liver cells; at the same time points, there were statistically significant differences in the control group (P < 0.01). Over time, NF-κB expression increased; this was statistically lower (P < 0.05) at 14 weeks in the intervention group compared to the model group, but significantly increased (P < 0.05) compared with the control group; RT-PCR showed that AT1R mRNA expression increased gradually in the model group; at 14 wk, the expression was significantly different compared with expression at 10 weeks as well as at 6 weeks (P < 0.05). In the model group, AT1R mRNA expression was significantly higher than at the same time point in the control group (P < 0.01).
CONCLUSION: With increasing severity of NAFLD, NF-κB activity is enhanced, and the inhibition of NF-κB activity may reduce AT1R mRNA expression in NAFLD.
Core tip: Angiotensin II receptor type 1 (AT1R) is closely associated with the process of non-alcoholic fatty liver disease (NAFLD) fibrosis. As the nuclear transcription factor which is closely related to the tissue inflammation and fibrosis, when the activity of nuclear factor (NF)-κB was inhibited, AT1R mRNA expression was reduced, and the degrees of inflammation and fibrosis gradually reduced, indicating that NF-κB might play a key role throughout the course of NAFLD and that the NF-κB inhibitor might be effective in the treatment of the disease, while the exact mechanism still requires further study.
- Citation: Tan DY, Shi HY, Li CP, Zhong XL, Kang M. Effect of nuclear factor-κB and angiotensin II receptor type 1 on the pathogenesis of rat non-alcoholic fatty liver disease. World J Gastroenterol 2015; 21(19): 5877-5883
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5877.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5877
With social and economic development, the incidence of non-alcoholic fatty liver disease (NAFLD) has increased over the years, to the point that it has become a common chronic liver disease that endangers human health. Worldwide epidemiological surveys have shown that the incidence rate of adult NAFLD is 20%-33%; this rate could reach up to 75% in obese individuals or those with type 2 diabetes. A recent study found that the disease also exhibits a trend of developing in younger people; therefore, NAFLD has gained more attention in Western countries and other regions of the world[1-3]. Pathological changes are mainly due to inflammation, necrosis, or apoptosis in liver cells, leading to steatohepatitis, liver fibrosis, and cirrhosis.
Nuclear factor-κB (NF-κB) is a nuclear protein factor that participates in the regulation of a variety of protein genes and causes disease through the induction of cytokines, which are related to immunity, inflammation, and fibrosis; NF-κB thus plays an important role in inflammation and the immune response[4-6]. Some studies have shown that the liver renin-angiotensin-aldosterone system (RAAS) is closely related to the development of liver fibrosis[7]. Therefore, an angiotensin II receptor type 1 (AT1R) antagonist could inhibit the development of experimental liver fibrosis[8,9]. In this study, pyrrolidine dithioformate (PDTC) was used to inhibit the activity of NF-κB in NAFLD rats, with the aim to explore the changes in angiotensin (Ang) II and AT1R mRNA expression, and to investigate the regulation of NF-κB towards Ang II AT1R mRNA expression in NAFLD rats.
Thirty-six healthy adult SPF-level male SD rats, weighing 150 g ± 30 g, were randomly divided into the control group (n = 18, fed a normal diet) and the model group (n = 18, fed a high-fat diet: 2% cholesterol, 0.5% sodium cholate, 0.2% propylthiouracil, 5% sucrose, 10% lard, and 82.3% basal diet, freshly prepared by the experimental Animal Center at Luzhou Medical College). The rats had free access to water. Six rats of each group were killed after 6, 10, and 14 wk. Serum and liver tissues were isolated and stored at -80 °C. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Luzhou Medical College.
The liver tissues were fixed with 10% paraformaldehyde for 1 d, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). Liver histopathological changes were observed by light microscopy.
The radioimmunoassay kit and radioimmunoassay counter were used to perform the detection. NF-κB protein expression detection was performed by immunohistochemistry according to the instructions of the SP immunohistochemical staining kit and DAB staining kit (Shengzhen Chemical Co. Ltd., Shengzhen, China). NF-κB mainly appeared as brown staining in the cell membrane or cytoplasm, and the nuclear membrane was occasionally stained. Five high-power fields were imaged from each section (with > 500 cells in each field) to calculate the positive rate (i.e., number of positive cells/total cells × 100%).
Total RNA was extracted according to the instructions of the RNA extraction kit. The integrity of the RNA was analyzed, the concentration and purity were measured, and then the extracted RNA was diluted to the same concentration in accordance with the measured concentration; 0.5 μg was prepared for reverse transcription (RT)-polymerase chain reaction amplification according to the instructions in the First Strand cDNA Synthesis Kit (Beijing Biomed Co. Ltd., Beijing, China). The upstream primer sequence of the AT1R gene was: 5’-ACG TGT CTC AGC ATC GAC CGC TAC C-3’ and the downstream primer sequence was: 5’-AGA ATG ATA AGG AAA GGG AAC AAG AA-3’. The upstream primer of the internal reference β-actin gene was: 5’-GAG GGA AAT CGT GCG TGA C-3’ and the downstream primer was: 5’-CTG GAA GGT GGA CAG TGA G-3’. The length of the targeted AT1R mRNA amplification fragment was about 278 bp, and the RT reaction parameters were: 30 °C for 10 min, 42 °C for 20 min, 99 °C for 5 min, and 4 °C for 5 min. Then, 1 μL of the cDNA product, 1 μL of the upstream and downstream primers (10 μM), 12.5 μL of 2 × MasterMix, and 9.5 μL of ddH2O were mixed together to a total volume of 25 μL for the PCR. The reaction parameters were: AT1R: pre-denaturation at 94 °C for 5 min, denaturation at 94 °C for 35 s, annealing at 53 °C for 30 s, and extension at 72 °C for 1 min; after 30 cycles, extension was performed at 72 °C for 5 min.
SPSS17.0 statistical software was used, with P < 0.05 considered to indicate statistical significance. The data are expressed as the mean ± SD, and comparisons among different time points for the control and model groups were made using analysis of variance. Homogeneity of variance test was also performed, and data sets with homogeneity of variance were then submitted to single-factor variance analysis, while those without homogeneity of variance were submitted to non-parametric tests.
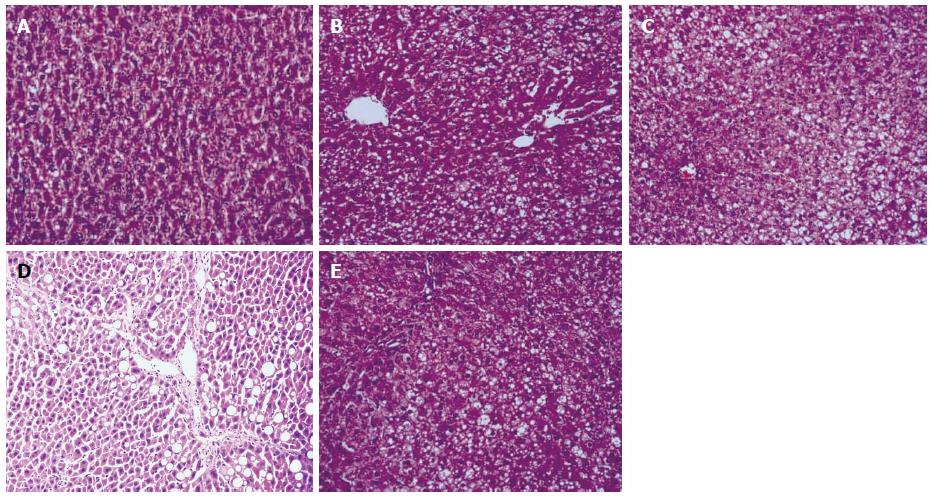
The morphology of the liver tissue in the control group after 6, 10, and 14 wk was normal based on HE staining. In the model group, after 6 wk, liver cells within the hepatic lobules exhibited more than 33% fatty degeneration, with no significant inflammatory cell infiltration in the portal area and acinus. There was no significant formation of collagen fibers and fibrosis, indicating the stage of liver cell steatosis. After 10 wk, the steatotic liver cells in the model group accounted for more than 50% of the total cells, and in some rats the ratio reached more than 66%, with significant ballooning degeneration in the acinar 3 band. Spotty hepatocellular necrosis could be seen in the acinus, and inflammatory cells appeared in the portal area, indicating non-alcoholic hepatitis. After 14 wk, various degrees of lobular and portal inflammation could be seen in the model group, and extensive ballooning degeneration could be seen in the acinar 3 band. At the same time, periportal degeneration, spotty necrosis, and focal necrosis were also found, with partial necrosis being integrated into a larger area. Inflammation was apparent in and around the portal area. Four rats exhibited hepatocellular fibrosis. In the intervention group, lobular inflammation could be seen after 14 wk and spotty necrosis appeared in the acinus, with some inflammation in the periportal area. One rat exhibited hepatocellular fibrosis (Figure 1).
Throughout the model, the serum Ang II concentration gradually increased, and was higher than the concentration in the control group at the same time; the differences were statistically significant (P < 0.05). The Ang II concentration in the intervention group was lower than in the model group after 14 wk, but higher than the level in the control group (P < 0.05) (Table 1).
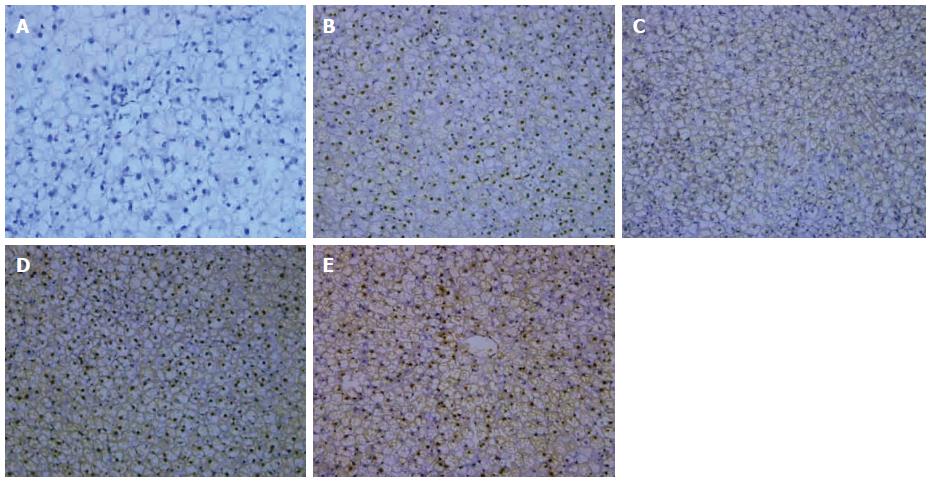
Yellow or buffy yellow-stained NF-κB, located in the cytoplasm and/or the nucleus, identified NF-κB positive cells. In the model group, apparent NF-κB activation could be seen after 6, 10, and 14 wk in the hepatocytes, and expression increased throughout the model. In the intervention group, NF-κB expression after 14 wk was significantly decreased when compared with that in the model group, although still higher when compared with the control group (Figure 2; Table 2).
The relative expression level of AR1T mRNA in the model group at each time point was higher than in the control group, and the relative expression level gradually increased throughout the model. The relative expression in the intervention group after 14 wk was higher than that in the control group at the same time point, but lower than that in the model group at the same time point.
With improvements in living standards and social change, the incidence of NAFLD has risen. The pathogenesis of NAFLD is driven by oxidative stress and lipid peroxidation damage, leading to the dysfunction of liver cells and mitochondrial activity, the activation and proliferation of hepatic stellate cells (HSC), and increased release of inflammatory cytokines, followed by inflammation, necrosis, and apoptosis, which then develops into steatohepatitis, fibrosis, and cirrhosis[10-12].
The RAAS is an important neuroendocrine system, as it maintains the balance of blood pressure, water, and electrolytes; Ang II is the most important bioactive substance in the RAAS. Some studies have shown that there was also RAAS in the partial liver[13]. Recent studies have also found that liver fibrosis is connected with RAAS activation[14]. The biological effects of Ang II are exerted by acting on specific receptors. The Ang II receptors include four G protein-coupled receptor subtypes, namely AT1R, AT2R, AT3R, and AT4R; AT1R and AT2R are the best understood. Yang et al[15] found that the TGF-β content in wild-type mouse liver tissue is higher than in AT1Ra-deficient mice, and the degree of inflammation and fibrosis was much greater in wild-type than in AT1Ra-deficient mice, suggesting that AT1R plays an important role in the process of liver fibrosis. In the current study, it was shown that AT1R mRNA expression increased in liver tissue during the process of liver steatosis, and the processes of inflammation and fibrosis aggravated this. Most biological effects of Ang II are mediated by AT1R through a series of signal transduction pathways. At the same time, it can also act as a pro-inflammatory cytokine in various inflammatory processes. The results shown here indicate that AT1R is involved in the development of NAFLD from steatosis to hepatic fibrosis. When damaging factors affect the liver, this stimulates AT1R mRNA transcription, and when Ang II binds to AT1R, it conducts a signal via the G protein-coupled pathway and the mitogen-activated protein kinase pathway, thereby activating downstream factors and promoting inflammation. During the stages of steatosis and hepatitis, AT1R accelerates the synthesis of inositol phosphate, which causes the sarcoplasmic reticulum to release calcium into the cytoplasm. This has an important role in the inflammatory response and oxidative stress, resulting in liver cell degeneration and necrosis through multiple ways, and thus leading to hepatic steatosis and liver cell inflammation. In the stage of liver fibrosis, AT1R promotes the transcription of proto-oncogenes, inducing mesenchymal cells and fibroblasts to proliferate abnormally, leading to increased collagen synthesis and fibrosis[16]. Meanwhile, AT1R activation also induces hepatic stellate cell proliferation and upregulates TGF-β1 levels[17], thus playing an important role in liver fibrosis.
Recent studies have found that the activation of NF-κB is closely related to the process of liver inflammation and fibrosis. The key to NF-κB activation is IκB degradation. During the second stage of NAFLD, the products of reactive oxygen species and lipid peroxidation significantly increase, which activates the NF-κB complex; first, through the mitogen-activated protein kinase (MAP kinase, MAPK) family, serine kinase (IKK) catalyzes the phosphorylation[18-22]. When the IKK complex combines with membrane coupled receptors, two types of autophosphorylation occur to activate the serine kinase[23-26]. Subsequently, under the effect of IKK, IκB is phosphorylated and, via pantothenic acid, the proteasome, and other mediators, the 3D structure of activated IκB is destroyed, exposing the amino acid sequence which is then recognized by the pantothenic acid ligase. Pantothenic acidification then occurs, and NF-κB is thus hydrolyzed and activated. Increased activity of NF-κB promotes the expression of COX-2, IL-6, and IL-8 by HSC, which increase liver inflammation, and further activates NF-κB through inflammatory mediators, thereby maintaining HSC activation and eventually leading to the occurrence of liver fibrosis. Ang II induces NF-κB, resulting in the activation of a large number of target genes, such as cytokines, chemokines, adhesion molecules, and TNF-α. Ji et al[27-29] found that the intrahepatic RAAS system can mediate hepatic fibrosis by activating the NF-κB and AP-1 pathways, and AECI and ARB may play an anti-liver fibrosis role by inhibiting NF-κB activity. Activated NF-κB can also act on angiotensinogen, further affecting the local hepatic RAAS system and leading to liver fibrosis. In the intervention group in this experiment, it was found that by inhibiting NF-κB activity, the pathological changes were better than in the model group, and AT1R mRNA expression was significantly lower than in the model group, which further indicates the presence of this kind of relationship.
AT1R is closely associated with the process of NAFLD fibrosis[30,31]. Since this nuclear transcription factor is closely related to tissue inflammation and fibrosis, when the activity of NF-κB was inhibited, AT1R mRNA expression was reduced, and the degree of inflammation and fibrosis gradually reduced as well, indicating that NF-κB might play a key role throughout the course of NAFLD and an NF-κB inhibitor might be effective in the treatment of this disease. However, the exact mechanism still needs further study.
The pathogenesis of non-alcoholic fatty liver diseases (NAFLD) is not clear. Many studies have shown that nuclear factor (NF)-κB and the angiotensin II receptor type 1 (AT1R) may participate in its pathogenesis.
NF-κB participates in the regulation of a variety of protein genes, and thus plays an important role in inflammation and the immune response. An AT1R antagonist could inhibit the development of experimental liver fibrosis, but the role of this pathway in NAFLD is not clear.
Pyrrolidine dithioformate (PDTC) was used to inhibit the activity of NF-κB in NAFLD rats with the aim to explore the changes in angiotensin II (Ang II) and AT1R mRNA expression, and to investigate the regulation of NF-κB regarding Ang II and AT1R mRNA expression in NAFLD rats.
NF-κB might play a key role throughout the course of NAFLD, and an NF-κB inhibitor might be effective in the treatment of this disease. It would be helpful to develop a new drug for the treatment of NAFLD.
NF-κB is a nuclear protein factor that can cause disease by inducing cytokines which are related to immunity, inflammation, and fibrosis, thus playing an important role in inflammation and the immune response.
AT1R is closely associated with the process of NAFLD fibrosis. NF-κB might play a key role throughout the course of NAFLD, and an NF-κB inhibitor might be effective in the treatment of this disease.
P- Reviewer: Kalinowski A, Van Cutsem E S- Editor: Yu J L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Paredes AH, Torres DM, Harrison SA. Nonalcoholic fatty liver disease. Clin Liver Dis. 2012;16:397-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950-954. [PubMed] |
| 6. | Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic Fatty liver disease: metabolic complications and therapeutic tools. Front Immunol. 2014;5:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Grünhage F, Nattermann J. Viral hepatitis: human genes that limit infection. Best Pract Res Clin Gastroenterol. 2010;24:709-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Serviddio G, Sastre J, Bellanti F, Viña J, Vendemiale G, Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 2008;29:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Kim MS, Kung S, Grewal T, Roufogalis BD. Methodologies for investigating natural medicines for the treatment of nonalcoholic fatty liver disease (NAFLD). Curr Pharm Biotechnol. 2012;13:278-291. [PubMed] |
| 11. | Wang Z, Xu M, Peng J, Jiang L, Hu Z, Wang H, Zhou S, Zhou R, Hultström M, Lai EY. Prevalence and associated metabolic factors of fatty liver disease in the elderly. Exp Gerontol. 2013;48:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Björnsson E, Angulo P. Non-alcoholic fatty liver disease. Scand J Gastroenterol. 2007;42:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Kato J, Koda M, Kishina M, Tokunaga S, Matono T, Sugihara T, Ueki M, Murawaki Y. Therapeutic effects of angiotensin II type 1 receptor blocker, irbesartan, on non-alcoholic steatohepatitis using FLS-ob/ob male mice. Int J Mol Med. 2012;30:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int. 2012;32:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Yang L, Bataller R, Dulyx J, Coffman TM, Ginès P, Rippe RA, Brenner DA. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol. 2005;43:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Shirai Y, Yoshiji H, Noguchi R, Kaji K, Aihara Y, Douhara A, Moriya K, Namisaki T, Kawaratani H, Fukui H. Cross talk between toll-like receptor-4 signaling and angiotensin-II in liver fibrosis development in the rat model of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2013;28:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Chayama K. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009;50:1226-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Peng X, Zhang P, Wang X, Chan J, Zhu M, Jiang M, Tuthill C, Wan Y, Dragoi AM, Chu WM. Signaling pathways leading to the activation of IKK and MAPK by thymosin alpha1. Ann N Y Acad Sci. 2007;1112:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Hsu WH, Chen TH, Lee BH, Hsu YW, Pan TM. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem Toxicol. 2014;64:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Sessler T, Healy S, Samali A, Szegezdi E. Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling. Pharmacol Ther. 2013;140:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Ma W, Tian S, Fan Z, Ma X, Yang X, Zhao Q, Tan K, Chen H, Chen D. RanBPM interacts with TβRI, TRAF6 and curbs TGF induced nuclear accumulation of TβRI. Cell Signal. 2014;26:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008;19:187-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, Weng X. Resveratrol modulates autophagy and NF-κB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Deng YR, Ma HD, Tsuneyama K, Yang W, Wang YH, Lu FT, Liu CH, Liu P, He XS, Diehl AM. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun. 2013;46:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Ji H, Meng Y, Zhang X, Luo W, Wu P, Xiao B, Zhang Z, Li X. Aldosterone induction of hepatic stellate cell contraction through activation of RhoA/ROCK-2 signaling pathway. Regul Pept. 2011;169:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. 2006;40:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Cui Y, Wang Q, Li X, Zhang X. Experimental nonalcoholic fatty liver disease in mice leads to cytochrome p450 2a5 upregulation through nuclear factor erythroid 2-like 2 translocation. Redox Biol. 2013;1:433-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [PubMed] |
| 31. | Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, Iida H, Kato S, Fujita K, Takahashi H. Association between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 2009;29:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |