Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5598
Peer-review started: November 26, 2014
First decision: December 26, 2014
Revised: January 12, 2015
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: May 14, 2015
Processing time: 173 Days and 17.3 Hours
AIM: To evaluate the impact of postoperative infectious complications on hepatocellular carcinoma following curative hepatectomy.
METHODS: We performed a retrospective analysis of 200 hepatocellular carcinoma patients who underwent hepatectomy at our institution between September 2003 and June 2011. The patients’ demographics, clinicopathological characteristics and postoperative infectious complications were analyzed. The Clavien-Dindo classification was adopted to assess the severity of complications. The dynamic change in the neutrophil-to-lymphocyte ratio, defined as the absolute neutrophil count divided by the absolute lymphocyte count, after surgery was also investigated. The observation endpoints for this study were recurrence-free survival and overall survival of the patients. Statistical analysis of the survival curves was performed using the Kaplan-Meier method and the log-rank test. The prognostic value of each variable for predicting prognosis was assessed via multivariate Cox proportional hazards regression analysis. The cutoff score for each variable was selected based on receiver operating characteristic curve analysis. All statistical tests were two-sided, and significance was set at P < 0.05.
RESULTS: The median age of the patients was 49 years, and the majority of patients were male (86%) and had been infected with hepatitis B virus (86%). The 30-d postoperative infectious complication rate was 34.0% (n = 68). Kaplan-Meier survival analysis revealed that postoperative infection was significantly correlated with tumor recurrence (P < 0.001). The postoperative intra-abdominal infection group exhibited a worse prognosis than the non-intra-abdominal infection group (P < 0.001). A significantly increased incidence of postoperative intra-abdominal infection was observed in the patients with hepatic cirrhosis (P = 0.028), concomitant splenectomy (P = 0.007) or vascular invasion (P = 0.026). The patients who had an elevated postoperative neutrophil-to-lymphocyte ratio change (> 1.643) clearly exhibited poorer recurrence-free survival than those who did not (P = 0.009), although no significant correlation was observed between overall survival and the change in the postoperative neutrophil-to-lymphocyte ratio. Based on multivariate analysis, hepatitis B surface antigen positivity, Child-Turcotte-Pugh class B, an elevated postoperative neutrophil-to-lymphocyte ratio change and intra-abdominal infection were significant predictors of poor recurrence-free survival. Hepatic cirrhosis, the maximal tumor diameter and intra-abdominal infection were significant predictors of overall survival.
CONCLUSION: Postoperative intra-abdominal infection adversely affected oncologic outcomes, and the change in postoperative neutrophil-to-lymphocyte ratio was a good indicator of tumor recurrence in hepatocellular carcinoma patients after curative hepatectomy.
Core tip: Post-operative infectious complications adversely affect survival in various cancers. However, the prognostic value of postoperative infectious complications for patients with hepatocellular carcinoma remains unclear. We found that the occurrence of postoperative intra-abdominal infection was an independent predictor of tumor recurrence and poor overall survival. Postoperative neutrophil-to-lymphocyte ratio change, which indicated a systemic inflammatory response, was also an independent predictor of tumor recurrence.
- Citation: Ruan DY, Lin ZX, Li Y, Jiang N, Li X, Wu DH, Wang TT, Chen J, Lin Q, Wu XY. Poor oncologic outcomes of hepatocellular carcinoma patients with intra-abdominal infection after hepatectomy. World J Gastroenterol 2015; 21(18): 5598-5606
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5598
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, leading to 695900 deaths annually. Globally, nearly half of new HCC cases occur in China due to the prevalence of hepatitis B virus infection[1]. Surgical resection is considered a curable treatment for HCC, but the recurrence and the long-term prognosis of HCC remain unsatisfactory[2]. The 5-year recurrence-free survival rate has been reported to be approximately 30%[3-5]. Due to improvements in surgical techniques and perioperative management, the mortality rate of hepatectomy has significantly decreased in recent decades. However, the high incidence of postoperative infectious complications (ranging from 15.6% to 25.0% based on previous reports) remains a major problem, especially in patients with underlying hepatic disease, such as chronic hepatitis and liver cirrhosis[6,7].
Recently, the negative impact of infectious complications on prognosis has been demonstrated in various cancers. The study by Farid et al[8] suggested that postoperative sepsis was an independent predictor of both disease-free survival and overall survival in colorectal cancer patients with liver metastasis who underwent hepatic resection. Amin Andalib et al[9] analyzed a health care database of lung cancer patients after curative-intent surgery in Quebec, and discovered a strong negative correlation between major infectious complications and the long-term survival rate. However, the prognostic impact of postoperative infectious complications on patients with HCC remains unclear.
Inflammation and its associated immunomodulation have been suggested as causes of cancer progression. Systemic inflammation augments the adhesion of circulating tumor cells, thereby accelerating tumor metastasis[10], and bacterial antigens directly increase the metastatic ability of cancer cells[11,12]. Additional evidence has demonstrated the correlation between inflammation and poor oncologic outcomes of cancer patients. Markers of systemic inflammatory responses, such as C-reactive protein (CRP) and the preoperative monocyte count have been investigated as prognostic factors[13,14]. The neutrophil-to-lymphocyte ratio (NLR) is a novel marker of the systemic inflammatory response. An elevated NLR has been experimentally demonstrated to serve as a predictor of poor prognosis in cancer patients[15-18].
Long-term survival was reported to be poorer in HCC patients with operative complications after hepatectomy[19,20]. However, to date, few studies have focused on the potential association between postoperative infectious complications and oncologic outcome of HCC. This study aimed to evaluate the impact of postoperative infectious complications on recurrence-free survival (RFS) and overall survival (OS) among patients receiving curative hepatectomy for HCC.
A total of 200 HCC patients who underwent curative hepatectomy in an open procedure for HCC at our institution between September 2003 and June 2011 were included in this study. Patients with extrahepatic metastasis and patients receiving any neoadjuvant treatment such as transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) were excluded. Patients were also excluded if the histological diagnosis was not HCC or if the patient died within 30 d after surgery. The diagnosis of HCC was based on the serum α-fetoprotein (AFP) level, at least two radiological parameters [ultrasonography, computed tomography (CT) or magnetic resonance imaging (MRI)], and was ultimately confirmed postoperatively via histopathological diagnosis. The collected data included the patients’ demographic information, baseline clinicopathological characteristics, and laboratory and radiological results. The study protocol was approved by an Institutional Review Board. Informed consent was obtained according to the Declaration of Helsinki.
The following infectious complication characteristics were examined: (1) intra-abdominal infection; (2) respiratory infection; (3) wound infection; (4) urinary tract infection; and (5) venous catheter related-infection. Infectious complications were diagnosed based on symptoms with clinical/radiological signs, together with increased levels of inflammatory markers, positivity/negativity of sputum/fluid/blood cultures and requirement for treatment with antibiotics or further intervention. The Clavien-Dindo (C-D) classification[21] was adopted to assess these complications and only patients classified as grade II or higher were included in this analysis. In patients with infectious complications at multiple sites, the highest grade was recorded. All patients received antibiotic treatment after the diagnosis of infection.
Preoperative white blood cells and differential cell counts were measured within five days prior to the operation. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. Patients who were administered hematopoietic agents such as granulocyte colony-stimulating factor within one month before surgery were excluded. The postoperative NLR was obtained on the 7th day after surgery. The dynamic change in the NLR after surgery was defined as delta-NLR, which was quantitatively calculated by dividing the postoperative NLR value by the preoperative NLR value.
The follow-up examinations consisted of physical examinations, routine blood chemistry, serum AFP measurement and abdominal ultrasound, were performed once every 3-6 mo for the first 2 years and once every 6-12 mo thereafter. In cases with suspicious lesions on ultrasound or with elevated AFP, further examinations (contrast-enhanced ultrasound, CT or MRI) were conducted to confirm or rule out recurrence. The primary endpoint of this analysis was RFS, defined as the interval from the date of surgery to the date of the first recurrence, or the most recent follow-up that revealed no recurrence. The patients with recurrence received further individualized treatment, such as a second hepatectomy, chemoembolization, RFA, percutaneous ethanol injection or systemic therapy.
The statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, United States). Categorical variables were compared using the χ2 test or Fisher’s exact test, and those variables with statistical significance were validated by multivariate analysis. The RFS and OS curves were constructed using the Kaplan-Meier method, and the results were compared using the log-rank test. The prognostic value of each variable for predicting RFS or OS was assessed via multivariate Cox proportional hazards regression analysis. The cutoff value of delta-NLR was selected based on receiver operating characteristic (ROC) curve analysis. All statistical tests were two-sided, and significance was set at P < 0.05.
The characteristics of the studied patients are summarized in Table 1. The median follow-up duration was 32 mo (range: 4-103 mo). At the time of the most recent follow-up, 101 (50.5%) patients had developed tumor recurrence, 43 (21.5%) patients had died of HCC progression, and two (1.0%) patients had died of non-cancerous disease. The 1-, 3-, and 5-year OS rates for all the patients in this study were 96.0%, 83.8% and 81.3%, respectively, and the 1-, 3-, and 5-year RFS rates were 74.5%, 56.0% and 51.0%, respectively. Overall, the 30-d postoperative infectious complication rate was 34.0% (n = 68), and the peak time of infectious complications occurred within one to two weeks after surgery. Table 1 also details the specific number and categories of infectious complications. Five patients exhibiting C-D grade I wound complications were excluded from the analysis of infectious complications. No urinary tract infections or venous catheter related-infections were observed in this study.
| Characteristics | Total |
| No. of patients | 200 |
| Age, yr | |
| mean ± SD | 48 ± 11.601 |
| Median | 49 |
| Range | 22-75 |
| Gender, male | 172 (86.0) |
| Drinking | 46 (23.0) |
| Smoking | 68 (34.0) |
| HCC family history | 12 (6.0) |
| HBsAg (+) | 172 (86.0) |
| Alpha-fetoprotein, > 400 ng/mL | 75 (37.5) |
| Hepatic cirrhosis | 148 (74.0) |
| Child-Turcotte-Pugh class | |
| A | 186 (93.0) |
| B | 14 (7.0) |
| C | 0 |
| Tumor nodules | |
| Single | 174 (87.0) |
| Multiple | 26 (13.0) |
| Maximal tumor diameter, ≤ 5 cm | 143 (71.5) |
| Vascular invasion | 26 (13.0) |
| Infectious complications | |
| Respiratory infection | 43 (21.5) |
| Intra-abdominal infection | 18 (9.0) |
| Multiple sites of infection | 7 (3.5) |
| Total | 68 (34.0) |
| Severity (Clavien-Dindo grade) | |
| II | 56 (28.0) |
| IIIa | 11 (5.5) |
| IVa | 1 (0.5) |
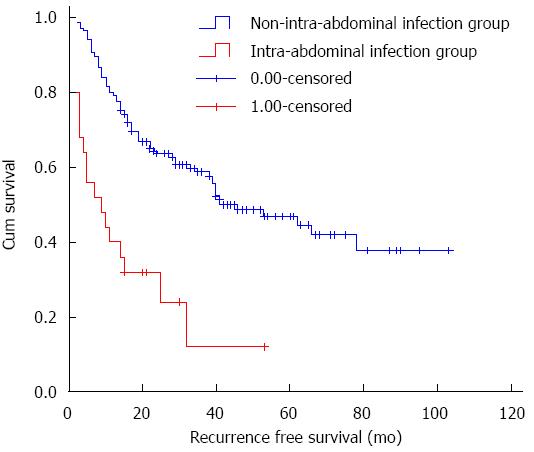
Kaplan-Meier survival analysis showed that postoperative infection was significantly correlated with tumor recurrence (P < 0.001), although no significant difference was detected between the different C-D grades (P = 0.798). Both intra-abdominal infection and infection at multiple sites were significantly associated with decreased RFS (P = 0.001 and P < 0.001), although the respiratory infection group was not significantly different from the non-infection group (P = 0.338). The source of infection in the patients with infection at multiple sites was the abdominal cavity, thus, we considered intra-abdominal infection as the primary investigated variable in this study. As shown in Figure 1, the difference between the groups with and without intra-abdominal infection was significant (log-rank test, P < 0.001). The 3-year RFS rate in the intra-abdominal infection group and the non-intra-abdominal infection group was 24.0% and 60.6%, respectively. Intra-abdominal infection was also associated with a poorer 3-year OS rate than non-intra-abdominal infection (52.0% vs 88.6%, P < 0.001; Figure 2). Based on further analysis, a significantly increased incidence of postoperative intra-abdominal infection was observed in patients with hepatic cirrhosis (P = 0.028), concomitant splenectomy (P = 0.007) or vascular invasion (P = 0.026) in univariate analysis, and these variables were all significant in multivariate analysis. However, the results showed no significant correlation between delta-NLR and any of the clinicopathological factors (Table 2). Figure 1 shows the RFS curve for HCC patients with and without post-operative intra-abdominal infection. The 3-year RFS rate in the intra-abdominal infection group and the non-intra-abdominal infection group was 24.0% (n = 6) and 60.6% (n = 106), respectively. The difference between these two groups was statistically significant (log-rank test, P < 0.001).
| Characteristics | Intra-abdominal infection (No.) | P value | Multivariate analysis | Delta-NLR | P value | ||
| No | Yes | P value | > 1.643 | ≤ 1.643 | |||
| Age, yr | |||||||
| < 60 | 138 | 19 | 0.745 | 108 | 48 | 0.268 | |
| ≥ 60 | 37 | 6 | 32 | 9 | |||
| Gender | |||||||
| Male | 151 | 21 | 0.759 | 122 | 48 | 0.587 | |
| Female | 24 | 4 | 18 | 9 | |||
| Drinking | |||||||
| No | 136 | 18 | 0.525 | 107 | 44 | 0.908 | |
| Yes | 39 | 7 | 33 | 13 | |||
| Smoking | |||||||
| No | 118 | 14 | 0.259 | 91 | 39 | 0.646 | |
| Yes | 57 | 11 | 49 | 18 | |||
| HCC family history | |||||||
| No | 165 | 23 | 0.649 | 133 | 53 | 0.733 | |
| Yes | 10 | 2 | 7 | 4 | |||
| HBsAg (+) | |||||||
| No | 26 | 2 | 0.540 | 18 | 10 | 0.393 | |
| Yes | 149 | 23 | 122 | 47 | |||
| Alpha-fetoprotein, | |||||||
| ≤ 400 ng/mL | 107 | 18 | 0.294 | 91 | 33 | 0.349 | |
| > 400 ng/mL | 68 | 7 | 49 | 24 | |||
| Hepatic cirrhosis | |||||||
| No | 50 | 2 | 0.0281 | 0.0431 | 36 | 16 | 0.734 |
| Yes | 125 | 23 | 104 | 41 | |||
| Child-Turcotte-Pugh | |||||||
| Class A | 163 | 23 | 0.689 | 130 | 53 | 1.000 | |
| Class B | 12 | 2 | 10 | 4 | |||
| Concomitant splenectomy | |||||||
| No | 165 | 19 | 0.0071 | 0.0161 | 127 | 54 | 0.565 |
| Yes | 10 | 6 | 13 | 3 | |||
| Tumor nodules | |||||||
| Single | 153 | 21 | 0.749 | 119 | 52 | 0.242 | |
| Multiple | 22 | 4 | 21 | 5 | |||
| Maximal tumor diameters | |||||||
| ≤ 5 cm | 126 | 17 | 0.679 | 102 | 38 | 0.385 | |
| > 5 cm | 49 | 8 | 38 | 19 | |||
| Vascular invasion | |||||||
| No | 156 | 18 | 0.0261 | 0.0061 | 119 | 52 | 0.242 |
| Yes | 19 | 7 | 21 | 5 | |||
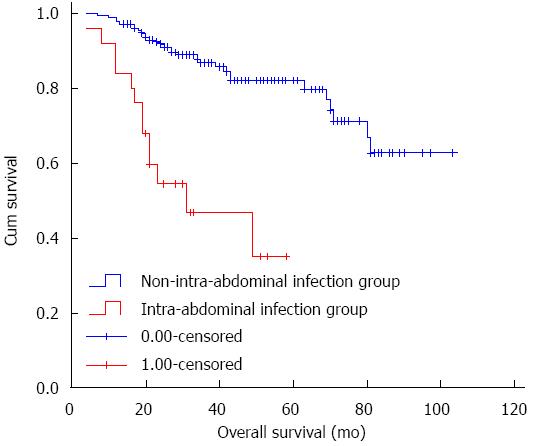
Figure 2 shows the OS curve for HCC patients with and without post-operative intra-abdominal infection. The 3-year OS rate in the intra-abdominal infection group and the non-intra-abdominal infection group was 52.0% (n = 13) and 88.6% (n = 155), respectively. The difference between these two groups was significant (log-rank test, P < 0.001).
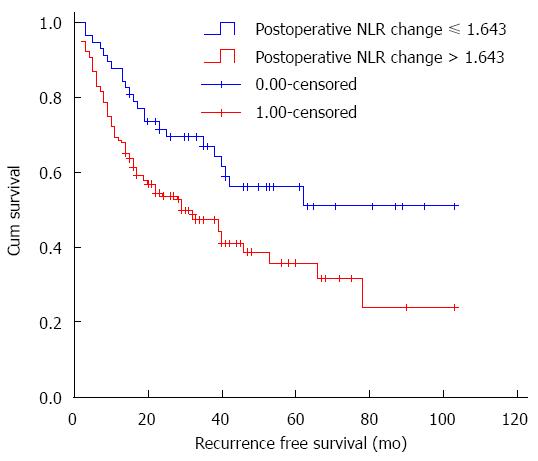
Postoperative NLR change, which was used as a measurement of inflammatory response, was another factor investigated in this study. The optimal cutoff value of delta-NLR for RFS was determined via receiver operating characteristic (ROC) curve. The area under the curve (AUC) was recorded as 0.513 (95%CI: 0.441-0.585). The delta-NLR value of 1.643 was used as the optimal cutoff value. This value resulted in the most appropriate sensitivity of 77.23% and specificity of 36.46%. The patients were stratified into the elevated (> 1.643) and non-elevated (≤ 1.643) delta-NLR groups. As shown in Figure 3, the 3-year RFS rate in the patients with an elevated delta-NLR was significantly poorer than that in the patients with a non-elevated delta-NLR (50.0% vs 68.4%, P = 0.009). Nevertheless, no significant correlation was detected between OS and postoperative NLR change.
Figure 3 shows the RFS curves of the HCC patients according to the postoperative NLR change (> 1.643 or ≤ 1.643). The 3-year RFS rate in the elevated group and the non-elevated group was 50.0% (n = 70) and 68.4% (n = 39), respectively. The elevated postoperative NLR change group exhibited significantly poorer RFS (log-rank test, P = 0.009).
The results of univariate and multivariate analysis are shown in Table 3. Indicators of underlying liver disease, such as the hepatitis B surface antigen (HBsAg) status and the Child-Turcotte-Pugh (CTP) class were identified as significant predictors of poor RFS. The postoperative NLR change and intra-abdominal infection were also identified as predictors of RFS. Multivariate analysis revealed HBsAg positivity, CTP class B, elevated delta-NLR and intra-abdominal infection as independent predictors of poor RFS. The tumor diameter displayed borderline significance. For OS, multivariate analysis identified hepatic cirrhosis, the maximal tumor diameter (> 5 cm) and intra-abdominal infection as independent predictors of poor OS.
| Category | 3-yr recurrence-free survival rate | Univariate analysis | Multivariate analysis | 3-yr OS rate | Univariate analysis | Multivariate analysis |
| P value | P value and HR (95%CI) | P value | P value and HR (95%CI) | |||
| Gender | 0.382 | 0.834 | ||||
| Male | 55.8% | 0.751 | 1.381 | 83.7% | 0.981 | 1.121 |
| Female | 57.1% | 0.670-2.849 | 85.7% | 0.385-3.267 | ||
| Age, yr | 0.100 | 0.765 | ||||
| < 60 | 57.3% | 0.496 | 1.508 | 83.4% | 0.763 | 0.887 |
| ≥ 60 | 51.2% | 0.925-2.460 | 86.0% | 0.402-1.954 | ||
| Smoking | 0.136 | 0.618 | ||||
| Yes | 51.5% | 0.076 | 1.482 | 83.8% | 0.374 | 1.237 |
| No | 58.3% | 0.883-2.485 | 84.1% | 0.536-2.854 | ||
| Drinking | 0.525 | 0.338 | ||||
| Yes | 52.2% | 0.443 | 0.829 | 87.0% | 0.740 | 0.632 |
| No | 57.1% | 0.466-1.477 | 83.1% | 0.247-1.615 | ||
| Family history | 0.199 | 0.091 | ||||
| Yes | 66.7% | 0.237 | 0.497 | 91.7% | 0.165 | 0.170 |
| No | 55.3% | 0.171-1.444 | 83.5% | 0.022-1.325 | ||
| HBsAg status | 0.0101 | 0.266 | ||||
| Negative | 82.1% | 0.006 | 3.019 | 92.9% | 0.057 | 2.374 |
| Positive | 51.7% | 1.309-6.959 | 82.6% | 0.517-10.895 | ||
| AFP, ng/mL | 0.279 | 0.972 | ||||
| > 400 | 54.4% | 0.184 | 0.782 | 82.7% | 0.948 | 1.012 |
| ≤ 400 | 58.7% | 0.501-1.220 | 84.8% | 0.508-2.018 | ||
| Hepatic cirrhosis | 0.635 | 0.0131 | 0.0311 | |||
| Yes | 53.4% | 0.176 | 1.128 | 80.4% | 3.233 | |
| No | 63.5% | 0.686-1.853 | 94.2% | 1.110-9.417 | ||
| CTP class | 0.0321 | 0.147 | ||||
| A | 58.1% | 0.011 | 2.130 | 83.9% | 0.262 | 2.321 |
| B | 28.6% | 1.068-4.246 | 85.7% | 0.745-7.233 | ||
| Delta-NLR | 0.0191 | 0.146 | ||||
| > 1.643 | 50.0% | 0.009 | 1.807 | 81.4% | 0.161 | 1.765 |
| ≤ 1.643 | 68.4% | 1.102-2.962 | 89.5% | 0.820-3.799 | ||
| Tumor nodules | 0.891 | 0.625 | ||||
| Single | 57.5% | 0.339 | 0.960 | 83.9% | 0.396 | 1.26 |
| Multiple | 46.2% | 0.530-1.736 | 84.6% | 0.499-3.185 | ||
| Maximal tumor diameter | 0.056 | 0.0191 | ||||
| > 5 cm | 47.4% | 0.075 | 1.573 | 75.4% | 0.0251 | 2.281 |
| ≤ 5 cm | 59.4% | 0.989-2.503 | 87.4% | 1.148-4.536 | ||
| Vascular invasion | 0.176 | 0.598 | 0.163 | 0.792 | ||
| Yes | 53.8% | 0.828 | 76.9% | 1.144 | ||
| No | 56.3% | 0.411-1.669 | 85.1% | 0.420-3.113 | ||
| Concomitant splenectomy | 0.721 | 0.308 | ||||
| Yes | 37.5% | 0.061 | 0.939 | 75.0% | 0.343 | 0.757 |
| No | 57.6% | 0.665-1.326 | 84.8% | 0.444-1.292 | ||
| Intra-abdominal infection | 0.0001 | 0.0001 | ||||
| Yes | 24.0% | < 0.001 | 3.308 | 52.0% | < 0.0011 | 5.357 |
| No | 60.6% | 1.806-6.061 | 88.6% | 2.494-11.507 | ||
The impact of postoperative infection on prognosis has been evaluated in various malignancies[8,22,23]. The presence of intra-abdominal and respiratory infections was reported to be associated with poorer long-term outcomes in patients who underwent hepatic resection for colorectal liver metastasis[8,14]. In the present study, we analyzed the data from a cohort of 200 HCC patients who underwent curative liver resection and demonstrated that postoperative intra-abdominal infection was an independent risk factor affecting RFS and OS.
Our results showed a relatively higher rate of postoperative infectious complications (34.0%) compared to the results of previous studies[3-5]. This difference may be due to distinct patient population characteristics, particularly the status of liver parenchyma. Most of our patients suffered from hepatic cirrhosis (74.0%), which highly correlated with postoperative intra-abdominal infection (Table 2). In addition to cirrhosis, vascular invasion and concomitant splenectomy correlated with an increased risk of postoperative intra-abdominal infection. However, the precise mechanism underlying these correlations remains unclear. The postoperative infectious complications were stratified according to severity using the C-D classification system in our cohort. No significant differences were detected in the outcomes of the patients according to the C-D grades, suggesting the homogeneity of the pro-neoplastic effects of inflammation on HCC carcinogenesis, despite differences in the severity of the postoperative complication.
The association between postoperative intra-abdominal infection and poor prognosis is complex and remains to be elucidated. Several possible mechanisms may explain the prognostic value of postoperative intra-abdominal infection. One possible mechanism is that the deregulated host immunological response during infection may promote tumorigenesis[10,24]. It has been well documented that infectious complications correlated with the excessive synthesis and release of proinflammatory cytokines, such as interleukin IL-1, IL-6 and TNFα, and these cytokines can affect the function and regulation of cytotoxic T lymphocytes, natural killer cells, and other immunocompetent circulating cells such as dendritic antigen-presenting cells and induce immunosuppression[25,26]. Cell-mediated immune function was also suppressed by the post-surgical stress response. As a result, the residual malignant cells may progress rapidly during the periods of relative immunosuppression caused by postoperative infection and post-surgical stress. In addition, infectious complications may stimulate the release of angiogenesis-regulating chemokines, growth factors and proteases, which are major contributors to tumor-related angiogenesis[27]. Furthermore, it has recently been demonstrated that bacterial antigens, which are frequently involved in postoperative infectious complications, exert a direct effect on the metastatic capacity of cancer cells[11].
The presence of a systemic inflammatory response can be evaluated based on both the elevation of the CRP level and the NLR[14,28]. Because the CRP level was not routinely measured at our center, we assessed the prognostic significance of the NLR as a potential objective biomarker representing the perioperative change in the inflammatory response. The patients with an elevated NLR more frequently exhibited lymphocytopenia and neutrophilic leukocytosis, shifting the balance to a pro-tumor inflammatory response. Various studies have demonstrated that an elevation in the preoperative NLR correlated with a poor clinical outcome in HCC patients treated by different methods[29-32]. The postoperative NLR was also considered to be a useful marker that displayed remarkable prognostic value[33]. However, neither the preoperative nor postoperative NLR reflects the dynamic change in the balance between the host inflammatory response and the immune response after curative surgery. Recently, the postoperative NLR change was found to be an independent prognostic factor for patients with small HCCs receiving RFA. The patients with a decreased NLR exhibited a higher survival rate than those with an increased NLR[30]. Our investigation showed a similar result among the HCC patients who underwent liver resection. The postoperative NLR change was an independent factor for RFS. Neither preoperative nor postoperative NLR was significantly associated with RFS or OS.
Our results should be interpreted with caution as this retrospective study contains some limitations. First, this study was based on retrospective data. Second, these data were collected from a single institution. Thus, further validation in prospective studies enrolling patients from additional institutions is needed. Third, we chose to stratify the patients according to a delta-NLR of > 1.643 or ≤ 1.643 based on ROC curve analysis. However, it is uncertain whether a different cutoff value would serve as a better predictor of disease recurrence for these HCC patients or whether NLR would be better categorized into “high”, “intermediate”, and “low” groups rather than two groups.
In conclusion, the results of our study suggest that postoperative intra-abdominal infection has an adverse effect on the oncologic outcomes (RFS and OS) of HCC patients after hepatectomy. The postoperative NLR change (delta NLR), a simple and objective inflammation index, is an independent predictor of tumor recurrence in HCC patients.
The authors thank Heng Zhang and Fan Zhang for their contribution in this study.
Surgical resection is considered a curable treatment for hepatocellular carcinoma (HCC), but the postoperative recurrence rate and long-term prognosis is still unsatisfactory. Many clinicopathological characteristics have been proved to be associated with the prognosis of HCC. Recently, the association between inflammatory response and cancer progression has become a research focus. It is reported that postoperative infectious complications affect prognosis in various cancers and that inflammation and its associated immunomodulation can cause cancer progression. However, to date, there is little evidence of the potential association between postoperative infectious complications and the oncologic outcome of HCC after hepatectomy. The neutrophil-to-lymphocyte ratio (NLR) is a novel marker of the systemic inflammatory response. An elevated NLR has been experimentally demonstrated to serve as a predictor of poor prognosis in cancer patients.
Recent studies suggested that postoperative infectious complications were an independent predictor for both disease-free survival and overall survival after surgery in various types of cancers, such as gastric cancer, esophageal cancer, and lung cancer, and laboratory research has provided evidence that host immunological response during infection may promote tumorigenesis. NLR, as a novel marker of inflammation, had been demonstrated to be a predictor of clinical outcome in HCC patients treated with different methods.
This study showed a clear association between postoperative intra-abdominal infection and prognosis and clarified the prognostic value of dynamic NLR change as an inflammatory marker for HCC patients after hepatectomy.
The study results suggest that intra-abdominal infection and elevated delta-NLR were significantly associated with poor recurrence-free survival and overall survival. These parameters could be used in prognosis prediction, postoperative monitoring and follow up.
The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The dynamic change in the NLR after surgery was defined as delta-NLR, which was quantitatively calculated by dividing the postoperative NLR value by the preoperative NLR value.
The authors provide data to demonstrate that postoperative intra-abdominal infection and postoperative NLR change are prognostic indicators for HCC patients with curative surgery. This study is overall interesting, the outline is organized clearly and the manuscript is written well.
P- Reviewer: Uemura M, Wang B, Zhou GW S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25528] [Article Influence: 1823.4] [Reference Citation Analysis (7)] |
| 2. | Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115-4127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 316] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (4)] |
| 3. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [PubMed] |
| 4. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Suzuki T, Shimamura T, Furukawa H, Matsushita M, Todo S. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Meguro M, Mizuguchi T, Kawamoto M, Nishidate T, Ishii M, Tatsumi H, Kimura Y, Furuhata T, Hirata K. Highest intraoperative lactate level could predict postoperative infectious complications after hepatectomy, reflecting the Pringle maneuver especially in chronic liver disease. J Hepatobiliary Pancreat Sci. 2014;21:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, Prasad KR. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Andalib A, Ramana-Kumar AV, Bartlett G, Franco EL, Ferri LE. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol. 2013;8:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Doan HQ, Bowen KA, Jackson LA, Evers BM. Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Res. 2009;29:2473-2478. [PubMed] |
| 12. | Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Paik KY, Lee IK, Lee YS, Sung NY, Kwon TS. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. 2014;46:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, Prasad KR, Toogood GJ, Lodge JP. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer. 2007;96:222-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res. 2012;32:3535-3538. [PubMed] |
| 16. | Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, Kim YJ. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 18. | Kayadibi H, Sertoglu E, Uyanik M, Tapan S. Neutrophil-lymphocyte ratio is useful for the prognosis of patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20:9631-9632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kaibori M, Ishizaki M, Matsui K, Kwon AH. Postoperative infectious and non-infectious complications after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Kusano T, Sasaki A, Kai S, Endo Y, Iwaki K, Shibata K, Ohta M, Kitano S. Predictors and prognostic significance of operative complications in patients with hepatocellular carcinoma who underwent hepatic resection. Eur J Surg Oncol. 2009;35:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 22. | Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H, Decker G, Nafteux P. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 24. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8294] [Article Influence: 487.9] [Reference Citation Analysis (0)] |
| 25. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5746] [Article Influence: 239.4] [Reference Citation Analysis (0)] |
| 26. | Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 426] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 29. | Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8:e58184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 32. | Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q, Chen GH. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |