Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5575
Peer-review started: June 30, 2014
First decision: August 6, 2014
Revised: August 22, 2014
Accepted: September 29, 2014
Article in press: September 30, 2014
Published online: May 14, 2015
Processing time: 323 Days and 23.8 Hours
AIM: To determine the association between the neutrophil to lymphocyte (N/L) ratio and the degree of liver fibrosis in patients with chronic hepatitis B (CHB) infection.
METHODS: Between December 2011 and February 2013, 129 consecutive CHB patients who were admitted to the study hospitals for histological evaluation of chronic hepatitis B-related liver fibrosis were included in this retrospective study. The patients were divided into two groups based on the fibrosis score: individuals with a fibrosis score of F0 or F1 were included in the “no/minimal liver fibrosis” group, whereas patients with a fibrosis score of F2, F3, or F4 were included in the “advanced liver fibrosis” group. The Statistical Package for Social Sciences 18.0 for Windows was used to analyze the data. A P value of < 0.05 was accepted as statistically significant.
RESULTS: Three experienced and blinded pathologists evaluated the fibrotic status and inflammatory activity of 129 liver biopsy samples from the CHB patients. Following histopathological examination, the “no/minimal fibrosis” group included 79 individuals, while the “advanced fibrosis” group included 50 individuals. Mean (N/L) ratio levels were notably lower in patients with advanced fibrosis when compared with patients with no/minimal fibrosis. The mean value of the aspartate aminotransferase-platelet ratio index was markedly higher in cases with advanced fibrosis compared to those with no/minimal fibrosis.
CONCLUSION: Reduced levels of the peripheral blood N/L ratio were found to give high sensitivity, specificity and predictive values in CHB patients with significant fibrosis. The prominent finding of our research suggests that the N/L ratio can be used as a novel noninvasive marker of fibrosis in patients with CHB.
Core tip: Reduced levels of the peripheral blood neutrophil to lymphocyte ratio were found to give high sensitivity, specificity and predictive values in chronic hepatitis B (CHB) patients with significant fibrosis. The neutrophil to lymphocyte ratio can be used as a novel noninvasive marker of fibrosis in patients with CHB.
- Citation: Kekilli M, Tanoglu A, Sakin YS, Kurt M, Ocal S, Bagci S. Is the neutrophil to lymphocyte ratio associated with liver fibrosis in patients with chronic hepatitis B? World J Gastroenterol 2015; 21(18): 5575-5581
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5575.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5575
More than 350 million humans globally suffer from chronic hepatitis B virus (CHB) infection[1,2]. Assessment of the degree of liver fibrosis is crucial for prognostic and therapeutic decisions in patients with CHB[3]. Liver biopsy is currently the gold standard for estimating the seriousness of liver fibrosis. The accuracy of this invasive procedure is influenced by technical and methodological interventions, for example fibrosis stage is misclassified in nearly 20% of patients[4]. Non-invasive methods such as the Fibro Test, mean platelet volume, FibroIndex and Hepascore are now favorable as an alternative to liver biopsy for predicting liver fibrosis[5-12]. However, some of these methods are not readily available and are not cost-effective.
Liver fibrosis is an inevitable process in CHB. Many inflammatory cytokines, such as transforming growth factor-beta (TGF-beta) and platelet-derived growth factor, have been shown to activate hepatic stellate cells and result in advanced extracellular matrix deposition, which will eventually lead to liver fibrosis[13-16]. The neutrophil to lymphocyte (N/L) ratio is a noninvasive and inexpensive marker of inflammation which can be simply acquired from the complete blood count. This marker combines data from two distinct pathways, lymphocytes which portray the regulatory pathway and neutrophils which cause ongoing inflammation[17,18]. Alkhouri et al[19] demonstrated that in individuals with nonalcoholic fatty liver disease, the N/L ratio was related to histological changes and can be used to identify patients with progressive disease. In the literature, it has been shown that in addition to chronic inflammatory diseases, malignancy, infections, cardiac diseases, diabetes, renal and/or hepatic failure, metabolic syndrome, thyroid disease, and many drugs may easily influence the N/L ratio[17,19-21].
In this research, we aimed to determine the association between the N/L ratio and the severity of liver fibrosis in patients with CHB infection.
Between December 2011 and February 2013, 129 consecutive patients with CHB, who were admitted to the study hospitals for histological assessment of liver fibrosis, were included in this retrospective study. The participants were chosen from three hospitals in Turkey: Hitit University Hospital, Gulhane Military Medical School Ankara Hospital and Gulhane Military Medical School Haydarpasa Training Hospital.
All were treatment naïve CHB patients. Those who regularly drank > 20 g alcohol/wk, those with antibiotic and/or antiinflammatory drug use, decompensated liver disease, renal failure, thyroid disease, co-infection, chronic inflammatory disease, diabetes, hepatocellular carcinoma and other malignancies, previous liver surgery or liver transplantation were excluded from the study.
Demographic, clinical and laboratory data were collected. In addition to liver biopsy, hematological and standard biochemical parameters were tested, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, gamma glutamyl transpeptidase (GGT), albumin, alpha-1 globulin, alpha-2 globulin, beta globulin, gamma globulin, the albumin/globulin ratio, prothrombin time (PT), and alpha fetoprotein (AFP). The NLR was calculated by dividing the neutrophil count by the lymphocyte count. APRI, AAR, API and CDS were determined according to the following accepted formulas:
AAR: AST/ALT
API: Age (years): < 30 = 0; 30 - 39 = 1; 40 - 49 = 2; 50 - 59 = 3; 60 - 69 = 4; > 70 = 5. PLT count (109/L): ≥ 225 = 0; 200 - 224 = 1; 175 - 199 = 2; 150 - 174 = 3; 125 - 149 = 4; ≤ 125 = 5. API is the sum of the above
CDS: PLT count (109/L): > 340 = 0; 280 - 339 = 1; 220 - 279 = 2; 160 - 219 = 3; 100 - 159 = 4; 40 - 99 = 5; < 40 = 6. ALT/AST ratio: > 1.7 = 0; 1.2 - 1.7 = 1; 0.6 - 1.19 = 2; < 0.6 = 3. INR: < 1.1 = 0; 1.1 - 1.4 = 1; > 1.4 = 2
CDS is the sum of the above
APRI: [(AST/ULN)/PLT (109/L)] × 100
Liver biopsy samples were acquired with a 17 G needle using an ultrasonography-guided technique. The obtained biopsy samples were fixed in 4% buffered formalin and then inserted in paraffin. Biopsy samples were prepared for microscopic evaluation using hematoxylin and eosin and Masson trichrome stain. In the case of an inadequate number of portal tracts (< 6) and/or length (< 1.5 cm), liver biopsy samples were excluded from the final analysis. All biopsy samples were evaluated by the same three experienced pathologists and these pathologists were blinded to the clinical data of the patients.
The histological activity was assessed according to the Histological Activity Index score and the fibrosis score was estimated according to the METAVIR Scoring System. The patients were divided into two groups based on the fibrosis score: patients with a score of F0 or F1 were chosen as the “no/minimal fibrosis” group, while patients with a score of F2, F3, or F4 were chosen as the “advanced fibrosis” group[22].
Data was analyzed using the Statistical Package for Social Sciences 18.0 for Windows and expressed as mean ± SD for normally distributed variables, as median and range for non-normally distributed variables, and count and percent for categorical variables. Categorical variables were compared using the χ2 test or Fisher’s exact test and continuous variables were compared using the Student’s t-test or Mann-Whitney test as appropriate. Receiver operating characteristic curve analysis was used to assess the likely usefulness of the N/L ratio in identifying patients with liver fibrosis. The cut-off values which raised both sensitivity and specificity were preferred. A P value of < 0.05 was accepted as statistically significant.
The mean age of the patients was 43.9 ± 17.6 years (92 males and 37 females). 92 patients (71.4%) had CHB with negative HBe antigen, and 37 patients (28.6%) had CHB with positive HBe antigen. After evaluating the biopsy samples in accordance with the METAVIR scoring system, the liver fibrosis stage was F0 in 37 cases (28.7%), F1 in 42 (32.6%), F2 in 19 (14.7%), F3 in 26 (20.2%), and F4 in 5 (3.9%). The “no/minimal fibrosis” group included 79 patients, and the “advanced fibrosis” group included 50 patients. The demographic, biochemical, and histological features of the patients are shown in Table 1.
| Characteristic | |
| n | 129 |
| Sex (male/female) | 92/37 |
| Age (yr) | 43.9 ± 17.6 (range, 18-81) |
| Hemoglobin (g/dL) | 14.7 (13.4-15.6) |
| White blood cell (× 103μL) | 6300 (5400-7400) |
| Platelet (× 103μL) | 217000 (180000-266000) |
| Red blood cell distribution width | 13.2 (12.5-13.5) |
| Mean corpuscular volume | 89.2 (85-91.3) |
| Platelet distribution width | 15.8 (12.9-16.3) |
| Aspartate aminotransferase (U/L) | 41 (28-67) |
| Alanine aminotransferase (U/L) | 54 (33-99) |
| HBeAg (+) | 37 |
| HBVDNA (IU/mL) | 676500 (89000-13113084) |
| Histology activity index | 6 (4-8) |
| Fibrosis | |
| 0 | 37 (28.7) |
| 1 | 42 (32.6) |
| 2 | 19 (14.7) |
| 3 | 26 (20.2) |
| 4 | 5 (3.9) |
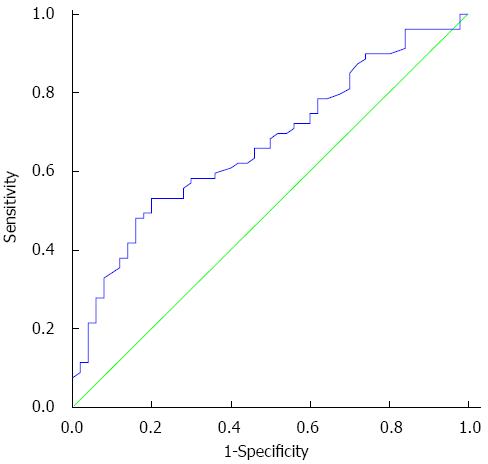
The N/L ratio values were assessed in the two groups. Mean N/L ratio values were notably lower in patients with advanced fibrosis compared to patients with no/minimal fibrosis (P = 0.001). ROC curve analysis revealed that the optimum cut-off point of ≤ 1.9 had the highest sensitivity (80.0%) and specificity (53.2%) for the identification of fibrosis ≥ 2 in CHB patients (Figure 1) with an area under the curve (AUC) of 0.67 (95%CI: 0.58-0.76).
No significant difference was found between the two groups regarding sex. However, a significant difference was found between the two groups in terms of age. The study groups were divided into subgroups according to sex, age, HAI and METAVIR score. Mean age was 52.28 ± 4.7 years for males and 46.63 ± 3.26 years for females (P = 0.243). Median HAI score was 7 (3-12) for females and 7 (2-11) for males (P = 0.177). Median METAVIR score was 3 (0-6) for females, and 2 (0-6) for males (P = 0.277). The mean platelet and neutrophil counts were significantly lower in the advanced fibrosis group when compared with the no/minimal fibrosis group. Mean corpuscular volume was significantly higher in patients with advanced fibrosis compared with patients with no/minimal fibrosis. The mean serum AST, ALT, and total bilirubin levels were significantly higher in cases with advanced fibrosis. The mean values of APRI and the AAR index were significantly higher in patients with significant fibrosis. The HAI index was significantly higher in patients with advanced fibrosis (Table 2).
| Fibrosis < 2 | Fibrosis≥2 | ||
| n | 79 | 50 | |
| Age (yr) | 37 (23-53) | 55 (37.8-65) | < 0.001 |
| Sex (male/female) | 57/22 | 35/15 | 0.80 |
| HbeAg (+) | 23 | 14 | 0.94 |
| HBV DNA (IU/mL) | 460000 (47219-9643500) | 1215000 (165250-18492000) | 0.13 |
| HAI | 4 (3-6) | 8 (7-10) | < 0.001 |
| Hemoglobin (g/dL) | 14.7 (13.5-15.7) | 14.8 (13.2-15.6) | 0.76 |
| White blood cell (× 103μL) | 6300 (5600-7450) | 5650 (4950-7350) | 0.32 |
| Platelet (× 103μL) | 235000 (185500-270000) | 198500 (167750-242500) | 0.047 |
| Red blood cell distribution width | 13.4 (12.5-13.7) | 13.0 (12.5-13.4) | 0.51 |
| Mean corpuscular volume | 88 (83.8-92) | 90.7 (87.2-92.8) | 0.006 |
| Platelet distribution width | 15 (12.7-16.5) | 16.1 (13.6-16.2) | 0.46 |
| Neutrophil | 50.7% (5.3-62.4) | 41.7% (3.0-54.5) | 0.005 |
| Lymphocyte | 25.2% (2.4-33.0) | 23.6% (2.1-35.2) | 0.92 |
| Neutrophil lymphocyte ratio | 1.9 (1.3-2.6) | 1.5 (1.1-1.8) | 0.001 |
| Prothrombin time (INR) | 1.05 (0.95-1.10) | 1.05 (0.99-1.13) | 0.43 |
| Aspartate aminotransferase (U/L) | 33 (24-52) | 49 (41-109) | < 0.001 |
| Alanine aminotransferase (U/L) | 52 (28-85) | 54.5 (42-137) | 0.03 |
| Albumin (g/dL) | 4.3 (4.1-4.5) | 4.3 (4.2-4.7) | 0.16 |
| AAR | 0.73 (0.56-0.93) | 0.79 (0.67-1.07) | 0.03 |
| API | 3 (1-4) | 3 (2-5.25) | 0.21 |
| CDS | 3 (3-4.75) | 4 (3-5) | 0.12 |
| APRI | 0.35 (0.21-0.55) | 0.73 (0.45-1.0) | < 0.001 |
In this research, we evaluated the N/L ratio as a surrogate marker for liver fibrosis severity in patients with CHB. Our results revealed that CHB patients with advanced fibrosis have a significantly lower N/L ratio than CHB patients with no/minimal fibrosis. Thus, decreased levels of the peripheral blood N/L ratio were found to give high sensitivity, specificity and predictive values in CHB patients with advanced fibrosis. In light of our findings, it is suggested that the N/L ratio is a novel noninvasive marker of fibrosis in patients with CHB.
In patients with CHB, identification of the degree of liver fibrosis is necessary for antiviral treatment. Liver biopsy is an invasive procedure and is the gold standard in the evidence-based therapy of CHB patients. However, non-invasive methods for the evaluation of liver fibrosis degree are now favorable in patients with CHB[23-25].
Lymphomononuclear cells play a pivotal role in inflammatory pathways during the development of cirrhosis[26]. In our study we noted that the neutrophil count was markedly lower in patients with advanced fibrosis compared with patients with no/minimal fibrosis. It is debatable whether the N/L ratio exactly reflects the mononuclear inflammation occurring at the tissue level. However, a recent paper by Alkhouri et al[27] showed that the N/L ratio was elevated in patients with nonalcoholic steatohepatitis (NASH) and significant fibrosis compared with patients who did not have NASH.
The N/L ratio is a non-complex and an easily available index of systemic inflammatory response which correlates with prognosis in advanced disease states. In the literature, the N/L ratio has been studied in various inflammatory states and neoplastic diseases such as ulcerative colitis, Crohn’s disease, acute pancreatitis, colorectal cancer, breast neoplasms, lung cancer and hepatocellular carcinoma[28-34]. Furthermore, in the light of current reports, the N/L ratio is practical for estimating survival after coronary interventions[34] and non-ST-segment elevation myocardial infarction[35]. Moreover, it has been demonstrated that this ratio may be efficacious in predicting outcomes in individuals following liver resection or liver transplantation for hepatocellular carcinoma[31,36]. Our current findings showed that N/L ratio values were considerably lower in cases with advanced liver fibrosis.
Even though prothrombin time (PT) is suggested to be prolonged with the progression to liver cirrhosis, some researchers have shown that prolonged PT is not an exact marker of fibrosis[10,37-39]. In our study, we noted that PT was not significantly prolonged in patients with advanced liver fibrosis.
Although there are studies which suggest that low platelet counts are related to advanced hepatic fibrosis, other studies have shown opposite findings[5,37-45]. In the current research, we found that the mean platelet count was considerably lower in patients with advanced fibrosis.
It was previously demonstrated that serum AST level was not closely related to fibrosis degree in patients with CHB. In the literature, a few studies have suggested that AST is an exact predictor of hepatic fibrosis[10,37,39,42-45]. In the present study, we found a relationship between serum AST levels and hepatic fibrosis. On the other hand, despite the suggestion that serum ALT level is not associated with the fibrosis score in patients with CHB[10,37,39,40,42-44], we found an association between serum ALT level and fibrosis in our study.
In previous studies, it was shown that individuals with higher serum HBV DNA levels had higher fibrosis scores[37,39-41,44-46]. However, no association was found between serum HBV DNA levels and hepatic fibrosis scores in our research.
In conclusion, the present study has shown, for the first time, that in patients with CHB, the N/L ratio is strongly associated with histological severity and can be used to identify patients with advanced disease. If our data are confirmed in future studies, we believe that a standardized cut-off value for the N/L ratio would simplify the identification of advanced fibrosis in patients with CHB. Thus, we suggest that the N/L ratio, a low-cost and useful test, provides a beneficial and speedy evaluation of fibrosis in patients with CHB. In light of our findings, it is suggested that the N/L ratio, in combination with other noninvasive parameters, may assist in identifying individuals at high risk of having advanced and progressive disease.
Assessment of the degree of liver fibrosis is crucial for prognostic and therapeutic decisions in patients with chronic hepatitis B. Liver biopsy is currently the gold standard for estimating the seriousness of liver fibrosis. The accuracy of this invasive procedure is influenced by technical and methodological interventions, for example fibrosis stage is misclassified in nearly 20% of patients. The neutrophil to lymphocyte (N/L) ratio is a noninvasive and inexpensive marker of inflammation which can be simply acquired from the complete blood count.
The N/L ratio combines data from two distinct pathways, lymphocytes that portray the regulatory pathway and neutrophils which cause ongoing inflammation. It has been shown that in individuals with nonalcoholic fatty liver disease, the N/L ratio is strongly related to histological changes and can be used to identify cases with progressive disease. In addition to chronic inflammatory diseases, malignancy, infections, cardiac diseases, diabetes, renal and/or hepatic failure, metabolic syndrome, thyroid disease, and many drugs may influence the N/L ratio.
In this research, the authors evaluated the N/L ratio as a surrogate marker of liver fibrosis severity in patients with chronic hepatitis B virus (CHB) infection. Those results revealed that CHB patients with advanced fibrosis have significantly lower N/L ratios than CHB patients with no/minimal fibrosis. Thus, decreased levels of peripheral blood N/L ratio were found to give high sensitivity, specificity and predictive values in CHB patients with advanced fibrosis. Those findings, it is suggested that the N/L ratio is a novel noninvasive marker of fibrosis in patients with CHB.
The present study has shown, for the first time, that in patients with CHB, the N/L ratio is strongly associated with histological severity and can be used to identify patients with advanced disease. If those data are confirmed in future studies, the authors believe that a standardized cut-off value for the N/L ratio would simplify the identification of advanced fibrosis in patients with CHB. Thus, the authors suggest that the N/L ratio, a low-cost and useful test, provides a beneficial and speedy evaluation of fibrosis in patients with CHB.
In patients with CHB, the identification of liver fibrosis degree is necessary for antiviral treatment. Liver biopsy is an invasive procedure, and is the gold standard in the evidence-based therapy of CHB patients. Non-invasive methods for the evaluation of liver fibrosis degree are now favorable in patients with CHB. The N/L ratio is a non-complex and easily available index of systemic inflammatory response, which correlates with prognosis in advanced disease states. In the literature, the N/L ratio has been studied in various inflammatory states and neoplastic diseases such as ulcerative colitis, Crohn’s disease, acute pancreatitis, colorectal cancer, breast neoplasms, lung cancer and hepatocellular carcinoma.
The manuscript investigated the association between N/L ratio and the severity of the liver fibrosis in patients with chronic hepatitis B infection. Their results showed that N/L ratio was decreased in CHB patients with significant fibrosis. The study was innovation and might provide a noel non-invasive marker of fibrosis in CHB patients. It is a well-designed study that offers more to our knowledge in this very interesting field.
P- Reviewer: Ding XF, Savopoulos CG, Zhu F S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 2. | Yu MW, Hsu FC, Sheen IS, Chu CM, Lin DY, Chen CJ, Liaw YF. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Hui AY, Chan HL, Wong VW, Liew CT, Chim AM, Chan FK, Sung JJ. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol. 2005;100:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Kim BK, Kim HS, Park JY, Kim do Y, Ahn SH, Chon CY, Park YN, Han KH, Kim SU. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e41964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, Boisson RC, Bosson JL, Guyader D, Renversez JC. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol. 2012;56:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Jarcuska P, Janicko M, Veselíny E, Jarcuska P, Skladaný L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105-117. [PubMed] |
| 10. | Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 759] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 12. | de Lédinghen V, Vergniol J, Barthe C, Foucher J, Chermak F, Le Bail B, Merrouche W, Bernard PH. Non-invasive tests for fibrosis and liver stiffness predict 5-year survival of patients chronically infected with hepatitis B virus. Aliment Pharmacol Ther. 2013;37:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Wang H, Lafdil F, Wang L, Yin S, Feng D, Gao B. Tissue inhibitor of metalloproteinase 1 (TIMP-1) deficiency exacerbates carbon tetrachloride-induced liver injury and fibrosis in mice: involvement of hepatocyte STAT3 in TIMP-1 production. Cell Biosci. 2011;1:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1293] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 15. | Murawaki Y, Ikuta Y, Idobe Y, Kitamura Y, Kawasaki H. Tissue inhibitor of metalloproteinase-1 in the liver of patients with chronic liver disease. J Hepatol. 1997;26:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647-1659. [PubMed] |
| 17. | Avanzas P, Quiles J, López de Sá E, Sánchez A, Rubio R, García E, López-Sendón JL. Neutrophil count and infarct size in patients with acute myocardial infarction. Int J Cardiol. 2004;97:155-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Alkhouri N, Tamimi TA, Yerian L, Lopez R, Zein NN, Feldstein AE. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig Dis Sci. 2010;55:2644-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 614] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 21. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1416] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 23. | Hanna RF, Kased N, Kwan SW, Gamst AC, Santosa AC, Hassanein T, Sirlin CB. Double-contrast MRI for accurate staging of hepatocellular carcinoma in patients with cirrhosis. AJR Am J Roentgenol. 2008;190:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Montazeri G, Estakhri A, Mohamadnejad M, Nouri N, Montazeri F, Mohammadkani A, Derakhshan MH, Zamani F, Samiee S, Malekzadeh R. Serum hyaluronate as a non-invasive marker of hepatic fibrosis and inflammation in HBeAg-negative chronic hepatitis B. BMC Gastroenterol. 2005;5:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Park SH, Kim CH, Kim DJ, Suk KT, Cheong JY, Cho SW, Hwang SG, Lee YJ, Cho M, Yang JM. Usefulness of multiple biomarkers for the prediction of significant fibrosis in chronic hepatitis B. J Clin Gastroenterol. 2011;45:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Calvaruso V, Craxì A. Fibrosis in chronic viral hepatitis. Best Pract Res Clin Gastroenterol. 2011;25:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 414] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 30. | Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, Mulet-Margalef N, Felip E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 32. | Markar SR, Karthikesalingam A, Falzon A, Kan Y. The diagnostic value of neutrophil: lymphocyte ratio in adults with suspected acute appendicitis. Acta Chir Belg. 2010;110:543-547. [PubMed] |
| 33. | Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, Ozderin YO, Beyazit Y, Kayacetin E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. 2012;36:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 358] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 36. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | Schmilovitz-Weiss H, Tovar A, Halpern M, Sulkes J, Braun M, Rotman Y, Tur-Kaspa R, Ben-Ari Z. Predictive value of serum globulin levels for the extent of hepatic fibrosis in patients with chronic hepatitis B infection. J Viral Hepat. 2006;13:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Liu WP, Xu DJ, Zhao LR, Lu ZH, Wang YH, Lang ZW, Wang GQ. [The prediction and validation of liver fibrosis by a noninvasive model and validation in patients with chronic hepatitis B]. Zhonghua Neike Zazhi. 2008;47:308-312. [PubMed] |
| 39. | Zhou K, Gao CF, Zhao YP, Liu HL, Zheng RD, Xian JC, Xu HT, Mao YM, Zeng MD, Lu LG. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2010;25:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Ekiz F, Yüksel O, Koçak E, Yılmaz B, Altınbaş A, Çoban S, Yüksel I, Üsküdar O, Köklü S. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis B. J Clin Lab Anal. 2011;25:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, Forouzanfar MH, Abedian S, Tavangar SM, Mohamadkhani A. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol. 2006;101:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Pan JJ, Yang CF, Chu CJ, Chang FY, Lee SD. Prediction of liver fibrosis in patients with chronic hepatitis B by serum markers. Hepatogastroenterology. 2007;54:1503-1506. [PubMed] |
| 43. | Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, Mak B, Oo AM, Lim SG. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, Cho SW, Han KH, Chon CY, Moon YM. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int. 2007;27:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Chen YP, Hou JL, Dai L, Wang JL, Zhu YF. [Ultrasonic scores combined with blood indexes for screening and predicting compensated liver cirrhosis in chronic hepatitis B patients]. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:2157-2160. [PubMed] |
| 46. | Vardar R, Gunsar F, Sertoz R, Ozacar T, Nart D, Barbet FY, Karasu Z, Ersoz G, Akarca US. The relationship between HBV-DNA level and histology in patients with naive chronic HBV infection. Hepatogastroenterology. 2010;57:908-912. [PubMed] |