Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5630
Peer-review started: December 1, 2014
First decision: December 26, 2014
Revised: January 21, 2015
Accepted: February 12, 2015
Article in press: May 4, 2015
Published online: May 14, 2015
Processing time: 168 Days and 18.4 Hours
AIM: To retrospectively evaluate our experience with the diagnosis and surgical resection of esophageal gastrointestinal stromal tumors (GISTs).
METHODS: Between January 2003 and August 2014, five esophageal GIST cases were admitted to our hospital. In this study, the hospital records, surgery outcomes, tumor recurrence and survival of these patients were retrospectively reviewed.
RESULTS: The median age of the patients was 45.6 years (range: 12-62 years). Three patients presented with dysphagia, and one patient presented with chest discomfort. The remaining patient was asymptomatic. Four patients were diagnosed with esophageal GISTs by a preoperative endoscopic biopsy. Three patients underwent esophagectomy, and two patients underwent video-assisted thoracoscopic surgery. The mean operating time was 116 min (range: 95-148 min), and the mean blood loss was 176 mL (range: 30-300 mL). All tumors were completely resected. The mean length of postoperative hospital stay was 8.4 d (range: 6-12 d). All patients recovered and were discharged successfully. The median postoperative follow-up duration was 48 mo (range: 29-72 mo). One patient was diagnosed with recurrence, one patient was lost to follow-up, and three patients were asymptomatic and are currently being managed with close radiologic and clinical follow-up.
CONCLUSION: Surgery is the standard, effective and successful treatment for esophageal GISTs. Long-term follow-up is required to monitor recurrence and metastasis.
Core tip: Gastrointestinal stromal tumors (GISTs) commonly occur in the stomach and small intestine but are less frequent in the esophagus. To our knowledge, there are few detailed reports concerning the diagnosis and surgical treatment of esophageal GISTs. The main purpose of the present study was to present our experience with the diagnosis and surgical resection of esophageal GISTs.
- Citation: Zhang FB, Shi HC, Shu YS, Shi WP, Lu SC, Zhang XY, Tu SS. Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World J Gastroenterol 2015; 21(18): 5630-5634
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5630
Gastrointestinal stromal tumors (GISTs) are common mesenchymal neoplasms arising from the digestive tract and abdomen and were initially described by Mazur and Clark[1] in 1983. Since their discovery, GISTs have been identified in a number of locations, including the esophagus, stomach, jejunum, ileum and colon[2-13]. Furthermore, according to a study by Miettinen[14], the most common sites of GISTs are the stomach (60%-70%) and small intestine (20%-30%)[14-16]. In contrast, esophageal GISTs are rare and account for fewer than 2% of all GISTs[17-19]. In the past, the origin of esophageal GISTs was initially reported to be smooth muscle[20]. However, following an immunohistochemical-analysis-based study, it has become well established that GISTs arise from pluripotent stem cells or interstitial cells[21].
We therefore analyzed the clinical features, histological and immunohistochemical characteristics of five cases of esophageal GISTs and reviewed our experience with the diagnosis and surgical treatment of patients with esophageal GISTs.
Date was retrospectively retrieved for five patients who underwent surgical resection of esophageal GISTs at the Department of Thoracic Surgery, Clinical College of Yangzhou University, Yangzhou, China between January 2003 and August 2014. This retrospective study was approved by our Institutional Review Board, and waiver of consent was obtained. Standard preoperative work-up included routine blood routine examination, biochemical examination, chest and abdominal computed tomography (CT) scanning, esophageal barium meal, esophagoscopy and endoscopic ultrasound (EUS) for elective patients.
Clinicopathologic information form hospital records and the database of the clinical research institute, including patient gender, age, clinical presentation, location and size of lesions, operative time, operative findings, operative blood loss, tumor size, tumor characteristics (benign or malignant) and recurrence during routine follow-up were retrieved for all cases. The histological and immunohistochemical characteristics of the tumors, including CD34, CD117, Desmin, S-100 and SMA expression, were also reviewed. The diagnosis of malignant GISTs was made according to the previously published paper of Emory[17]. In brief, diagnostic criteria for GISTs were assessed for the presence of the following: (1) esophageal GISTs > 5.5 cm in diameter; (2) mitotic index > 5 mitotic figures/50 high-power fields; and (3) invasive behavior and metastasis.
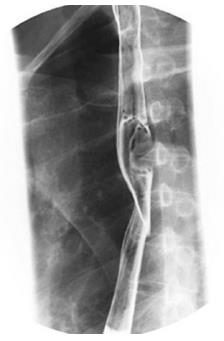
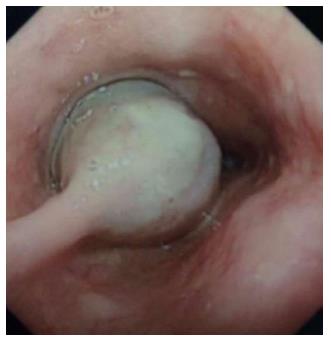
Of the five cases, all were males, with a mean age of 45.6 years (range: 12-62 years). Four patients were symptomatic, three presented with dysphagia and one presented with chest discomfort. The remaining patient was asymptomatic. Four patients were preoperatively diagnosed with esophageal GISTs and one with esophageal leiomyoma by endoscopic biopsy. Representative findings from esophageal barium meals showed a larger tumor in the esophagus (Figure 1), and esophagoscopy revealed a large tumor in the esophagus (Figure 2). One, two and two tumors occurred in the upper, middle and lower third of the esophagus, respectively. Data of the clinical features are shown in Table 1.
| Feature | Patient No. 1 | Patient No. 2 | Patient No. 3 | Patient No. 4 | Patient No. 5 |
| Gender | Male | Male | Male | Male | Male |
| Age | 12 | 49 | 48 | 57 | 62 |
| Presentation | Dysphagia | Chest discomfort | Dysphagia | Dysphagia | Asymptomatic |
| Location of lesions | Middle | Middle | Distal | Upper | Distal |
| Surgical procedure | Thoracotomy | Thoracotomy | Thoracotomy | VATS | VATS |
| Operating time (min) | 148 | 110 | 122 | 105 | 95 |
| Blood loss (mL) | 300 | 30 | 300 | 150 | 100 |
| Size of lesion (cm) | 6 × 4 | 2 × 1.5 | 6 × 5 | 3 × 2 | 1 × 2 |
| Postoperative hospital stay (d) | 12 | 6 | 10 | 7 | 7 |
| Time of intrathoracic drain (d) | 6 | 4 | 4 | 3 | 3 |
| Cellular pattern | Spindle | Spindle | Spindle | Spindle | Spindle |
| Mitosis per 50 high power fields | 10 | 0 | 6 | 1 | 0 |
| CD34 | + | + | + | + | - |
| CD117 | + | + | + | + | + |
| Desmin | - | - | - | - | - |
| S-100 | - | - | - | - | - |
| SMA | - | - | - | - | - |
| Diagnosis | Malignant | Benign | Malignant | Benign | Benign |
| Follow-up (mo) | - | 29 | 40 | 51 | 72 |
| Recurrence | - | No | Yes | No | No |
| Current status | - | NED | Dead | NED | NED |
All patients had no history of diabetes mellitus, hypertensive disease or coronary disease. Other physical examinations, including electrocardiogram, lung function examination and echocardiogram, were normal. The serum concentrations of Na+, K+, Ca2+ and glucose were all within the normal limits. Distant metastasis was not found by CT of the chest and abdomen.
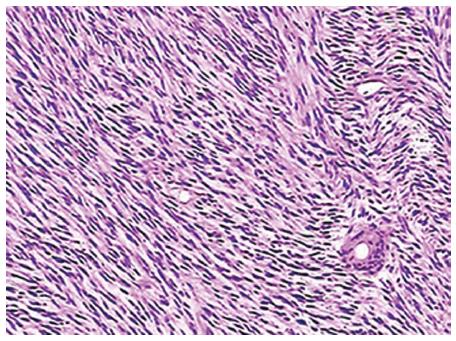
Three patients underwent esophagectomy, whereas the other two patients (No. 4 and No. 5) underwent tumor resection by VATS. The mean operating time was 116 min (range: 95-148 min), and the mean blood loss was 176 mL (range: 30-300 mL). All tumors were completely resected. The tumor size was available for five patients, and ranged from 2 to 6 cm, with a mean size of 3.4 cm. The hematoxylin-eosin stained GISTs showed a rich variety of spindle cells (Figure 3). The immunohistochemical reactions for CD 117 were positive in all patients, and four patients were positive for CD 34 (Table 1). The mean length of postoperative hospital stay was 8.4 d (range: 6-12 d). All patients recovered and were discharged successfully.
The mean duration of follow-up was 48 mo (range, 29-72 mo). One patient (No. 3) was followed for 35.3 mo post-surgery, and evidence of a recurrent tumor in the bilateral lung was observed. However, this patient was not treated with imatinib and died after 5 mo. One patient was lost to follow-up, and three patients were asymptomatic and are currently being managed with close radiologic and clinical follow-up.
GISTs are common neoplasms of the digestive tract, and they frequently occur in the stomach and small intestine and less frequently in the esophagus. In the early stages of esophageal GISTs, patients often do not exhibit clear clinical symptoms. As the tumor grows, patients present with different symptoms that are dependent on tumor location and size, including progressive dysphagia, chest discomfort, reflux and heartburn feeling.
To our knowledge, the largest single center experience describing the surgical treatment of primary esophageal GISTs was described by Lee[17]. In recent years, the characteristics of GISTs have become increasingly recognized. Due to the submucosal location and rarity, it is not possible to determine whether a mass is benign or malignant using imaging examinations, including endoscopic ultrasound, esophageal barium imaging, chest X-rays and CT. However, imaging examinations are beneficial for assessing GISTs and improving detection of distant metastases. Preoperatively, the diagnosis of GISTs may be confirmed through endoscopy, combined with typical histomorphological findings, including atypical spindle shaped neoplastic cells within red endochylema, as well as through immunohistochemical staining for a CD 117+/EMA- pattern. However, if a well-circumscribed, rounded or elliptical, submucosal tumor is detected during endoscopic procedures, a biopsy is rarely performed due to tumor cells releasing into thoracic cavity, leading to metastases[17]. Meanwhile, a biopsy might cause uncontrolled bleeding. With the advancement of technology and computers, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) may be beneficial for the diagnosis of GISTs and might play a crucial role in the assessment and prediction of treatment responses in the future. The importance of FDG-PET consists of its ability to detect distant metastases and its high negative predictive value.
The differential diagnosis of an esophageal GIST includes malignant and benign tumors, including leiomyoma, hemangioma, schwannoma, leiomyosarcoma and papillary epithelioma. Primarily, they share similar clinical characteristics, endoscopic observation and radiographic features. Thus, histology and immunohistochemistry are the most significant tools for differentiating between these tumors. For example, GISTs are c-kit (CD117)-positive and SMA-negative. Thus, the presence of different markers in GISTs distinguishes them from other tumors.
The most successful and effective treatment for GISTs is surgery[22-24]. In the past, the majority of esophageal tumor excisions have been approached via thoracotomy. With the development of thoracoscopic surgery, surgery is increasing being performed by thoracoscopy due to decreased pain and blood[25-27]. There is some skepticism for malignant GISTs concerning lymph node clearance and efficacy due to the rarity of cases. Therefore, continuous efforts focusing on the benefits of VATS over open surgery are needed for more comparative studies. According to Lee, patients with well-circumscribed, benign tumors less than 5 cm in diameter should undergo VATS, and patients with invasive, aggressive tumors greater than 5 cm may require biopsy and esophagectomy[4]. In the present case, surgical approaches were prepared depending on the characteristics of the tumor. If the tumor was benign, a total tumor excision was performed. For a malignant tumor, an esophagectomy and lymph node dissection involving radical dissection of the mediastinum was performed. Overall, three patients underwent esophagectomy, and two patients underwent VATS in our study. A 12-year-old boy, one of our rare cases, presented to our department with dysphagia for three months. Barium swallow was performed, which showed the presence of a tumor 6 cm in diameter at the middle thoracic esophagus. Reconstruction of the gastroesophageal junction after tumor resection was difficult and resulted in developmental immaturity of the digestive tract.
Based on the ability of imatinib to improve outcomes in GISTs, treatment strategies complementary to radical surgery have been extensively used. The optimal duration of preoperative imatinib may be as long as 6–12 mo[28]. However, due to tumor necrosis and cystic changes, preoperative imatinib may result in rupture or bleeding and increase the fragility of GISTs[29]. Adjuvant imatinib in patients with resection of GIST has consistently been shown to prevent recurrences and increase survival in many clinical studies[30,31]. However, all cases in these studies have been from the stomach or intestine. Because of the rarity of esophageal GISTs, the effectiveness of imatinib requires more comparative studies.
In conclusion, tumor resection was successfully performed and the diagnosis of GISTs was determined based on immunohistochemical findings. Long-term follow-up with radiological imaging is required to monitor the recurrence and metastasis of this type of tumor.
Gastrointestinal stromal tumors (GISTs) commonly occur in the gastrointestinal tract, including the stomach and small bowel, but are less frequent in the esophagus. Esophageal GISTs are often asymptomatic. As the tumor grows, patients present with different symptoms depending on the tumor location and size. Diagnosis and management of esophageal GISTs remain challenging.
Due to the rarity of esophageal GISTs, few studies have focused on their characteristics. Therefore, we described our experience with the diagnosis and surgical treatment of patients with esophageal GISTs.
Based on this study, the use of immunohistochemistry is an effective means of diagnosis of esophageal GISTs in patients. The authors focused on the treatment of esophageal GISTs, especially surgery, which may be the most effective treatment.
Surgery may be the most effective treatment for esophageal GISTs. Future randomized controlled trials between surgery and imatinib are needed to address the quality of life issues.
This survey was done as a good work, but sample size is low and it is only a descriptive study. I think this article can be accepted as letter to editor or case series.
P- Reviewer: Liu YC, Rafeey M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [PubMed] |
| 2. | Macías-García F, Parada P, Martínez-Lesquereux L, Pintos E, Fraga M, Domínguez-Muñoz JE. Gastrointestinal stromal tumors (GISTs) of the colon. Rev Esp Enferm Dig. 2010;102:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Hassan I, You YN, Dozois EJ, Shayyan R, Smyrk TC, Okuno SH, Donohue JH. Clinical, pathologic, and immunohistochemical characteristics of gastrointestinal stromal tumors of the colon and rectum: implications for surgical management and adjuvant therapies. Dis Colon Rectum. 2006;49:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Beltrán MA, Tapia RA, Cortés VJ, Larraín C, Koscina V, Rioseco MP, Molina M, Vera A. Multiple primary gastrointestinal stromal tumors presenting in jejunum and ileum. Indian J Surg. 2013;75:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Wan P, Li C, Yan M, Yan C, Zhu ZG. Laparoscopy-assisted versus open surgery for gastrointestinal stromal tumors of jejunum and ileum: perioperative outcomes and long-term follow-up experience. Am Surg. 2012;78:1399-1404. [PubMed] |
| 7. | Nakayama Y, Kadowaki K, Higure A, Hisaoka M, Yamaguchi K. Synchronous sporadic gastrointestinal stromal tumors in the stomach and jejunum: report of a case. Case Rep Gastroenterol. 2013;7:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Scherübl H, Faiss S, Knoefel WT, Wardelmann E. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J Gastrointest Endosc. 2014;6:266-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 9. | Cai R, Ren G, Wang DB. Synchronous adenocarcinoma and gastrointestinal stromal tumors in the stomach. World J Gastroenterol. 2013;19:3117-3123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Huang H, Liu YX, Zhan ZL, Liang H, Wang P, Ren XB. Different sites and prognoses of gastrointestinal stromal tumors of the stomach: report of 187 cases. World J Surg. 2010;34:1523-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Katoh T, Itoh Y, Mohri T, Suzuki H. Endoscopic enucleation of gastrointestinal stromal tumors of the stomach: report of five cases. World J Gastroenterol. 2008;14:2609-2611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, Ueda K, Ishida K, Naka T, Ono K. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006;30:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Mochizuki Y, Kodera Y, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Inada K, Kato T. Treatment and risk factors for recurrence after curative resection of gastrointestinal stromal tumors of the stomach. World J Surg. 2004;28:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 15. | Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211-222. [PubMed] |
| 16. | Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Lee HJ, Park SI, Kim DK, Kim YH. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg. 2009;87:1569-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (33)] |
| 18. | von Rahden BH, Stein HJ, Feussner H, Siewert JR. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. Surg Endosc. 2004;18:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Jiang P, Jiao Z, Han B, Zhang X, Sun X, Su J, Wang C, Gao B. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. Eur J Cardiothorac Surg. 2010;38:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (33)] |
| 20. | Emory TS, Sobin LH, Lukes L, Lee DH, O’Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Takahashi T, Nakajima K, Miyazaki Y, Miyazaki Y, Kurokawa Y, Yamasaki M, Miyata H, Takiguchi S, Nishida T, Mori M. Surgical Strategy for the Gastric Gastrointestinal Stromal Tumors (GISTs) Larger Than 5 cm: Laparoscopic Surgery is Feasible, Safe, and Oncologically Acceptable. Surg Laparosc Endosc Percutan Tech. 2015;25:114-118. [PubMed] |
| 23. | Blum MG, Bilimoria KY, Wayne JD, de Hoyos AL, Talamonti MS, Adley B. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg. 2007;84:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (33)] |
| 24. | Markakis CG, Spartalis ED, Liarmakopoulos E, Kavoura EG, Tomos P. Esophageal gastrointestinal stromal tumor: diagnostic complexity and management pitfalls. Case Rep Surg. 2013;2013:968394. [PubMed] |
| 25. | Qiu WQ, Zhuang J, Wang M, Liu H, Shen ZY, Xue HB, Shen L, Ge ZZ, Cao H. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. 2013;14:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Jeong IH, Kim JH, Lee SR, Kim JH, Hwang JC, Shin SJ, Lee KM, Hur H, Han SU. Minimally invasive treatment of gastric gastrointestinal stromal tumors: laparoscopic and endoscopic approach. Surg Laparosc Endosc Percutan Tech. 2012;22:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Dholakia C, Gould J. Minimally invasive resection of gastrointestinal stromal tumors. Surg Clin North Am. 2008;88:1009-118, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU, DeLaney TF, Ganjoo KN, George S, Gonzalez R, Heslin MJ. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:951-960. [PubMed] |
| 29. | Yip D, Zalcberg J, Ackland S, Barbour AP, Desai J, Fox S, Kotasek D, McArthur G, Smithers BM. Controversies in the management of gastrointestinal stromal tumors. Asia Pac J Clin Oncol. 2014;10:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 971] [Article Influence: 60.7] [Reference Citation Analysis (1)] |
| 31. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |