Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5719
Peer-review started: October 10, 2014
First decision: November 14, 2014
Revised: November 30, 2014
Accepted: December 19, 2014
Article in press: January 5, 2015
Published online: May 14, 2015
Processing time: 220 Days and 14.9 Hours
AIM: To investigate whether prophylactic abdominal drainage is necessary after pancreatic resection.
METHODS: PubMed, Web of Science, and the Cochrane Library were systematically searched to obtain relevant articles published before January 2014. Publications were retrieved if they met the selection criteria. The outcomes of interest included: mortality, morbidity, postoperative pancreatic fistula (POPF), clinically relevant pancreatic fistula (CR-PF), abdominal abscess, reoperation rate, the rate of interventional radiology drainage, and the length of hospital stay. Subgroup analyses were also performed for pancreaticoduodenectomy (PD) and for distal pancreatectomy. Begg’s funnel plot and the Egger regression test were employed to assess potential publication bias.
RESULTS: Nine eligible studies involving a total of 2794 patients were identified and included in this meta-analysis. Of the included patients, 1373 received prophylactic abdominal drainage. A fixed-effects model meta-analysis showed that placement of prophylactic drainage did not have beneficial effects on clinical outcomes, including morbidity, POPF, CR-PF, reoperation, interventional radiology drainage, and length of hospital stay (Ps > 0.05). In addition, prophylactic drainage did not significantly increase the risk of abdominal abscess. Overall analysis showed that omitting prophylactic abdominal drainage resulted in higher mortality after pancreatectomy (OR = 1.56; 95%CI: 0.93-2.92). Subgroup analysis of PD showed similar results to those in the overall analysis. Elimination of prophylactic abdominal drainage after PD led to a significant increase in mortality (OR = 2.39; 95%CI: 1.22-4.69; P = 0.01).
CONCLUSION: Prophylactic abdominal drainage after pancreatic resection is still necessary, though more evidence from randomized controlled trials assessing prophylactic drainage after PD and distal pancreatectomy are needed.
Core tip: The elimination of prophylactic abdominal drainage resulted in an increase in mortality rate after pancreatic resection, especially in patients who underwent pancreaticoduodenectomy (PD). Therefore, prophylactic abdominal drainage is still necessary after pancreatic resection. Randomized controlled trials assessing the value of prophylactic abdominal drainage after PD and distal pancreatectomy are required to provide more powerful evidence. Based on current evidence, future prophylactic abdominal drainage may not be routine due to advances in surgical techniques and perioperative management. Moreover, drainage strategy after pancreatic resection should be tailored based on the characteristics of each patient.
- Citation: Dou CW, Liu ZK, Jia YL, Zheng X, Tu KS, Yao YM, Liu QG. Systematic review and meta-analysis of prophylactic abdominal drainage after pancreatic resection. World J Gastroenterol 2015; 21(18): 5719-5734
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5719.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5719
Prophylactic abdominal drainage, which is considered routine and mandatory after pancreatic resection, is a highly invasive surgery with a morbidity rate as high as 40%. This traditional practice is based on the concept that prophylactic drainage can evacuate anastomotic leakage fluid and abdominal collections. Drainage fluid can serve as a warning sign of anastomotic leakage, intra-abdominal hemorrhage, and abdominal infection. Therefore, it can facilitate the early detection and timely management of postoperative complications. However, prospective studies have demonstrated that prophylactic drainage does not result in any benefit after other abdominal surgeries including cholecystectomy[1], hepatectomy[2,3], and colorectal surgery[4]. Some surgeons have begun to question the significance of prophylactic drainage after pancreatic resection, and studies assessing the value of prophylactic drainage after pancreatic resection have been conducted.
As early as 1992, Jeekel[5] reported that 22 patients who underwent Whipple’s procedure without abdominal drainage had acceptable postoperative outcomes. The study concluded that abdominal drainage was not mandatory after Whipple’s procedure. In the following two decades, comparative studies were conducted to examine the value of prophylactic abdominal drainage. A retrospective study by Heslin et al[6] showed that intra-abdominal drainage did not significantly reduce the rate of major complications after pancreaticoduodenectomy (PD). In addition, a prospective cohort study by Fisher et al[7] demonstrated that a no drainage policy was associated with a decreased incidence of delayed gastric emptying and wound infection. The first randomized controlled trial (RCT) by Conlon et al[8] showed that omitting prophylactic drainage was not associated with a significant increase in mortality or morbidity. However, Correa-Gallego et al[9] showed that eliminating routine abdominal drainage resulted in a higher mortality rate based on the results of 739 patients. The latest randomized prospective multicenter trial by Van Buren et al[10] demonstrated that omitting prophylactic drainage after PD was associated with higher morbidity and mortality.
The results of these studies are conflicting, and the value of prophylactic abdominal drainage in pancreatic resection has been intensively debated in recent years. Therefore, this meta-analysis was conducted to clarify whether prophylactic drainage is necessary for all patients after pancreatic resection.
PubMed, Web of Science, and the Cochrane Library were searched to obtain relevant articles published up until January 2014. The following medical subject heading (Mesh) terms were used in combination with Boolean operators AND or OR: pancreaticoduodenectomy, Whipple, pancreatectomy, pancreatic resection, drain, and drainage. Furthermore, the references in relevant articles were screened manually to identify additional eligible studies. No language restriction was imposed during the electronic search.
Studies eligible for this meta-analysis had to fulfill the following inclusion criteria: (1) comparative study evaluating the efficacy of prophylactic abdominal drainage after pancreatic resection; (2) at least one postoperative outcome was reported, including mortality, morbidity, pancreatic fistula, abdominal abscess, interventional radiology drainage, reoperation rate, or length of hospital stay; and (3) the study was published as a full-length article.
Abstracts, letters, case reports, editorials, expert opinions, reviews, animal studies, and articles not reporting outcomes of interest were excluded.
The outcomes of interest included mortality, morbidity, postoperative pancreatic fistula (POPF), clinically relevant pancreatic fistula (CR-PF), abdominal abscess, reoperation rate, the rate of interventional radiology drainage, and the length of hospital stay. Table 1 shows the definition of clinical outcomes in each study.
| Author | Mortality | Morbidity | POPF | Abdominal abscess |
| Heslin et al[6] | NA | NA | Drain output at a rate of ≥ 30 mL/d or more and lasting for more than 7 d | Abdominal collection associated with fever and a positive culture requiring either percutaneous or operative drainage yielding positive cultures |
| Conlon et al[8] | Deaths within 30 d of surgery | NA | Drain output on postoperative day 5 or > 30 mL and amylase level > 150 IU/L and/or three times greater than the serum value | Abdominal collection associated with fever and a positive culture requiring either surgical or radiologic drainage |
| Fisher et al[7] | Deaths within 30 d of surgery. | CTCAE (v4.0)[35] | ISGPF[36] | Abdominal collection with a positive Gram stain or cultures |
| Paulus et al[26] | NA | NA | ISGPF[36] | Abdominal collection associated with fever, abnormal blood routine test, and positive cultures |
| Adham et al[29] | Deaths within 90 d of surgery | Clavien classification[37] | ISGPF[36] | Abdominal collection associated with fever and a positive culture requiring surgical drain or interventional treatment |
| Correa-Gallego et al[9] | Deaths within 90 d of surgery | CTCAE (v4.0)[35] | Clinical signs and symptoms with amylase-rich drainage > 50 mL/d beyond postoperative day 10 | Clinical signs and symptoms or radiologic diagnosis of abdominal abscess or peritonitis |
| Lim et al[27] | Clavien classification[37] | Clavien classification[37] | ISGPF[36] | NA |
| Mehta et al[28] | Deaths within 30 d of surgery | Clavien classification[37] | ISGPF[36] | NA |
| Van Buren et al[10] | Deaths within 90 d of surgery | CTCAE (v4.0)[35] | ISGPF[36] | NA |
The titles and abstracts of the search results were scanned for potentially eligible studies. Then, the full texts of potentially eligible studies were screened to determine whether they should be included based on the selection criteria. Data from the included articles were extracted independently by two reviewers, and inconsistencies were resolved by consensus. The quality of RCTs was assessed using the Cochrane Risk of Bias Tool, while that of cohort studies and the case-control study was assessed using the modified Newcastle-Ottawa Scale.
This meta-analysis was conducted based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement, using Review Manager 5 software (The Cochrane Collaboration, Oxford, United Kingdom). Dichotomous variables are presented as ORs with 95%CI, while continuous variables are presented as mean difference (MD) with 95%CI. If the mean value was not available, median values were converted to means for pooled analysis based on Hozo’s method[11]. If the standard deviation (SD) was not available and the range value was available, the SD was calculated from the range value according to Hozo’s formula[11]. If the SD was not reported and the P value or interquartile range value was available, the SD was calculated from the P value or interquartile range value based on the Cochrane Collaboration guidelines[12]. The statistical tests were two-sided, and P < 0.05 was regarded as statistically significant. The Cochrane Q test was conducted to assess statistical heterogeneity; P < 0.1 indicated statistically significant heterogeneity, thus the fixed-effects model was used. Otherwise, the random-effects model was employed. The I2 statistic, which was transformed from the Cochrane Q test [I2 = 100% × (Q-df)/Q], was also used to assess statistical heterogeneity. An I2 value of < 25% indicated low heterogeneity, a value of > 50% indicated high heterogeneity and a value between 25 and 50% indicated moderate heterogeneity[12]. To determine the influence of non-RCTs (NRCTs) on pooled results, we performed a restricted analysis of RCTs. A sensitivity test was conducted by reanalyzing the data after removing each trial to assess the robustness of pooled results.
As distal pancreatectomy (DP) and PD are two distinct operations, subgroup analyses were conducted to evaluate the efficacy of prophylactic abdominal drainage in PD and in DP. Begg’s funnel plot and the Egger’s regression test were employed to assess potential publication bias in this meta-analysis. A P > 0.1 in the Egger’s test indicated no significant publication bias.
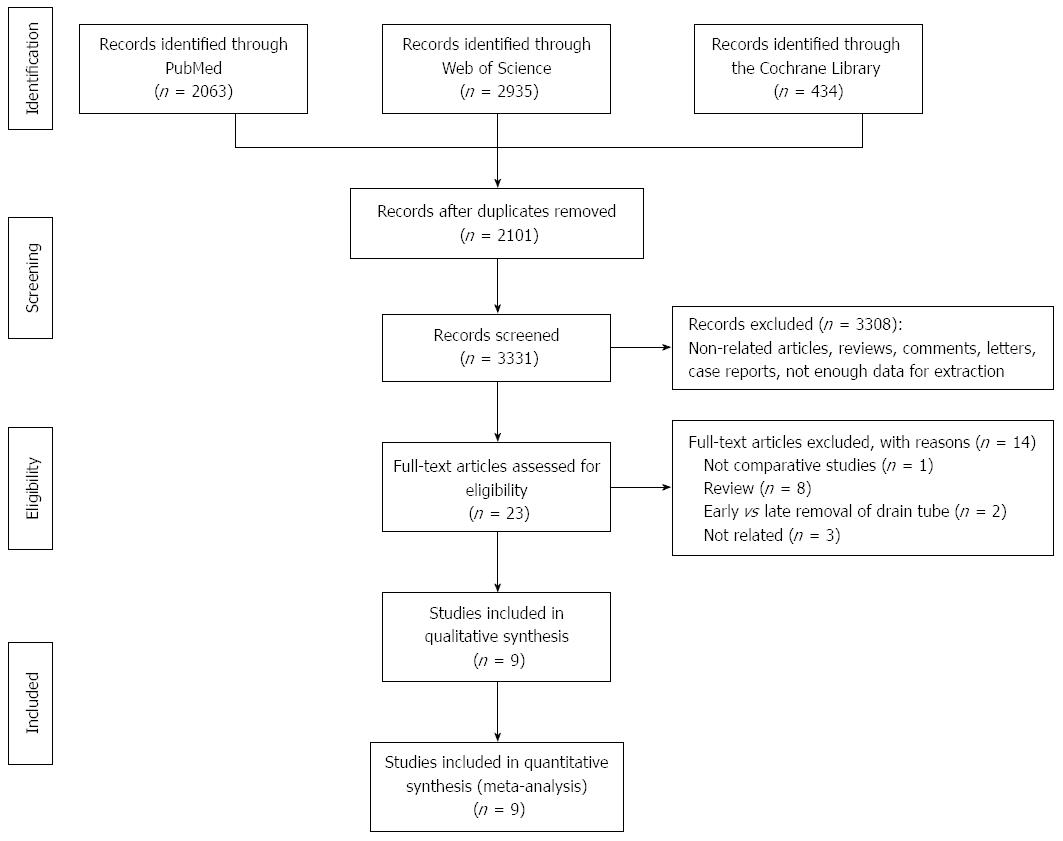
Figure 1 illustrates the process of study selection. Initially, a total of 5432 articles were obtained through a search of PubMed (n = 2063), Web of Science (n = 2935) and the Cochrane Library (n = 434). After scanning the titles and abstracts, 2101 duplicates and 3308 irrelevant articles were excluded. Full texts of the remaining 23 potentially eligible articles were screened for detailed assessment. One study[5] was excluded due to lack of a control group. Two studies[13,14] comparing early removal with late removal of drain tubes and eight review articles[15-22] were excluded. Three articles[23-25] were irrelevant and were excluded after full-text assessment. Nine studies[6-10,13,26-28] were finally included in the meta-analysis.
Basic information on the nine included studies, such as author, year of publication, country, study design, baseline demographics, and surgical procedures are listed in Table 2. Of the nine included studies, two[8,10] were RCTs, one study[27] was a case-control study and the other six[6,7,9,26,28,29] were observational cohort studies. A total of 2794 patients were included in this meta-analysis; 1373 patients received prophylactic abdominal drainage and the remaining 1421 patients did not. The average age of the patients in each study ranged from 62 to 69 years, and the percentage of males varied from 29.6 to 58.1. In four studies[6,10,27,28], the type of pancreatic resection was uniformly PD. In the other four studies[7,8,10,26,29], the type of surgery included DP, PD, and other types of pancreatic resection. The study conducted by Correa-Gallego et al[9] included two distinct subgroups of patients. Patients in one subgroup underwent PD, while those in the other subgroup underwent DP.
| Author, year | Country | Design | No. of patients | Group | Age (yr) | Male:female | Operation type: No. of patients |
| Heslin et al[6] | United States | OCS | 89 | Drain | 65 ± 2 | 18:20 (58.1) | PD: 51 |
| 1998 | No drain | 65 ± 2 | 32:19 | PD: 38 | |||
| Conlon et al[8] | United States | RCT | 179 | Drain | 66 (23-81) | 46:42 (49.7) | PD: 73, DP: 15 |
| 2001 | No drain | 69 (33-87) | 43:48 | PD: 66, DP: 25 | |||
| Fisher et al[7] | United States | OCS | 228 | Drain | 63 (53-72) | 78:101 (40.7) | PD: 123, DP: 56 |
| 2011 | No drain | 59 (51-70) | 19:40 | PD: 30, DP: 17 | |||
| Paulus et al[26] | United States | OCS | 59 | Drain | 52 (44-66) | NA | DP: 39 |
| 2012 | No drain | 58 (52-68) | NA | DP: 30 | |||
| Adham et al[29] | France | OCS | 242 | Drain | 61.5 (20-85) | 66:64 (52.4) | PD: 79, DP: 29, Others: 22 |
| 2013 | No drain | 66.5 (19-85) | 61:51 | PD: 69, DP: 37, Others: 6 | |||
| Correa-Gallego et al[9] | United States | OCS | 739 (Subgroup A of PD) | Drain | NA | NA | PD: 386 |
| 2013 | No drain | NA | NA | PD: 353 | |||
| 350 (Subgroup B of DP) | Drain | NA | NA | DP: 154 | |||
| No drain | NA | NA | DP: 196 | ||||
| Lim et al[27] | France | OCS | 54 | Drain | 62 (40-76) | 8:19 (29.6) | PD: 27 |
| 2013 | No drain | 62 (38-78) | 8:19 | PD: 27 | |||
| Mehta et al[28] | United States | OCS | 709 | Drain | 60 | 130:121 | PD: 251 |
| 2013 | No drain | 62.5 | 232:236 | PD: 458 | |||
| Van Buren et al[10] | United States | RCT | 137 | Drain | 62.1 ± 11.7 | 37:31 | PD: 68 |
| 2013 | No drain | 64.3 ± 12.6 | 38:31 | PD: 69 |
Basic medical information (including comorbidity, preoperative treatment, preoperative biochemical tests, pathology, length of operation, and estimated blood loss) was also reported in the nine included studies. A summary of the comparability of patients in the two groups in each study is shown in Table 3. Most aspects were comparable between the two groups in each study.
| Author | Comorbidity | Preoperative treatment | Preoperative biochemical test | Pathology | Length of operation | Estimated blood loss | Texture of pancreas | Diameter of pancreatic duct |
| Heslin et al[6] | Comparable | Comparable | Comparable | Comparable | NA | Comparable | NA | NA |
| Conlon et al[8] | NA | Comparable | NA | Comparable | Comparable | Comparable | NA | NA |
| Fisher et al[7] | Significant difference | NA | Significant difference | Comparable | Comparable | Significant difference | Comparable | Comparable |
| Paulus et al[26] | NA | NA | NA | Comparable | Comparable | Comparable | Comparable | |
| Adham et al[29] | Comparable | Comparable | Comparable | Comparable | NA | NA | NA | NA |
| Correa-Gallego et al[9] | ||||||||
| PD subgroup | NA | Comparable | NA | Comparable | Significant difference | Significant difference | Significant difference | Comparable |
| DP subgroup | NA | NA | NA | Comparable | Significant difference | Significant difference | NA | Significant difference |
| Lim et al[27] | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable |
| Mehta et al[28] | Comparable | Comparable | Comparable | Comparable | Significant difference | Significant difference | NA | Significant difference |
| Van Buren et al[10] | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable |
The risk of bias in six cohort studies and one case-control study were assessed by the modified Newcastle-Ottawa scale. In the six cohort studies[6,7,9,26,28,29], the exposed cohorts in most studies were representative of the patients who had prophylactic drainage after pancreatic resection. The non-exposed cohorts were selected from the same patient base as the exposed cohorts in most studies. The cohort study by Heslin et al[6] reported an independent assessment of the clinical outcomes. Two studies[6,26] did not have a clear description of the length of follow-up. In the case control study[27], the comparability of cases and controls was ensured by one-to-one matching. The risk of bias in the two RCTs[8,10] was evaluated by the Cochrane Risk of Bias Tool. The two RCTs had a low risk of bias in random sequence generation, allocation concealment and selective reporting. The study by Van Buren et al[10] did not report the blinding assessment of clinical outcomes. The results of the quality assessment for the nine included studies are shown in Table 4.
| Cohort studies | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Comparability between the two cohorts | Assessment of outcome | Length of follow-up |
| Heslin et al[6] | Potential selection bias | Same patient base | Surgical record | No restriction/matching | Independent assessment | NM |
| Paulus et al[26] | Representative | Same patient base | Surgical record | No restriction/matching | Surgical record | NM |
| Fisher et al[7] | Representative | Different patient base | Surgical record | No restriction/matching | Surgical record | 30 d |
| Adham et al[29] | Representative | Same patient base | Surgical record | No restriction/matching | Surgical record | 90 d |
| Correa-Gallego et al[9] | Representative | Same patient base | Surgical record | No restriction/matching | Surgical record | 90 d |
| Metha et al[28] | Representative | Same patient base | Surgical record | No restriction/matching | Surgical record | 90 d |
| Case-control study | Representativeness of the cases | Selection of Controls | Ascertainment of exposure | Comparability of cases and controls | Assessment of outcome | Definition of Controls and cases |
| Lim et al[27] | Potential selection bias | Hospital control | Surgical record | One to one matching | Surgical record | Surgical record |
| RCTs | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
| Conlon et al[8] | Low risk | Low risk | High risk | Low risk | Unclear risk | Low risk |
| Van Buren et al[10] | Low risk | Low risk | High risk | Unclear risk | Low risk | Low risk |
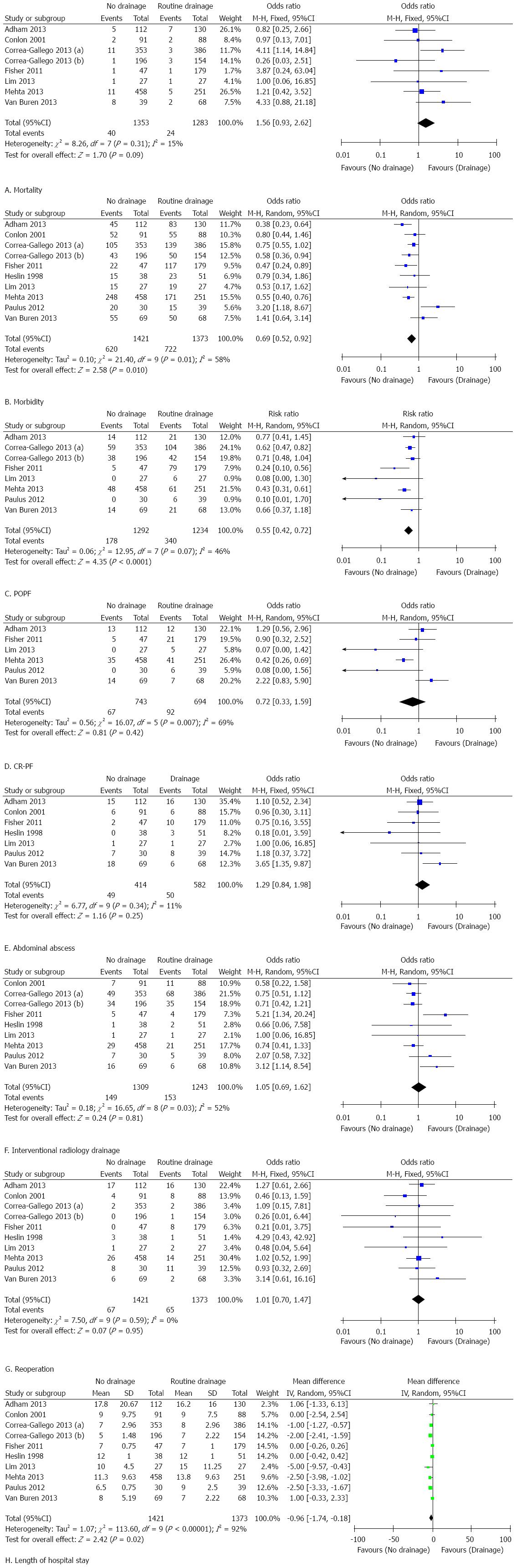
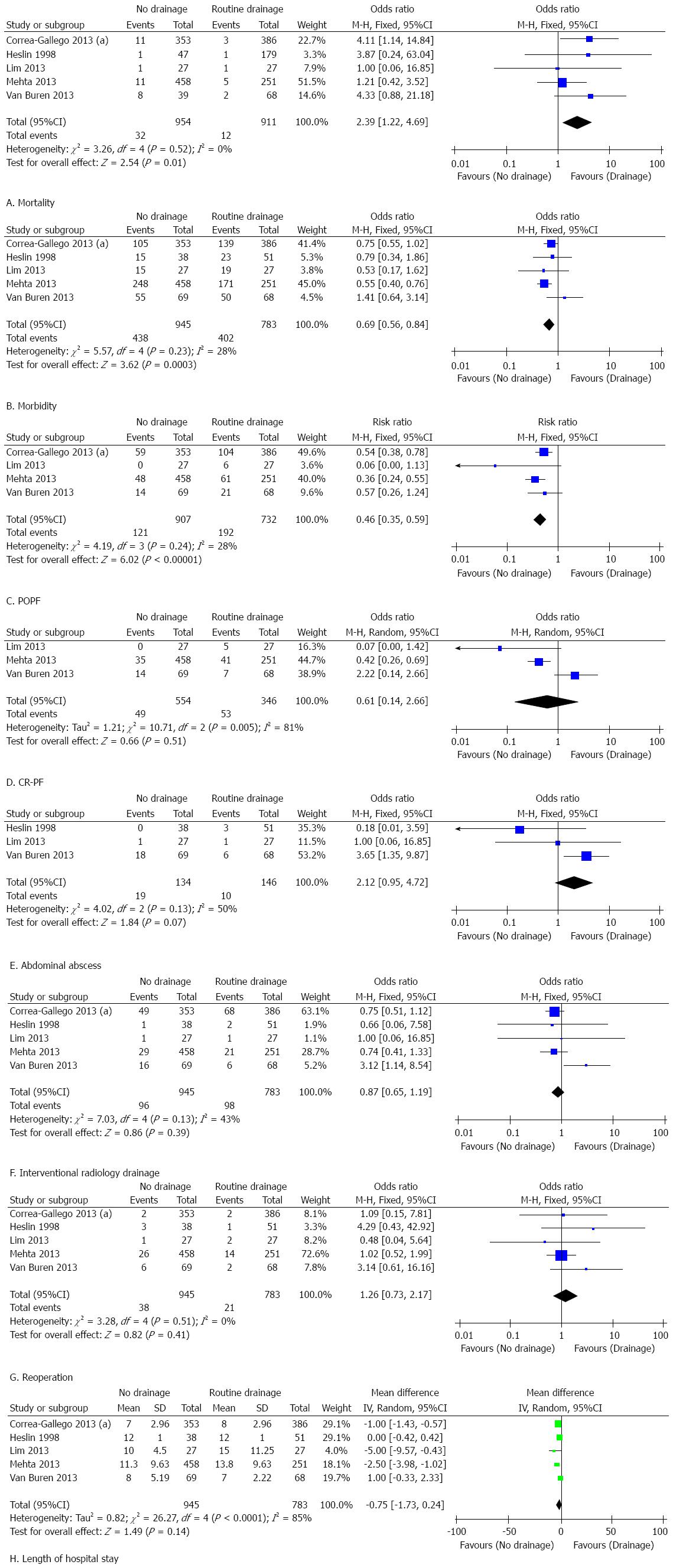
The results of this meta-analysis are summarized in Table 5. Forest plots representing the results of the overall analysis are displayed in Figure 2. Forest plots representing the results of subgroup analyses of PD and DP are shown in Figures 3 and 4.
| Outcome of interest | Studies | Patients | Results | Pooled estimates | P value | P value for HG | I2 | ||
| No drainage | Drainage | No drainage | Drainage | (95%CI) | |||||
| Mortality | |||||||||
| Overall analysis | 7 | 1353 | 1283 | 2.96% | 1.87% | 1.56 (0.93-2.62) | 0.09 | 0.31 | 15% |
| Restricted analysis of RCTs | 2 | 160 | 150 | 6.25% | 2.56% | 2.55 (0.79-8.30) | 0.12 | 0.25 | 26% |
| Subgroup analysis of PD | 5 | 954 | 911 | 3.35% | 1.32% | 2.39 (1.22-4.69) | 0.01 | 0.52 | 0% |
| Overall morbidity | |||||||||
| Overall analysis | 9 | 1421 | 1373 | 43.54% | 52.59% | 0.69 (0.52-0.92) | 0.01 | 0.01 | 58% |
| Restricted analysis of RCTs | 2 | 160 | 150 | 66.88% | 67.31% | 1.00 (0.58-1.72) | 1.00 | 0.26 | 20% |
| Subgroup analysis of PD | 5 | 945 | 783 | 46.35% | 51.34% | 0.69 (0.56-0.84) | < 0.01 | 0.23 | 28% |
| Subgroup analysis of DP | 2 | 226 | 193 | 27.88% | 33.68% | 1.29 (0.24-6.81) | 0.76 | < 0.01 | 89% |
| POPF | |||||||||
| Overall analysis | 7 | 1292 | 1234 | 13.78% | 27.55% | 0.55 (0.42-0.72) | < 0.01 | 0.07 | 46% |
| Subgroup analysis of PD | 4 | 907 | 732 | 13.34% | 26.23% | 0.46 (0.35-0.59) | < 0.01 | 0.24 | 28% |
| Subgroup analysis of DP | 2 | 226 | 193 | 16.81% | 24.87% | 0.39 (0.07-2.21) | 0.29 | 0.17 | 46% |
| CR-PF | |||||||||
| Overall analysis | 6 | 743 | 694 | 9.02% | 13.26% | 0.72 (0.33-1.59) | 0.42 | < 0.01 | 69% |
| Subgroup analysis of PD | 3 | 554 | 346 | 8.84% | 15.32% | 0.61 (0.14-2.66) | 0.51 | < 0.01 | 81% |
| Abdominal abscess | |||||||||
| Overall analysis | 7 | 414 | 582 | 11.84% | 8.59% | 1.29 (0.84-1.98) | 0.25 | 0.34 | 11% |
| Restricted analysis of RCTs | 2 | 160 | 150 | 15.00% | 7.70% | 1.95 (0.53-7.16) | 0.32 | 0.09 | 65% |
| Subgroup analysis of PD | 3 | 134 | 146 | 14.18% | 6.85% | 2.12 (0.95-4.72) | 0.07 | 0.13 | 50% |
| Interventional radiology drainage | |||||||||
| Overall analysis | 8 | 1309 | 1243 | 11.38% | 12.31% | 1.05 (0.69-1.62) | 0.81 | 0.03 | 52% |
| Restricted analysis of RCTs | 2 | 160 | 150 | 14.38% | 10.90% | 1.35 (0.26-6.97) | 0.72 | 0.02 | 81% |
| Subgroup analysis of PD | 5 | 945 | 783 | 10.16% | 12.52% | 0.87 (0.65-1.19) | 0.39 | 0.13 | 43% |
| Subgroup analysis of DP | 2 | 226 | 193 | 18.14% | 20.73% | 1.03 (0.38-2.80) | 0.95 | 0.13 | 57% |
| Reoperation | |||||||||
| Overall analysis | 9 | 1421 | 1373 | 4.71% | 4.73% | 1.01 (0.70-1.47) | 0.95 | 0.59 | 0% |
| Restricted analysis of RCTs | 2 | 160 | 150 | 16.67% | 6.41% | 1.11 (0.17-7.29) | 0.91 | 0.07 | 70% |
| Subgroup analysis of PD | 5 | 945 | 783 | 4.02% | 2.68% | 1.26 (0.73-2.17) | 0.41 | 0.51 | 0% |
| Subgroup analysis of DP | 2 | 226 | 193 | 3.54% | 6.22% | 0.80 (0.29-2.17) | 0.66 | 0.46 | 0% |
| Length of hospital stay | |||||||||
| Overall analysis | 9 | 1421 | 1373 | - | - | -0.96 [-1.74-(-0.18)] | 0.02 | < 0.01 | 92% |
| Restricted analysis of RCTs | 2 | 160 | 150 | - | - | 0.78 (-0.40-1.97) | 0.19 | 0.49 | 0% |
| Subgroup analysis of PD | 5 | 945 | 783 | - | - | -0.75 (-1.73-0.24) | 0.14 | < 0.01 | 85% |
| Subgroup analysis of DP | 2 | 226 | 193 | - | - | -2.10 [-2.46-(-1.73)] | < 0.01 | 0.29 | 11% |
Seven of nine studies reported mortality. The mortality rate in the non-prophylactic drainage group was higher than that in the prophylactic drainage group (2.96% vs 1.87%), although the difference was not statistically significant (OR = 1.56, 95%CI: 0.93-2.92; P = 0.09; I2 = 15%). Pooled analysis of the two RCTs showed consistent results. The sensitivity test showed that eliminating prophylactic drainage was associated with a significant increase in mortality (OR = 1.83, 95%CI: 1.02-3.28; P = 0.04; I2 = 13%), after excluding the study by Adham et al[29].
All nine studies reported morbidity. Pooled results favored the elimination of prophylactic drainage due to significantly lower morbidity in the non-drainage group (OR = 0.69, 95%CI: 0.52-0.92; P = 0.01; I2 = 58%). However, restricted analysis of the two RCTs showed that omitting prophylactic drainage was not associated with reduced morbidity (OR = 1.00, 95%CI: 0.58-1.72; P = 1.00; I2 = 58%). The sensitivity test demonstrated that the difference in morbidity between the groups was not significant after excluding the study by Mehta et al[28] (OR = 0.73, 95%CI: 0.53-1.01; P = 0.06; I2 = 60%).
Seven studies reported the rate of POPF and CR-PF. The pooled results suggested that omitting prophylactic drainage was associated with a significantly lower rate of POPF (OR = 0.55, 95%CI: 0.42-0.72; P < 0.01; I2 = 46%). The difference in the rate of CR-PF was not statistically different between the groups (OR = 0.72, 95%CI: 0.33-1.59; P = 0.42; I2 = 69%). Restricted analysis of the RCTs showed consistent results. The sensitivity test for POPF and CR-PF confirmed the above results.
Seven studies compared the rate of abdominal abscess formation between the two groups and showed discordant results. Prophylactic drainage did not lead to a significantly higher rate of abdominal abscess formation (OR = 1.29, 95%CI: 0.84-1.98; P < 0.25; I2 = 11%). Restricted analysis of the RCTs confirmed this result. This was unchanged after the sensitivity test.
Eight studies reported the rate of postoperative interventional drainage. The frequency of interventional radiology drainage was not different between the groups (OR = 1.05, 95%CI: 0.69-1.62; P = 0.81; I2 = 52%). This result was also supported by restricted analysis of the two RCTs. The result was not altered after the sensitivity test.
All nine studies reported the rate of reoperation after pancreatic resection. Four studies reported higher reoperation rates in the non-prophylactic drainage group, while the other five studies reported the opposite results. The reoperation rates were similar (OR = 1.01, 95%CI: 0.70-1.47; P = 0.95; I2 = 0%) between the two groups. Restricted analysis of the RCTs showed a similar result. The robustness of this result was confirmed by the sensitivity test.
Nine studies reported the length of hospital stay. Pooled results showed that eliminating prophylactic drainage resulted in a significantly shorter length of hospital stay [MD = -0.96, 95%CI: -1.74-(-0.18); P = 0.02]. Noteworthy was the high statistical heterogeneity between the studies (I2 = 92%). Restricted analysis of the RCTs showed consistent results. However, the results were altered after removing the study by Mehta et al[28] (MD = -0.80, 95%CI: -1.61-0.01; P = 0.05; I2 = 93%) or the study by Paulus et al[26] (MD = -0.74, 95%CI: -1.53-0.05; P = 0.07; I2 = 91%).
Subgroup analysis of patients who underwent PD: Mortality in the non-prophylactic drainage group after PD was significantly higher than that in the prophylactic drainage group with a pooled estimate of 2.39 (95%CI: 1.22-4.69; P = 0.01; I2 = 0%). This indicated that omission of prophylactic drainage after PD resulted in significantly higher mortality. However, patients who underwent PD without prophylactic abdominal drainage had a significantly lower rate of morbidity (OR = 0.69, 95%CI: 0.56-0.84; P < 0.01; I2 = 28%) and POPF (OR = 0.46, 95%CI: 0.35-0.59; P < 0.01; I2 = 28%). In terms of CR-PF (OR = 0.61, 95%CI: 0.14-2.66; P = 0.51; I2 = 81%), abdominal abscess (OR = 2.12, 95%CI: 0.95-4.72; P = 0.07; I2 = 50%), reoperation rate (OR = 1.26, 95%CI: 0.73-2.17; P = 0.41; I2 = 0%), postoperative interventional drainage (OR = 0.87, 95%CI: 0.65-1.19; P = 0.39; I2 = 43%) and length of hospital stay (MD = -0.75, 95%CI: -1.73-0.24; P = 0.14; I2 = 85%), no significant differences were observed between the groups.
Subgroup analysis of patients who underwent DP: Only two studies were eligible for the subgroup analysis of patients who underwent DP. No significant difference was found between the groups with respect to morbidity (OR = 1.29, 95%CI: 0.24-6.81; P = 0.76; I2 = 89%), POPF (OR = 0.39, 95%CI: 0.07-2.21; P = 0.29; I2 = 46%), reoperation (OR = 0.80, 95%CI: 0.29-2.17; P = 0.66; I2 = 0%), or interventional radiology drainage (OR = 1.03, 95%CI: 0.38-2.80; P = 0.95; I2 = 57%). Eliminating prophylactic drainage was associated with a significantly reduced length of hospital stay [MD = -2.10, 95%CI: -2.46-(-1.73); P < 0.01; I2 = 11%].
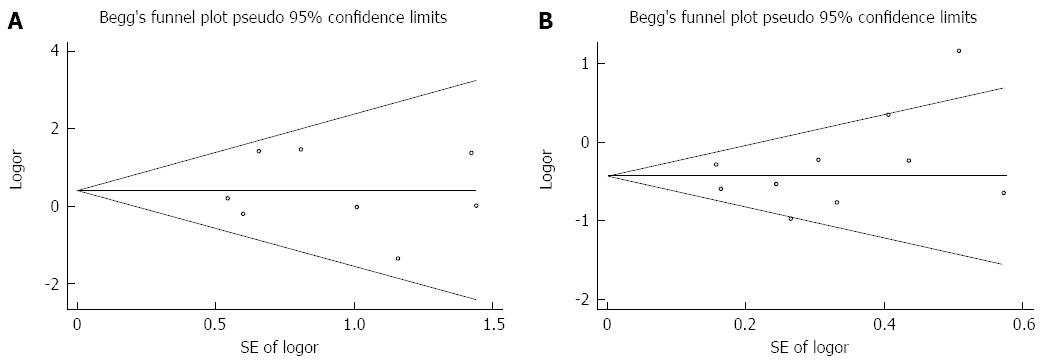
Begg’s funnel plots depicting publication bias are presented in Figure 5. The Egger’s test confirmed that there was no publication bias regarding mortality (P = 0.87) or morbidity (P = 0.32).
Prophylactic drainage at the operative bed after pancreatic resection is a closely held belief for most pancreatic surgeons based on the rationale that it contributes to the early detection of intra-abdominal complications and evacuation of abdominal collections. In contrast, placement of an abdominal drainage tube potentially increases the risk of infection[6,8] and leads to injury of visceral organs[30]. The development of imaging modalities has helped doctors identify intra-abdominal complications early and perform interventional radiology drainage to effectively evacuate abdominal collections[31]. Moreover, the results from comparative studies evaluating the efficacy of prophylactic drainage after pancreatic resection are inconsistent. Therefore, the necessity for prophylactic abdominal drainage after pancreatic resection in all patients has been challenged and its value after pancreatic resection is controversial.
This study shows that prophylactic drainage after pancreatic resection does not improve clinical outcomes in terms of morbidity, POPF, CR-PF, abdominal abscess, reoperation, interventional radiology drainage, or length of hospital stay. However, our results demonstrate that the elimination of prophylactic abdominal drainage results in increased mortality after pancreatic resection. Subgroup analysis of PD patients also shows that omitting prophylactic drainage results in a significantly higher mortality. Therefore, our pooled analysis supports the conclusion that it is unsafe to abandon prophylactic drainage after pancreatic resection, especially in patients undergoing PD.
The most recent RCT by Van Buren et al[10] showed that omitting prophylactic drainage resulted in higher morbidity. This meta-analysis found that the rate of morbidity was significantly lower in patients without prophylactic drainage tubes. However, the pooled results of two RCTs showed comparable morbidity between the groups. Therefore, the actual effect of prophylactic drainage on overall morbidity requires more evidence from RCTs.
In this meta-analysis, POPF was significantly lower in patients without prophylactic drainage. Subgroup analysis of patients who underwent PD showed consistent results. Grobmyer et al[32] proposed that closed drainage suction could generate high negative pressure which possibly contributed to surrounding tissue damage and the formation of a fistula. This may be a possible explanation for increased POPF in drained patients. We put forward another possible explanation: for patients with prophylactic drainage, the fluid from drainage tubes facilitates the detection of mild postoperative complications, which have no obvious symptoms. Mild complications can easily be ignored in patients without prophylactic drainage. Radiologic examinations are not usually performed in patients without clinical symptoms suggestive of intra-abdominal complications. This likely results in a significantly lower rate of overall morbidity and POPF in patients without prophylactic drainage. The discrepancy between the results of POPF and CR-PF support the feasibility of the latter explanation. Although a significantly lower rate of POPF was observed in patients without prophylactic drainage, no significant difference was found between the groups regarding CR-PF, which caused obvious symptoms.
Prospective studies[6,8,33] have suggested that drainage tubes can potentially be utilized by various pathogens and increase the risk of infection, which will subsequently lead to the formation of an abdominal abscess. A prospective study of 104 consecutive patients compared early with late drain removal after pancreatic head resection and concluded that early drain removal reduced the risk of intra-abdominal infections[13]. Conlon et al[8] also reported that patients with routine drainage were more likely to develop abdominal abscesses. However, the pooled results of the present meta-analysis indicate that prophylactic drainage does not increase the risk of abdominal abscess.
Another concern related to abdominal drainage is the potential injury to visceral organs caused by the drainage tubes. Bae et al[30] reported the case of a 70-year-old man who underwent PD due to a distal common bile duct malignancy. The two drainage tubes placed at the pancreaticojejunostomy and choledochojejunostomy sites led to penetration of the jejunum. In this meta-analysis, injury of visceral organs directly caused by drainage tubes was not reported in the nine included studies. This evidence indicates that the risk of potential injury to visceral organs related to drainage tubes after pancreatic resection is minimal.
Based on the results of the subgroup analysis of patients who underwent DP, elimination of prophylactic drainage was associated with a significantly shorter length of hospital stay and had no adverse influence on other clinical outcomes including morbidity, POPF, interventional radiology drainage and reoperation. However, this conclusion is not robust as only two studies were included in this subgroup analysis. To determine whether prophylactic drainage can be safely eliminated after DP requires further investigation.
To date, two RCTs have been conducted to evaluate the efficacy of prophylactic abdominal drainage after PD and their results were conflicting. Patients without prophylactic abdominal drainage have been shown to have acceptable outcomes in several observational studies. Thus, more RCTs should be conducted to examine the value of prophylactic abdominal drainage after PD.
With the further development of surgical techniques and perioperative management, postoperative complications related to pancreatic resection will subsequently decrease. Therefore, we are optimistic that prophylactic abdominal drainage will not be routine practice in the future. Moreover, numerous studies have been conducted to evaluate the risk factors for POPF and other complications after pancreatic surgery. Callery et al[34] proposed a clinical risk score predicting pancreatic fistula after PD based on intraoperative bleeding, diameter of the pancreatic duct, texture of the pancreas, and pathologic diagnosis. Lim et al[27] suggested that it was safe to abandon the practice of abdominal drainage after PD in patients with low risk for POPF. Based on these studies, it is predicted that surgeons in the future will evaluate the risk of postoperative complications and tailor an appropriate drainage strategy for each patient, instead of performing prophylactic drainage for all patients undergoing pancreatic resection.
There are several limitations related to this study. First, most of the studies included in this meta-analysis were observational comparative studies[6,7,9,26-29]. An inevitable disadvantage of NRCTs is that the surgeon’s criteria for use of prophylactic drainage may be affected by surgery progression, including intraoperative bleeding, the length of operation, and other factors (as shown in Table 3). These factors during surgery will influence the incidence of postoperative complications and even the prognosis of patients. Therefore, we compared these influencing factors between the patients in the two groups. Most influencing factors were comparable between the patients in the two groups. Moreover, to further eliminate the influence of NRCTs on the pooled results, we performed a restricted analysis of the two RCTs. Most pooled results of the two RCTs were consistent with those in the overall analysis, indicating the soundness of the conclusion of this meta-analysis. Second, in most studies, the patients were not stratified by risk factors for POPF (including diameter and texture of the pancreatic duct, intraoperative bleeding, and pathology). Therefore, we were unable to perform a more detailed subgroup analysis in which the patients were stratified by these known risk factors. Third, detailed information on the included patients was not available in most published studies. This detailed patient information, such as the number of postoperative days to drain removal and the type and number of drainage tubes, can potentially affect the clinical outcome of patients. Studies in the future should provide more complete information on included patients to obtain a more detailed analysis. Fourth, the mean value and SD of the length of hospital stay were not available in some studies. We calculated the mean and SD from median, range, interquartile ranges or P value. This possibly influenced the accuracy and reliability of the results. Fifth, the definitions and grading systems for assessing postoperative complications were not universal between the studies. The operative techniques, anastomotic methods, and the perioperative management were not the same in each study. These factors increased the heterogeneity and hindered the comparison of clinical outcomes between the studies. Sixth, only two studies were included in the subgroup analysis that evaluated the need for prophylactic drainage in patients undergoing DP. Thus, the results of the subgroup analysis of DP were not reliable.
In conclusion, our results fail to show that patients receiving prophylactic abdominal drainage have improved outcomes in terms of morbidity, POPF, CR-PF, interventional treatments, and length of hospital stay. However, this meta-analysis demonstrates that the elimination of prophylactic drainage results in an increase in mortality rate after pancreatic resection, especially in patients who underwent PD. Placement of prophylactic drainage did not increase the risk of abdominal abscess. Based on present evidence, we conclude that prophylactic abdominal drainage is still necessary for patients undergoing pancreatic resection, especially for those undergoing PD. More RCTs assessing the value of prophylactic drainage after PD and DP are required to provide more powerful evidence. Based on this study and current evidence, we are optimistic that prophylactic abdominal drainage will not be routine practice in the future due to the development of surgical techniques and perioperative management. Moreover, instead of universally performing prophylactic drainage in all patients after pancreatic resection, the drainage strategy should be tailored based on the characteristics of each patient.
Prophylactic abdominal drainage is considered routine and mandatory after pancreatic resection, which is a highly invasive surgery. However, prospective studies have demonstrated that prophylactic drainage does not result in any benefit after other abdominal surgeries, including cholecystectomy, hepatectomy, and colorectal surgery. Some surgeons have questioned the significance of prophylactic drainage after pancreatic resection. Studies assessing the value of prophylactic drainage after pancreatic resection have been conducted. The results of these studies are conflicting, and the value of prophylactic abdominal drainage after pancreatic resection has been intensively debated in recent years.
Over the past two decades, studies have been performed to investigate the value of prophylactic abdominal drainage after pancreatic resection. In addition, several reviews have recently been published discussing this issue. However, these reviews were methodologically insufficient and thus did not reach a comprehensive conclusion.
This meta-analysis found that prophylactic drainage after pancreatic resection does not improve clinical outcome in terms of morbidity, postoperative pancreatic fistula, clinically relevant pancreatic fistula, abdominal abscess, reoperation, interventional radiology drainage, and length of hospital stay. The elimination of prophylactic abdominal drainage results in increased mortality after pancreatic resection. Subgroup analysis of pancreaticoduodenectomy (PD) patients also showed that omitting prophylactic drainage results in significantly higher mortality.
The results of this study suggest that it is unsafe to omit prophylactic drainage after pancreatic resection, especially in patients undergoing PD. Additional RCTs assessing the value of prophylactic drainage after PD and pancreatectomy are required to provide more powerful evidence.
Pancreatic resections, including PD and distal pancreatectomy, are highly invasive procedures for treating benign and malignant periampullary diseases. Prophylactic abdominal drainage is traditionally considered an effective method to evacuate anastomotic leakage fluid and abdominal collections after invasive abdominal surgery.
This is a well-performed meta-analysis of currently available studies on the value of prophylactic abdominal drainage after pancreatic resection. The authors found that prophylactic drainage after pancreatic resection did not improve clinical outcomes, including morbidity, postoperative pancreatic fistula, clinically relevant pancreatic fistula, abdominal abscess, reoperation, interventional radiology drainage, and length of hospital stay. However, eliminating prophylactic abdominal drainage results in increased mortality after pancreatic resection, especially for patients who underwent PD. This meta-analysis will provide useful guides for pancreatic surgeons.
P- Reviewer: Barneo L S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
| 1. | Monson JR, Guillou PJ, Keane FB, Tanner WA, Brennan TG. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery. 1991;109:740-746. [PubMed] |
| 2. | Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Fong Y, Brennan MF, Brown K, Heffernan N, Blumgart LH. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 165] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Hoffmann J, Shokouh-Amiri MH, Damm P, Jensen R. A prospective, controlled study of prophylactic drainage after colonic anastomoses. Dis Colon Rectum. 1987;30:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Jeekel J. No abdominal drainage after Whipple’s procedure. Br J Surg. 1992;79:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Heslin MJ, Harrison LE, Brooks AD, Hochwald SN, Coit DG, Brennan MF. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, Brunicardi FC. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford). 2011;13:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, Merchant N, Brennan MF. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487-493; discussion 493-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Correa-Gallego C, Brennan MF, D’angelica M, Fong Y, Dematteo RP, Kingham TP, Jarnagin WR, Allen PJ. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Ann Surg. 2013;258:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Van Buren G, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Vollmer C, Velanovich V, Riall T, Muscarella P. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 11. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6902] [Article Influence: 345.1] [Reference Citation Analysis (0)] |
| 12. | Higgins JPT, Green S, editors . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2008; Available from: http://www.cochrane-handbook.org. |
| 13. | Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 15. | Büchler MW, Friess H. Evidence forward, drainage on retreat: still we ignore and drain!? Ann Surg. 2006;244:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Yeo CJ. Pancreatic surgery 101: drain, no drain, early drain removal, or late drain removal. What are the data? Where do we go from here? Ann Surg. 2010;252:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Allen PJ. Operative drains after pancreatic resection--the Titanic is sinking. HPB (Oxford). 2011;13:595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Büchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Giovinazzo F, Butturini G, Salvia R, Mascetta G, Monsellato D, Marchegiani G, Pederzoli P, Bassi C. Drain management after pancreatic resection: state of the art. J Hepatobiliary Pancreat Sci. 2011;Epub ahead of print. [PubMed] |
| 20. | Nissen NN, Menon VG, Puri V, Annamalai A, Boland B. A simple algorithm for drain management after pancreaticoduodenectomy. Am Surg. 2012;78:1143-1146. [PubMed] |
| 21. | Kaminsky PM, Mezhir JJ. Intraperitoneal drainage after pancreatic resection: a review of the evidence. J Surg Res. 2013;184:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | van der Wilt AA, Coolsen MM, de Hingh IH, van der Wilt GJ, Groenewoud H, Dejong CH, van Dam RM. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB (Oxford). 2013;15:337-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Shinchi H, Wada K, Traverso LW. The usefulness of drain data to identify a clinically relevant pancreatic anastomotic leak after pancreaticoduodenectomy? J Gastrointest Surg. 2006;10:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Kim JK, Park JS, Hwang HK, Shin HW, Yoon DS. Drainage volume after pancreaticoduodenectomy is a warning sign of chyle leakage that inversely correlates with a diagnosis of pancreatic fistula. World J Surg. 2013;37:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Li SG, Shi WB, Mei JW, Wang JD, Shen J, Zhou XP, Wang XF. [The effect of drainage in cavities on preventing from grade B and C of the pancreatic fistula after pancreaticoduodenectomy]. Zhonghua Waike Zazhi. 2013;51:400-402. [PubMed] |
| 26. | Paulus EM, Zarzaur BL, Behrman SW. Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary? Am J Surg. 2012;204:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Lim C, Dokmak S, Cauchy F, Aussilhou B, Belghiti J, Sauvanet A. Selective policy of no drain after pancreaticoduodenectomy is a valid option in patients at low risk of pancreatic fistula: a case-control analysis. World J Surg. 2013;37:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. J Am Coll Surg. 2013;216:635-642; discussion 642-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Adham M, Chopin-Laly X, Lepilliez V, Gincul R, Valette PJ, Ponchon T. Pancreatic resection: drain or no drain? Surgery. 2013;154:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Bae SH, Lee TH, Lee SH, Lee SH, Park SH, Kim SJ, Kim CH. Drain Tube-Induced Jejunal Penetration Masquerading as Bile Leak following Whipple’s Operation. Case Rep Gastroenterol. 2011;5:295-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002;137:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Grobmyer SR, Graham D, Brennan MF, Coit D. High-pressure gradients generated by closed-suction surgical drainage systems. Surg Infect (Larchmt). 2002;3:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Burt BM, Brown K, Jarnagin W, DeMatteo R, Blumgart LH, Fong Y. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 35. | Services USDoHaH Common terminology criteria for adverse effects (CTCAE), version 4. 0. Available from: http://evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_85x11pdf.. |
| 36. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3514] [Article Influence: 175.7] [Reference Citation Analysis (34)] |
| 37. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24846] [Article Influence: 1183.1] [Reference Citation Analysis (0)] |