Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5635
Peer-review started: October 9, 2014
First decision: November 14, 2014
Revised: November 28, 2014
Accepted: December 19, 2014
Article in press: December 22, 2014
Published online: May 14, 2015
Processing time: 221 Days and 16.8 Hours
AIM: To determine if hyperpolarisation-activated nucleotide-gated (HCN) channels exist in human colon, and to investigate the expression of HCN channels in Hirschsprung’s disease.
METHODS: We investigated HCN1, HCN2, HCN3 and HCN4 protein expression in pull-through specimens from patients with Hirschsprung’s disease (HSCR, n = 10) using the proximal-most ganglionic segment and distal-most aganglionic segment, as well as in healthy control specimens obtained at the time of sigmoid colostomy closure in children who had undergone anorectoplasty for imperforate anus (n = 10). Fluorescent immunohistochemistry was performed to assess protein distribution, which was then visualized using confocal microscopy.
RESULTS: No HCN1 channel expression was observed in any of the tissues studied. Both HCN2 and HCN4 proteins were found to be equally expressed in the aganglionic and ganglionic bowel in HSCR and controls. HCN3 channel expression was found to be markedly decreased in the aganglionic colon vs ganglionic colon and controls. HCN2-4 channels were seen to be expressed within neurons of the myenteric and submucosal plexus of the ganglionic bowel and normal controls, and also co-localised to interstitial cells of Cajal in all tissues studied.
CONCLUSION: We demonstrate HCN channel expression in human colon for the first time. Reduced HCN3 expression in aganglionic bowel suggests its potential role in HSCR pathophysiology.
Core tip: Children with Hirschsprung’s disease experience symptoms of intestinal obstruction due to a spastically contracted aganglionic segment of distal bowel, however the mechanism for this spasticity is incompletely understood. Hyperpolarisation-activated nucleotide-gated channels play a key role in regulating cell excitability in both pacemaker and non-pacing cells. We examined expression of hyperpolarisation-activated nucleotide-gated (HCN) 1-4 in both Hirschsprung’s disease (HSCR) colon and healthy controls. While HCN-1 expression was absent, and HCN-2 and HCN-4 were expressed at similar levels in diseased and healthy bowel, we found HCN-3 to be reduced in aganglionic bowel in HSCR. We suggest deficient HCN3 channels may be involved in the pathophysiology of HSCR.
- Citation: O’Donnell AM, Coyle D, Puri P. Decreased expression of hyperpolarisation-activated cyclic nucleotide-gated channel 3 in Hirschsprung’s disease. World J Gastroenterol 2015; 21(18): 5635-5640
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5635.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5635
The enteric nervous system (ENS) is a complex network of neurons and glia, arranged in concentric rings of ganglionated plexuses within the walls of the gastrointestinal (GI) tract. Normal intestinal motility requires the coordinated interaction of the ENS, smooth muscle cells (SMCs) and interstitial cells of Cajal (ICCs). Hirschsprung’s disease (HSCR) is a congenital condition characterized by an absence of enteric ganglia in the bowel. This condition is caused by a failure of neural crest cell migration to the distal hindgut during the early stages of embryonic development, which results in a tonically contracted aganglionic segment. The pathophysiology of HSCR is poorly understood. It is recognized that the obstructive symptoms in HSCR are secondary to the abnormal motility of the distal narrowed segment of bowel, but there is still no explanation for the occurrence of the spastic or tonically contracted segment of bowel.
Hyperpolarisation-activated non-specific cation currents (Ih currents) are important for the regulation of cell excitability. The Ih current is typically seen as a slowly developing inward current that is activated by hyperpolarisation of the membrane beyond the resting potential. In non-pacing cells, Ih helps determine resting membrane properties and limits the extent of hyperpolarizing responses[1]. These currents are carried by channels of the hyperpolarisation-activated nucleotide-gated (HCN) family. Four mammalian genes that encode HCN isoforms (HCN 1-4) have been identified[2]. Different combinations of HCN channel subtypes have been reported to be expressed in various organs, which suggests that the role of these channels differs based on their anatomical location. To date, HCN channels have been found to be expressed in the heart[3], the central nervous system[4], the retina[5], the urinary system[6,7], and mouse colon[2,8].
The ENS contains a large number of neurons that are arranged in ganglionated plexuses within the wall of the GI tract. Electrophysiologically, there are two major types of neurons, S/Dogiel Type I neurons and AH/Dogiel Type II neurons. AH neurons are so named due to the “after-hyperpolarisation” that occurs following the action potential. Xiao et al[2] investigated the expression and localization of HCN isoforms in the mouse distal colon and showed that HCN2 was most highly expressed on AH/Dogiel Type II neurons, with lower levels of expression of HCN2 and HCN4 and a lack of HCN3 expression. It is believed that AH/Dogiel II neurons are excitatory neurons and exhibit the Ih current[2]. Recently, Yang et al[9] investigated the role of HCN2 channels in spontaneous rhythmic activity of GI tract in mice and showed that the HCN2 channels were located within the myenteric neurons. More recently, Shahi et al[8] reported existence of HCN channels in cultured interstitial cells of Cajal from the mouse colon and suggested that HCN channels participate in the regulation of pacemaker activity in the GI tract.
ICCs are pacemaker cells, which actively propagate electrical slow waves in the gastrointestinal smooth muscle. They mediate inhibitory and excitatory motor neurotransmission. Several investigators have reported altered distribution of ICCs in the aganglionic bowel[10]. As HCN channels are also pacemaker channels that regulate spontaneous rhythmic activity, we therefore hypothesized that HCN channel expression is impaired in HSCR and designed this study to investigate expression of HCN channels in normal human colon and in patients with HSCR.
This study was approved by the Ethics Medical Research Committee, Temple Street Children’s University Hospital (Ref. 13.003) and tissue samples were obtained with informed parental consent. HSCR specimens from 10 patients (9 male, 1 female, aged 3-14 mo) who underwent pull-through surgery were studied. These specimens were divided into aganglionic and ganglionic samples. Ganglionic samples were taken from the most proximal margin of the pull-through specimen, while ganglionic samples were taken from the most distal margin of the pull-through specimen. Normal control samples included 10 specimens from patients who underwent sigmoid colostomy closure following anorectoplasty for imperforate anus (7 boys, 3 girls, aged 7-21 mo). Tissue specimens were either snap-frozen in liquid nitrogen and stored at -80 °C for protein extraction or embedded in OCT Mounting Compound (VWR International, Leuven, Belgium) for immunofluorescence and stored at -80 °C until use.
Frozen blocks of HSCR colon and normal control samples were sectioned transversely at a thickness of 10 μm, mounted on SuperFrost® Plus slides (VWR International, Leuven, Belgium) and fixed with 10% buffered formalin for 5 min. Sections underwent cell membrane permeabilization with 1% TritonX-100 for 20 min at room temperature. After blocking with 10% normal goat serum (Sigma Aldrich Ltd, Arklow, Ireland) for 30 min to avoid non-specific absorption, sections were incubated with primary antibodies; rabbit anti-HCN1, rabbit anti-HCN2 (Abcam, Cambridge, United Kingdom), mouse anti-HCN3 and rabbit anti-HCN4 (Santa Cruz, United States), mouse anti-HuC/HuD (Molecular Probes), mouse anti-α-smooth muscle actin, rabbit anti-protein gene product 5.5 (Sigma Aldrich, Ireland), mouse anti-c-kit and rabbit anti-c-kit (Abcam, Cambridge, United Kingdom) and rabbit anti-TMEM (Santa Cruz), all used at dilution 1:100, overnight at 4 °C. Sections were then washed in PBS+0.05% Tween and incubated with corresponding secondary antibodies (goat anti-rabbit Alexa Fluor® 488, dilution 1:200 and goat anti-mouse Alexa Fluor® 647, dilution 1:200, Abcam, Cambridge, United Kingdom) for 1 h at room temperature. After washing, sections were counterstained with DAPI antibody, dilution 1:1000 (Roche Diagnostics GmbH, Mannheim, Germany) for 10 min, washed, mounted and cover-slipped with Fluorescent Mounting Medium (DAKO Ltd, Cambridgeshire, United Kingdom). All sections were independently evaluated by two investigators with a LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Specimens of HSCR colon and control colon were homogenized in RIPA buffer (Radio Immunoprecipitation Assay, Sigma-Aldrich Ltd., Wicklow, Ireland) containing 1% protease inhibitor cocktail (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland). Protein concentrations were determined using a Bradford assay (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland). A total volume of 20 μL Laemmli sample buffer (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland) containing 10 μg of protein was loaded in the 10% SDS-PAGE gel (NuPAGE Novex Bis-Tris gels, Invitrogen, Carlsbad, United States) for electrophoretic separation. The electrophoresis was performed in MES SDS running buffer (Invitrogen, Carlsbad, United States). Proteins were then transferred to 0.45 μm nitrocellulose membrane (Millipore Corporation, Billerica, United States) by western blotting. Following western blotting, the membranes were blocked in 3% BSA-0.05% Tween for 30 min before antibody detection. Primary antibodies; rabbit anti-HCN1 and rabbit anti-HCN2 (Abcam, Cambridge, United Kingdom) dilution 1:1000, and mouse anti-HCN3 and rabbit anti-HCN4 (Santa Cruz, United States) dilution 1:1000 were used and incubation was performed overnight at 4 °C. Following extensive washing (four times in PBS-0.05% Tween) the membranes were incubated with the appropriate secondary antibodies (goat anti-rabbit IgG, HRP-linked Antibody, dilution 1:10000, and goat anti-mouse IgG-HRP, dilution 1:10000, Abcam, Cambridge, United Kingdom) respectively followed by washing (four times in PBS-0.05% Tween). Detection was performed with the ECL plus chemiluminescence kit (Thermo, Fisher Scientific, Dublin, Ireland). We used GAPDH (mouse anti-GAPDH, dilution 1:1000, Abcam, Cambridge, United Kingdom) as an additional loading control.
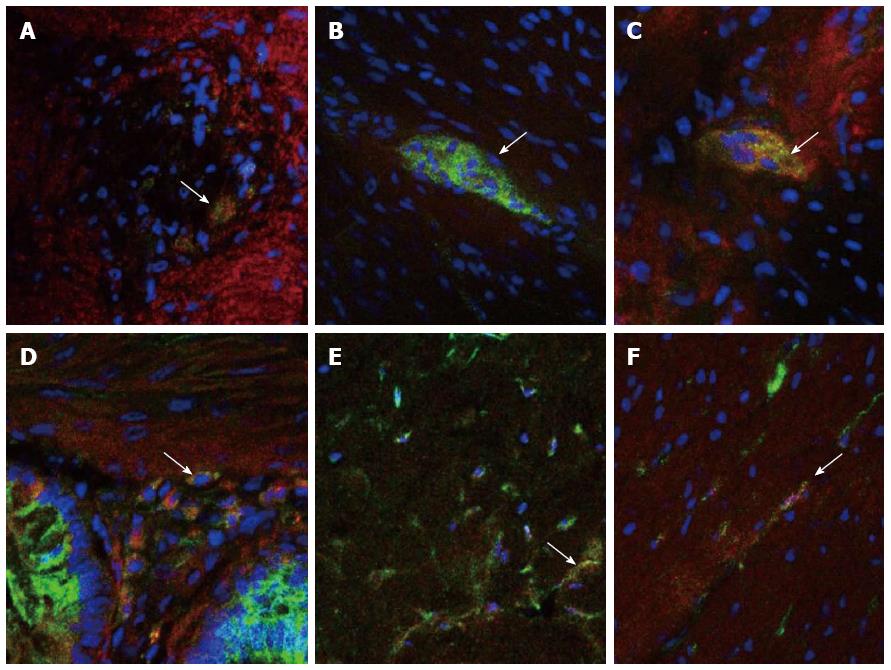
Immunofluorescence double-staining revealed that HCN2, HCN3 and HCN4 channels were expressed in neurons located within both the myenteric and submucosal plexuses of the normal human colon, as well as on ICCs within both the muscular and myenteric layers (Figure 1). In the HSCR tissue specimens, HCN3 channels were seen to be decreased in the ganglionic region compared to normal controls, with a further decrease evident within the aganglionic region. HCN2 and HCN4 channels appeared to be equally expressed in aganglionic, ganglionic and normal control specimens. No co-localisation was evident between any of the HCN channels and smooth muscle cells.
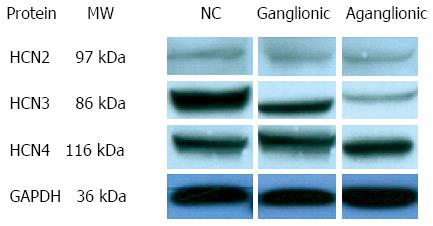
Western blot results showed that HCN1 protein expression was not evident in any of the tissues studied. HCN3 protein expression was found to be decreased in the ganglionic region compared to normal controls, with a further decrease evident within the aganglionic region (Figure 2). HCN2 and HCN4 channel proteins appeared to be equally expressed in aganglionic, ganglionic and normal control specimens.
The ENS contains more than 150 million nerve cells and constitutes an integrative neuronal network composed of intramural ganglia and interconnecting nerve fibres arranged in two major nerve plexuses, the submucosal plexus and the myenteric plexus. Mediation of GI motility, as well as the functions of resorption, secretion and immunity, is regulated by intrinsic reflex circuits orchestrated by the ENS. Similarly to the CNS, the functional integrity of the ENS depends on intact synaptic transmission and plasticity involving the synthesis, release and trafficking of a broad range of inhibitory and excitatory enteric neurotransmitters. Aberrations of enteric neurotransmission are associated with a wide spectrum of functional GI diseases characterized by severe disturbances of GI motility.
Over the last decade, several studies have reported the expression of HCN channels in the ENS of various species. In 2004, Xiao et al[2] showed the expression of HCN1, HCN2 and HCN4 isoforms in the enteric neurons of mice, rats and guinea-pigs. In rat and mouse ENS, HCN1 immunoreactivity was observed on Dogiel type II neurons. HCN2 channel immunoreactivity occurred in the majority of enteric neurons in the guinea-pig, rat and mouse, while immunoreactivity for the HCN4 protein was revealed on the cell membranes of many neurons, including Dogiel type II neurons, in the guinea-pig[2]. HCN4 was found to only be expressed by glial cells in the guinea-pig ENS. No HCN3 channel expression was found in any of the species studied. Reverse transcription-polymerase chain reaction revealed mRNA for all four HCN channels in the longitudinal muscle and myenteric plexus of mouse distal colon, while HCN2 was the most highly expressed HCN channel subtype in the myenteric plexus of mouse distal colon. HCN1 and HCN4 were found to be expressed at lower levels[2]. Intracellular recording to identify neurons with Ih currents revealed that AH neurons with Ih currents were HCN2 and HCN4 channel positive[2].
A study by Wang et al[11] in 2012 investigated the role of HCN channels in regulating the excitability of vagal and spinal gut afferents in the mouse small intestine. They measured the mechano-sensory response of mesenteric afferent activity in an ex vivo murine jejunum preparation. HCN channel activity was recorded through voltage and current clamp in acutely dissociated neurons of the dorsal root ganglia and nodose ganglia, which were retrogradely labeled from the small intestine through the injection of a fluorescent marker, DiI and examined by immunohistochemistry[11]. Their results showed that ramp distension of the small intestine evoked biphasic increases in the afferent nerve activity, reflecting the activation of low- and high-threshold fibers. Subsequent administration of two HCN blockers, CsCl and ZD7288, resulted in inhibiting the responses of low-threshold fibers to distension but showed no significant effects on the high-threshold responses. Double-labelled immunohistochemistry revealed differential expression of HCN isoforms in vagal and spinal afferents, with HCN2 and HCN3 being the more dominant isoforms expressed in DRG and NG, respectively[11].
In 2012, Si et al[12] demonstrated the expression of HCN channels on ICCs of the mouse gastric antrum, and they found that these channels were strongly affected by Cholecystokinin-8S (CCK-8S). They therefore concluded that extracellular calcium may be a trigger in the activation of HCN channels caused by CCK-8S in cultured ICCs.
Yang et al[9] performed an immunohistochemical study focusing on the distribution of HCN2-positive cells in the mouse GI tract. Their results showed that HCN2 channels were mainly located within the myenteric neurons of the ENS, with double-staining revealing that HCN2-positive neurons were labeled by ChAT, indicating that these HCN2-positive cells were cholinergic neurons. No co-localisation was evident using the anti-kit antibody, however it was noted that the neuronal processes of HCN2-positive neurons were in close proximity to ICCs within the myenteric plexus region tract[9]. Furthermore, several differences in the distribution of HCN2 in the stomach, small intestine and colon were partly consistent with the regional differences in the spontaneous rhythmic activities of these organs. The authors concluded that HCN2 channels may facilitate the release of Acetylcholine from cholinergic neurons to affect the GI peristalsis by acting on M receptors on the ICCs, despite the fact that the HCN2 channels are not directly involved in spontaneous slow-wave initiation by ICCs[9].
In a very recent study by Shahi et al[8] the authors used reverse transcription- polymerase chain reaction to assess the expression of mRNA transcripts for HCN channels in isolated murine colonic ICCs. Their results revealed that all four HCN channel subtypes were evident in the whole mount cultured colonic cells. However, in c-kit and Anoctamin-1- positive colonic ICCs, only mRNA transcripts for the HCN1 and HCN3 channel were expressed. Functionally HCN channels were found to be involved in the regulation, but not the initiation, of ICC pacemaker activity[8]. Additionally, they showed that HCN channels on ICCs in murine colon were tonically activated by basal cAMP production[8]. Of interest, however, was the prior observation by Stieber et al[13] who first cloned the human HCN3 channel in HEK293 cells, that HCN3, unlike HCN2 and HCN4, lacked a response to cAMP.
To our knowledge this is the first study reporting distribution and localization of HCN channels in the normal human colon and HSCR, the most common congenital gut motility disorder in children. Our results showed the presence of HCN2, HCN3 and HCN 4 channels in the neurons of both myenteric and submucosal plexuses and on ICCs within the normal human colon. A striking finding was the markedly reduced expression of HCN3 channels not only in the aganglionic bowel but also in the ganglionic bowel of patients with HSCR. Our group has previously reported decreased expression of ICCs both in the aganglionic and ganglionic bowel in HSCR patients[10,14]. Taken together, these findings suggest that decreased expression of HCN3 channels and ICCs in the aganglionic may contribute to motility dysfunction in HSCR by defective regulation of spontaneous pacemaker activity. HCN3 channels have already been shown to play a key role in the pacemaker activity in renal tissue. Hurtado et al[7] also demonstrated that HCN3-positive cells were coupled to smooth muscle cells by gap junctions and suggested that they play a role in the pathophysiology of renal motility disorders.
HSCR is characterized by an absence of neurons in the distal colon for varying distances. This aganglionic region is resected during surgery in order to restore normal bowel function in these patients. However, despite a properly performed pull-through procedure, our group and others have previously reported that up to half of all HSCR patients still suffer from persistent bowel symptoms such as severe constipation, soiling and enterocolitis[15-19]. These observations imply that, although the ganglionic segment in these patients is normally ganglionated, the presence of ganglion cells alone in the pull-through segment is not sufficient as an indicator of a satisfactory outcome. Other factors in these patients necessary for normal gut motility may be absent or deficient in the normoganglionic region. Decreased HCN3 channel expression observed in the normoganglionic colon in HSCR patients may be the cause of motility disturbances in some patients following a properly performed pull-through operation. Further studies are required to examine HCN channel expression in the proximal segment of resected bowel in HSCR patients to confirm if deficiency of HCN3 channel expression is the cause of persistent bowel symptoms in these patients.
In conclusion, we demonstrate for the first time the expression of HCN channels in the human colon. The decreased expression of HCN3 channels in the aganglionic and ganglionic bowel of HSCR patients suggests that HCN3 channels may play a role in the pathophysiology of this complex condition.
We wish to acknowledge the logistical assistance of the Departments of Histopathology in Our Lady’s Children’s Hospital, Crumlin and Children’s University Hospital, Temple St, Dublin in specimen collection. This research was funded by a grant from the National Children’s Research Centre/Children’s Medical Research Foundation, Our Lady’s Children’s Hospital, Crumlin.
Regulation of cell excitability in the gut wall is important in maintaining normal colonic motility. Hyperpolarisation-activated non-specific cation (Ih) currents are important in regulating cell excitability. These currents are carried by a family of channels known as hyperpolarisation-activated nucleotide-gated (HCN) channels.
HCN channel expression has been studied in several animal colon models. Importantly, HCN channels have been shown to have a role in the regulation of pacemaker electrical activity in the gut, a function which is often abnormal in gut motility disorders. Hirschsprung’s disease (HSCR) is the most common congenital gut motility disorder. Although the characteristic pathological findings in HSCR are well understood (aganglionosis and nerve cell hypertrophy), the mechanism underlying motility disturbance is not completely understood.
The family of HCN channels (HCN1-4) has been evaluated qualitatively and functionally in the gastrointestinal tract of mice, rats and guinea pigs in recent years. We have examined their expression in human colon for the first time, and shown HCN2, HCN3 and HCN4 to be expressed in the neurons of the myenteric and submucosal plexuses as well as in interstitial cells of Cajal, which are key pacemaker cells in gut motility.
In demonstrating reduced expression of HCN3 in Hirschsprung’s disease, this study provides the basis for future functional studies that may seek to determine its exact role in colonic dysmotility in HSCR and potentially in other gut motility disorders.
HCN channels are membrane channels involved in transmission of the Ih electrical current in the gut, a current which controls cell excitability. In this way, HCN channels contribute to the regulation of the spontaneous electrical rhythmicity that dictates gut motility.
The authors have explored the expression of HCNs in colon specimens of HSCR patients. They found the first time thant HCN3 markedly decreased in the aganglionic colon vs ganglionic colon and controls,while no HCN1 in colon and no difference of HCN2 and HCN4 expressions between normal and HSCR patients. The results implicated that HCN3 might be important for the pathophysiological development of the HSCR.
P- Reviewer: Cai KL, Crenn PP, Gazouli M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299-327. [PubMed] |
| 2. | Xiao J, Nguyen TV, Ngui K, Strijbos PJ, Selmer IS, Neylon CB, Furness JB. Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system. Neuroscience. 2004;129:603-614. [PubMed] |
| 3. | Moroni A, Gorza L, Beltrame M, Gravante B, Vaccari T, Bianchi ME, Altomare C, Longhi R, Heurteaux C, Vitadello M. Hyperpolarization-activated cyclic nucleotide-gated channel 1 is a molecular determinant of the cardiac pacemaker current I(f). J Biol Chem. 2001;276:29233-29241. [PubMed] |
| 4. | Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264-5275. [PubMed] |
| 5. | Müller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB. HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci. 2003;17:2084-2096. [PubMed] |
| 6. | Xue L, Li Y, Han X, Yao L, Yuan J, Qin W, Liu F, Wang H. Investigation of hyperpolarization-activated cyclic nucleotide-gated channels in interstitial cells of Cajal of human bladder. Urology. 2012;80:224.e13-224.e18. [PubMed] |
| 7. | Hurtado R, Bub G, Herzlinger D. A molecular signature of tissues with pacemaker activity in the heart and upper urinary tract involves coexpressed hyperpolarization-activated cation and T-type Ca2+ channels. FASEB J. 2014;28:730-739. [PubMed] |
| 8. | Shahi PK, Choi S, Zuo DC, Kim MY, Park CG, Kim YD, Lee J, Park KJ, So I, Jun JY. The possible roles of hyperpolarization-activated cyclic nucleotide channels in regulating pacemaker activity in colonic interstitial cells of Cajal. J Gastroenterol. 2014;49:1001-1010. [PubMed] |
| 9. | Yang S, Xiong CJ, Sun HM, Li XS, Zhang GQ, Wu B, Zhou DS. The distribution of HCN2-positive cells in the gastrointestinal tract of mice. J Anat. 2012;221:303-310. [PubMed] |
| 10. | Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928-933. [PubMed] |
| 11. | Wang YP, Sun BY, Li Q, Dong L, Zhang GH, Grundy D, Rong WF. Hyperpolarization-activated cyclic nucleotide-gated cation channel subtypes differentially modulate the excitability of murine small intestinal afferents. World J Gastroenterol. 2012;18:522-531. [PubMed] |
| 12. | Si X, Huang L, Gong Y, Lu J, Lin L. Role of calcium in activation of hyperpolarization-activated cyclic nucleotide-gated channels caused by cholecystokinin octapeptide in interstitial cells of cajal. Digestion. 2012;85:266-275. [PubMed] |
| 13. | Stieber J, Stöckl G, Herrmann S, Hassfurth B, Hofmann F. Functional expression of the human HCN3 channel. J Biol Chem. 2005;280:34635-34643. [PubMed] |
| 14. | Gfroerer S, Rolle U. Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int. 2013;29:889-897. [PubMed] |
| 15. | Menezes M, Corbally M, Puri P. Long-term results of bowel function after treatment for Hirschsprung’s disease: a 29-year review. Pediatr Surg Int. 2006;22:987-990. [PubMed] |
| 16. | Menezes M, Pini Prato A, Jasonni V, Puri P. Long-term clinical outcome in patients with total colonic aganglionosis: a 31-year review. J Pediatr Surg. 2008;43:1696-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Moore SW, Albertyn R, Cywes S. Clinical outcome and long-term quality of life after surgical correction of Hirschsprung’s disease. J Pediatr Surg. 1996;31:1496-1502. [PubMed] |
| 18. | Kaul A, Garza JM, Connor FL, Cocjin JT, Flores AF, Hyman PE, Di Lorenzo C. Colonic hyperactivity results in frequent fecal soiling in a subset of children after surgery for Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2011;52:433-436. [PubMed] |
| 19. | Levitt MA, Dickie B, Peña A. The Hirschsprungs patient who is soiling after what was considered a “successful” pull-through. Semin Pediatr Surg. 2012;21:344-353. [PubMed] |