Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5524
Peer-review started: August 8, 2014
First decision: August 27, 2014
Revised: September 16, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: May 14, 2015
Processing time: 283 Days and 18.3 Hours
AIM: To compare the efficacy and safety of tenofovir disoproxil fumarate (TDF) in Asian and non-Asian chronic hepatitis B (CHB) patients.
METHODS: The efficacy and safety of the initial 48 wk of treatment with TDF was compared in a post-hoc analysis of combined data from 217 Asians and 299 non-Asians included in Studies 102 and 103 and a post-approval, open-label trial (Study 123). Patient groups were compared according to baseline hepatitis B e antigen (HBeAg) status and viral load. The main outcome measures included the proportion of patients who achieved a hepatitis B virus (HBV) DNA level < 400 copies/mL at Week 48 of treatment. Secondary measures included: HBV DNA and alanine aminotransaminase (ALT) levels over time; proportion of patients with normal ALT levels; proportion of patients with HBeAg loss/seroconversion and proportion of patients with hepatitis B surface antigen loss/seroconversion; changes in liver histology. Safety and tolerability were evaluated by the occurrence of adverse events (AEs), serious AEs, laboratory abnormalities, discontinuation of the study drug due to AEs, or death. The primary efficacy and safety analysis set included all patients who were randomly assigned to treatment and received at least one dose of study drug.
RESULTS: At week 48, similar proportions of Asians and non-Asians reached HBV DNA < 400 copies/mL (96% of Asian and 97% of non-Asian patients with HBeAg-negative CHB and 83% of Asian and 79% of non-Asian patients with HBeAg-positive CHB had HBV DNA) and normal ALT (78% of Asian and 81% of non-Asian patients with HBeAg-negative CHB and 71% of Asian and 74% of non-Asian patients with HBeAg-positive CHB had normal ALT). On-treatment HBV DNA decline rates were similar between Asians and non-Asians regardless of baseline HBeAg status and viral load. HBV DNA decline during the first four weeks was 2.9 log10 copies/mL in HBeAg-negative Asians and non-Asians, and in HBeAg-positive non-Asians, and 3.1 log10 copies/mL in HBeAg-positive Asians. HBeAg loss and seroconversion was achieved in 14% of Asians vs 26% and 24%, respectively, in non-Asians. Liver histology improved in 77.2% of Asians and 71.5% of non-Asians. No resistance to TDF developed. No renal safety signals were observed.
CONCLUSION: TDF demonstrated similar viral suppression, normalization of ALT, improvements in liver fibrosis, and no detectable resistance in Asian and non-Asian patients regardless of baseline HBeAg status.
Core tip: Although a substantial proportion of the worldwide hepatitis B virus-infected population is Asian, this is often not reflected in clinical trial populations. Comparison of data from Asian and non-Asian patients is important to provide a better understanding of treatment outcome in Asian populations. Our analysis is the first and largest to compare early (48 wk) response to tenofovir disoproxil fumarate (TDF) in Asian and non-Asian patients during large-scale clinical studies. Overall, TDF demonstrated similar viral suppression, normalization of alanine aminotransaminase and improvements in liver fibrosis, with no resistance, in all cohorts, irrespective of baseline hepatitis B e antigen status and viral load.
- Citation: Pan CQ, Chan S, Trinh H, Yao A, Bae H, Lou L. Similar efficacy and safety of tenofovir in Asians and non-Asians with chronic hepatitis B. World J Gastroenterol 2015; 21(18): 5524-5531
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5524.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5524
Tenofovir disoproxil fumarate (TDF) is a potent nucleotide analog indicated for the treatment of chronic hepatitis B (CHB) in adults and adolescents 12 years of age and older[1]. TDF is recommended in treatment guidelines as a first-line therapy in patients with CHB including patients with resistance to lamivudine, telbivudine, adefovir or entecavir[2-4]. Randomized controlled trials have demonstrated the efficacy and safety of TDF in treatment-naïve and -experienced patients with hepatitis B e antigen (HBeAg)-positive and HBeAg-negative CHB[5-9]. Long-term studies have shown that sustained viral suppression with TDF is associated with regression of fibrosis and cirrhosis, and no drug resistance development[6,9,10]. Data from these trials describe a mixed patient population consisting predominantly of Caucasian and a limited number of Asian patients. CHB is diverse among different racial and ethnic groups in terms of mode of transmission and hepatitis B virus (HBV) genotype, which may result in differences in natural history and disease progression, and also response to therapy[4,11]. Since a substantial portion of the worldwide HBV-infected population is Asian and the TDF trials involve mostly non-Asian patients, comparing data between Asian and non-Asian patients is important and can provide a better understanding of treatment outcome for Asian patients.
The aim of the current analysis was to compare the efficacy and safety of TDF in Asian patients vs non-Asian patients with CHB during the first 48 wk of treatment. To allow for analysis of a large dataset, data from three previously published trials that had relatively similar eligibility criteria and baseline patient characteristics were combined; two pivotal trials (Studies 102 and 103)[5] and a post-approval community-based trial (Study 123)[12]. The enlarged dataset of more than 500 patients allowed comparison between Asian vs non-Asian, HBeAg-negative vs HBeAg-positive, and subgroups with varying baseline viral load. This report is the first comparative analysis of large subgroups of CHB patients receiving TDF treatment.
This was a post-hoc analysis of combined data from patients included in three clinical trials of TDF in patients with CHB (Studies 102, 103 and 123). Each trial has been analyzed independently and full details published elsewhere[5,12]. Briefly, Studies 102 (ClinicalTrials.gov identifier: NCT00117676) and 103 (ClinicalTrials.gov identifier: NCT00116805) were double-blind, randomized, active-controlled Phase 3 studies. These two studies each consisted of a double-blind phase in which HBeAg-negative (Study 102) and HBeAg-positive (Study 103) patients were randomized (2:1) to receive either TDF 300 mg or adefovir dipivoxil 10 mg orally once daily for 48 wk, followed by an open-label extension phase with TDF for up to 7 more years. For the present analysis, only the patients initially randomized to the TDF arms during the 48-wk double-blind phase were included. Study 123 (ClinicalTrials.gov identifier: NCT00736190) was a Phase 4, open-label, single-arm study in CHB patients (HBeAg-negative and HBeAg-positive) with self-reported Asian ancestry residing in the United States (Asian-Americans) treated with TDF for 48 wk at community-based sites. The treatment duration of Study 123 limits the present analysis to the first 48 wk of TDF treatment.
The study population consisted of patients from Study 102 and 103 who received TDF during the double-blind phase combined with the patients from Study 123. Key eligibility criteria were similar between the three trials except for HBV DNA levels (> 104 copies/mL in Study 123, > 105 copies/mL in Study 102 and > 106 copies/mL in Study 103), and alanine aminotransferase (ALT) levels [> upper limit of normal (ULN) in Study 102 and Study 123, and > 2 × ULN in Study 103, where ULN = 34 U/L for females and 43 U/L for males].
The main outcome measures included the proportion of patients who achieved an HBV DNA level < 400 copies/mL [Roche COBAS TaqMan; Roche Molecular Diagnostics, Pleasanton, CA, United States; lower limit of quantification (LLoQ): 2.2 log10 or 169 copies/mL] at week 48 of treatment. Secondary measures included: HBV DNA and ALT levels over time; proportion of patients with normal ALT levels (ULN: 34 U/L female, 43 U/L male); proportion of patients with HBeAg loss/seroconversion and proportion of patients with hepatitis B surface antigen (HBsAg) loss/seroconversion. Change in fibrosis at week 48 compared with baseline was also assessed; however results were not combined for all three studies because different methods to assess fibrosis were used. In Studies 102 and 103, fibrosis was assessed by liver biopsy in patients with paired baseline and week 48 data. Histology improvement was defined as at least a two-point reduction in the Knodell necroinflammatory score and no worsening in the Knodell fibrosis score. In Study 123, FibroTest® was used as a non-invasive marker of fibrosis. Patients were evaluated at baseline and at week 48. FibroTest® has been evaluated in a variety of liver diseases, including CHB, and has been validated in an Asian population[13,14].
Safety and tolerability were evaluated by the occurrence of adverse events (AEs), serious AEs (SAEs), laboratory abnormalities, discontinuation of the study drug due to AEs, or death. Hepatic flares were defined as elevation of ALT > 2 × baseline and 10 × ULN, or ALT elevation of one grade, or a measurement of twice a previous value, that was associated with abnormal laboratory parameters suggestive of worsening of hepatic function [a total bilirubin level ≥ 2 mg per deciliter (34 μmol/L) above the baseline value, a prothrombin time ≥ 2 s higher than the baseline value or an international normalized ratio ≥ 0.5 over baseline, or a serum albumin level ≥ 1 g per deciliter below the baseline value]. Specific markers of renal abnormalities included confirmed (defined as two consecutive visits) increase in serum creatinine (SCr) of at least 0.5 mg/dL above baseline value, serum phosphate values decrease to < 2 mg/dL, and creatinine clearance (CrCl) decrease to < 50 mL/min.
The methodology for resistance surveillance has been described in more detail elsewhere[5,12,15]. Briefly, genotypic testing [sequencing of the reverse transcriptase domain of the HBV polymerase gene (HBV pol/RT)] was performed for all patients at baseline, at week 48 if HBV DNA ≥ 400 copies/mL, and at discontinuation of TDF monotherapy. Phenotypic testing (in vitro assay) was performed for all patients post-baseline with amino acid substitution at conserved regions, changes at non-conserved (polymorphic) regions when the same change occurs in more than one patient, or at virologic breakthrough (confirmed > 1 log increase from nadir and/or ≥ 400 copies/mL after having < 400 copies/mL HBV DNA).
The primary efficacy and safety analysis set included all patients who were randomly assigned to treatment and received at least one dose of study drug. All categorical endpoints, including the primary efficacy endpoint, were summarized by number and percentage of patients meeting the endpoint. All continuous endpoints were summarized using descriptive statistics. For the primary efficacy analysis, missing data were considered failures of virologic response. Subgroup analyses of the primary efficacy endpoint included analyses by baseline HBeAg status and viral load. Demographic and baseline characteristics were compared with the use of a two-sided Mantel-Haenszel test for categorical data and a Wilcoxon rank-sum test for continuous data, with a significance level of 0.05.
In total, 219 Asian and 299 non-Asian patients were included in the current analysis: 127 Asian and 299 non-Asian patients from Studies 102 and 103 (TDF arms during the double-blind phase only) and 90 Asian patients from Study 123. Baseline characteristics are shown in Table 1.
| HBeAg-negative | HBeAg-positive | |||
| Asian | Non-Asian | Asian | Non-Asian | |
| n = 102 | n = 186 | n = 115 | n = 113 | |
| Male | 71 (70) | 144 (77) | 60 (52)b | 84 (73) |
| Age (yr, mean ± SD) | 44 ± 9.4 | 44 ± 10.9 | 34 ± 9.7 | 33 ± 11.9 |
| BMI (kg/m2, mean ± SD) | 24.1 ± 3.1b | 26.4 ± 4.5 | 23.0 ± 3.5b | 25.1 ± 5.5 |
| HBV DNA (log10 copies/mL, mean ± SD) | 6.5 ± 1.4a | 6.9 ± 1.3 | 8.4 ± 1.1b | 8.9 ± 1.0 |
| Median ALT, U/L (Q1, Q3) | 73 (45, 115) | 103 (67, 140) | 90 (56, 144) | 118 (88, 196) |
| Normal ALT1 | 17 (17)b | 12 (6.5) | 10 (9) | 5 (4) |
Asian patients had considerably lower mean body mass index (BMI) and lower mean baseline viral load compared with non-Asian patients in both HBeAg-negative and HBeAg-positive subgroups. Distribution of genotypes correlated with race. Overall, more than 90% of Asian patients with available data (n = 211) in this analysis had genotype B (41.2%) or C (53.6%) HBV, while more than 90% of non-Asian patients (n = 294) had genotype A (21.4%) or D (68.7%). Other genotypes were: Asians, genotype A 3.3%, genotype D 1.9%; non-Asians genotype B 1.0%, genotype C 2.7%, genotype E 3.0%, genotype F 2.3%.
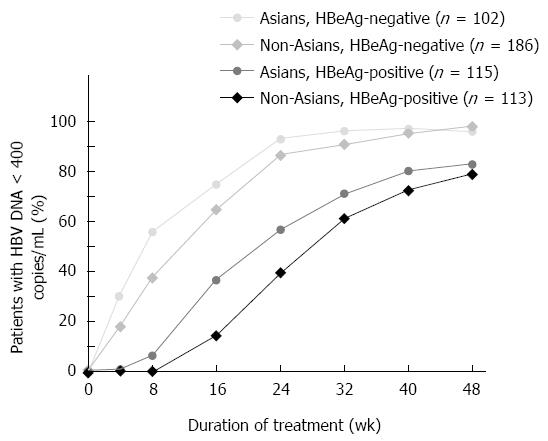
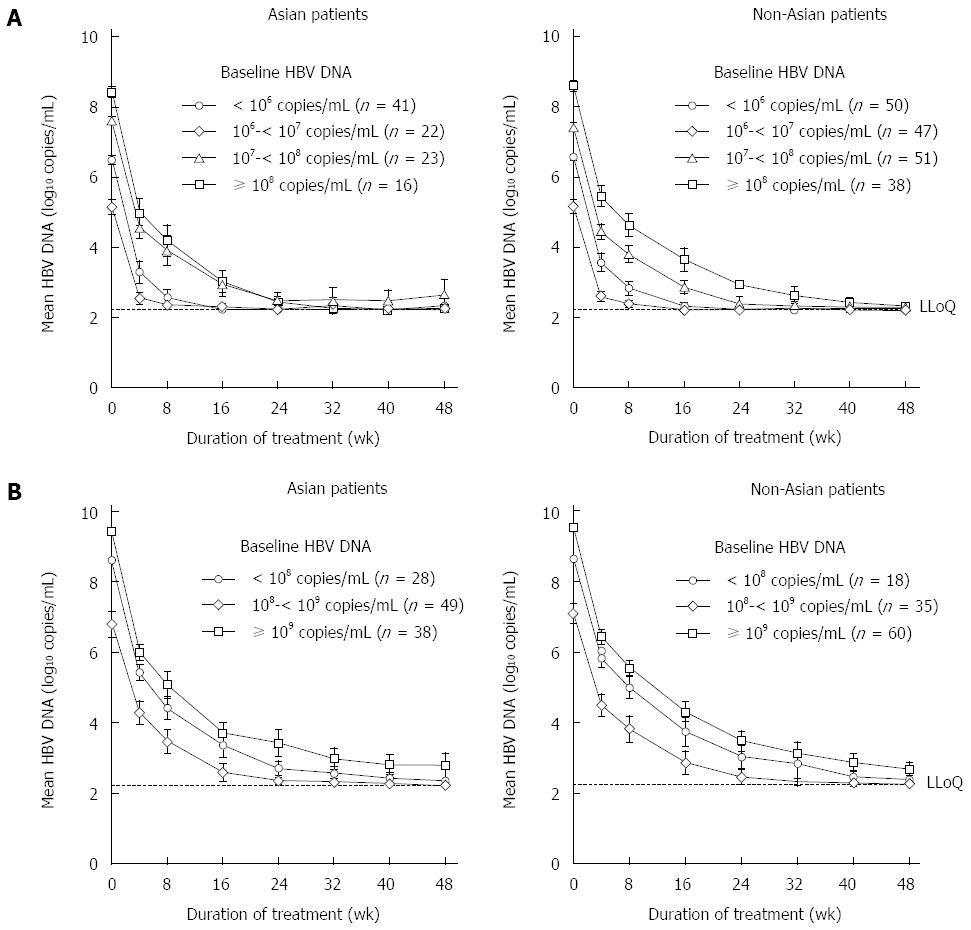
At week 48, 96% of Asian and 97% of non-Asian patients with HBeAg-negative CHB and 83% of Asian and 79% of non-Asian patients with HBeAg-positive CHB had HBV DNA < 400 copies/mL (Figure 1). Decline in HBV DNA following TDF treatment initiation was compared between Asian and non-Asian patients, according to baseline viral load and HBeAg status. HBeAg-negative Asian (n = 102) and non-Asian (n = 186) patients, and HBeAg-positive Asian (n = 115) and non-Asian patients (n = 113) were separately grouped according to their baseline viral load levels by 1-log10 (10-fold) intervals. Of particular note, approximately 20% of HBeAg-negative patients have baseline HBV DNA higher than 108 copies/mL compared with approximately 80% of HBeAg-positive patients. A similar pattern of decline in HBV DNA was seen in Asian and non-Asian patients, irrespective of baseline HBV DNA levels or HBeAg status (Figure 2). Declines in HBV DNA levels from baseline was rapid and profound, similar between Asian and non-Asian patients, at 2.9 to 3.1 log10 copies/mL (794 to 1259 fold) at week 4 and 3.4 to 4.0 log10 copies/mL (2512 to 10000 fold) at week 8 (Table 2).
| Weeks from baseline | HBeAg-negative | HBeAg-positive | ||
| Asians | Non-Asian | Asians | Non-Asian | |
| n = 102 | n = 186 | n = 115 | n = 113 | |
| Week 4 | -2.9 ± 0.82 | -2.9 ± 0.80 | -3.1 ± 0.76 | -2.9 ± 0.77 |
| Week 8 | -3.4 ± 1.04 | -3.5 ± 0.85 | -4.0 ± 1.07 | -3.8 ± 0.88 |
In all, 14% of HBeAg-positive Asian patients achieved HBeAg loss and 14% achieved HBeAg seroconversion. In the non-Asian group, 26% and 24% of patients achieved HBeAg loss and seroconversion, respectively. No Asian patient lost HBsAg during the course of the study. In the non-Asian group, HBsAg loss and seroconversion were seen in 4% and 2% of patients who were HBeAg-positive at baseline and subsequently had HBeAg loss during treatment, respectively, and in 0% of HBeAg-negative patients.
Baseline ALT levels varied widely among study participants. Median ALT levels declined similarly in Asian and non-Asian patients. At week 48, 78% of Asian and 81% of non-Asian patients with HBeAg-negative CHB and 71% of Asian and 74% of non-Asian patients with HBeAg-positive CHB had normal ALT.
As different methods were used to assess fibrosis in Studies 102 and 103 compared with Study 123, data could not be combined and are presented separately. In Studies 102 and 103, the majority of both Asian and non-Asian patients with available data showed a histologic response (Table 3). Most had improvement in necroinflammation without worsening in fibrosis (Knodell scoring). In the community-based Study 123, 30% of Asian-American HBeAg-negative patients were in FibroTest® stage 0 (F0) at baseline, compared with 29% at week 48; the proportion of patients in F4 decreased from 5% at baseline to 0% at week 48. In HBeAg-positive patients, 60% were in F0 at baseline compared with 67% at week 48; the proportion of patients in F4 decreased from 4% at baseline to 2% at week 48.
| Asian | Non-Asian | |
| Histology improvement1 | 95 (77.2) | 213 (71.5) |
| Knodell necroinflammatory score | ||
| Improvement | 99 (80.5) | 227 (76.2) |
| No change | 9 (7.3) | 38 (12.8) |
| Worsening | 2 (1.6) | 11 (3.7) |
| Missing | 13 (10.6) | 22 (7.4) |
| Knodell fibrosis score | ||
| Improvement | 3 (2.4) | 15 (5.0) |
| No change | 89 (72.4) | 227 (76.2) |
| Worsening | 18 (14.6) | 33 (11.1) |
| Missing | 13 (10.6) | 23 (7.7) |
The incidence of SAEs was lower in Asian (HBeAg-negative 2.0%; HBeAg-positive 3.5%) compared with non-Asian (HBeAg-negative 4.8%; HBeAg-positive 10.6%) patients, and this was statistically significant in the HBeAg-positive patient group (P = 0.04 Asians vs non-Asians). The incidence of Grade 3 or 4 laboratory abnormalities were similar between Asians (HBeAg-negative 10.8%; HBeAg-positive 21.7%) and non-Asians (HBeAg-negative 10.8%; HBeAg-positive 31.0%). No renal safety signals were observed in either Asians or non-Asians treated with TDF over the 48-wk analysis period despite a considerably lower BMI in Asian compared with non-Asian patients. Median SCr and CrCl remained within normal ranges throughout the study. No patient had a confirmed increase of SCr by ≥ 0.5 mg/dL, or a confirmed CrCl to < 50 mL/min. Five patients (one Asian and four non-Asian HBeAg-negative) had confirmed phosphate levels < 2 mg/dL. All cases were transient and none required phosphate supplementation. No case was considered clinically significant by investigators. The rate of treatment discontinuation due to AEs was low (< 2%) in both Asians and non-Asians.
No resistance to TDF developed in either Asian or non-Asian patients over 48 wk of treatment. In all, 24 (11%) Asian and 29 (10%) non-Asian patients were included in resistance analysis at week 48 due to viremia. Of these, 15 Asian and 23 non-Asians had HBV DNA > 400 copies/mL at week 48 without evidence of virologic breakthrough (defined as confirmed HBV DNA increase of > 1 log10 from nadir and/or to > 400 copies/mL after having < 400 copies/mL), six in each group had HBV DNA > 400 copies/mL at week 48 with evidence of virologic breakthrough, and three Asian patients had HBV DNA > 400 copies/mL at early discontinuation. Two Asian and two non-Asian patients were found to have conserved site changes. Clinical isolates from patients with conserved site changes or virologic breakthrough did not have reduced in vitro sensitivity to TDF.
This combined analysis included a large dataset of CHB patients treated with TDF for 48 wk, allowing a subgroup comparison between 217 Asian patients (102 HBeAg-negative and 115 HBeAg-positive) and 299 non-Asian (186 HBeAg-negative and 113 HBeAg-positive) patients. Treatment with TDF resulted in rapid virologic suppression, irrespective of baseline HBV DNA levels or HBeAg status, and a similarly high proportion of both Asian and non-Asian patients achieved normal ALT. No patient, including those with virologic breakthrough, developed amino acid substitutions associated with resistance to TDF. Although Asian patients had considerably lower BMI when compared with non-Asians in this study, there were no clinically meaningful differences in safety findings between the two groups, including no decline in renal function. The only difference in treatment outcome noted was a lower serologic response in Asian patients compared with non-Asians at week 48.
This combined analysis was limited to the first year of TDF treatment because the duration of Study 123 was 48 wk. However, data during the first year timeframe provide practical and useful information to clinicians and their CHB patients who are just starting TDF treatment.
Traditionally, post-hoc analysis of datasets combined across studies may be viewed as being less rigorous. However, the current analysis allowed the construction of a large sample size of patients who are under-represented in clinical trials sufficient to permit subgroup analysis and stratification. Comparing various outcome measures between subgroups confirmed and added insights to previous results from smaller datasets.
In keeping with previous reports[5,12], this combined analysis showed a higher proportion of HBeAg-negative patients achieved HBV DNA < 400 copies/mL after 48 wk of TDF treatment compared with HBeAg-positive patients, regardless of racial group. Rapid virologic suppression was seen in all patient groups in the current study when stratified by baseline viral load, with a similar rate of HBV DNA decline upon commencement of TDF treatment. Given that baseline viral load was higher overall in HBeAg-positive patients and the rate of HBV DNA decline was similar regardless of baseline HBV DNA or HBeAg status, it is to be expected fewer HBeAg-positive patients would achieve the pre-determined endpoint of HBV DNA < 400 copies/mL after 48 wk of treatment. In general, patients with higher baseline viral load would be expected to take longer to reach a targeted lower level of HBV DNA. This is in keeping with a recent report that evaluated the antiviral response after 240 wk of TDF treatment in patients included in the pivotal TDF Phase 3 studies who had high baseline viral load (HBV DNA ≥ 9 log10 copies/mL)[16]. The majority of patients (91.5%) in this high viral load group were HBeAg-positive and took longer to achieve HBV DNA < 400 copies/mL than those with baseline HBV DNA < 9 log10 copies/mL. Encouragingly, by week 96, the percentages of patients with HBV DNA < 400 copies/mL were similar in both groups[16]. These results emphasize that while antiviral efficacy in clinical trials is often reported as percentage of patients achieving undetectable HBV DNA at a pre-specified time point, this outcome measure may not be the most informative for everyday practices. Given that our study showed similar kinetics of decline irrespective of viral load and HBeAg status, our findings suggest that longer term treatment for patients with high viral load at baseline would eventually result in undetectable HBV DNA.
Asian patients in the current analysis showed a lower rate of HBeAg loss/seroconversion over 48 wk than did non-Asian patients. However, HBeAg serology response has been shown to increase with continuous TDF treatment; after five years, the rate of HBeAg loss/seroconversion was 47%/40% in Asian patients, similar to 50%/41% in non-Asian patients[17].
Over the 48-wk treatment period, 4% of non-Asian patients who were HBeAg-positive at baseline and who had HBeAg loss during treatment also achieved HBsAg loss and 2% HBsAg seroconversion. Although no Asian patient showed an HBsAg response during the study period, a three-year follow up of Asian patients from Study 123 showed that one patient lost HBsAg (1%)[18].
One limitation of the present study is the lack of liver histology data in the community-based Study 123. However, this is a reflection of current clinical practice, where fewer patients undergoing a liver biopsy. In the pivotal trials, Study 102 and 103, histologic improvement was shown in a majority of patients after 48 wk of TDF treatment. This improvement was further demonstrated by evidence of regression of fibrosis and cirrhosis in a majority of patients after 240 wk of therapy[6].
In summary, TDF provides potent suppression of HBV DNA, with normalization of ALT, improvements in liver fibrosis and no detectable resistance development in Asian patients with either HBeAg-positive or HBeAg-negative CHB over the first 48 wk of treatment. Efficacy is similar to that for non-Asian patients. Treatment was well tolerated, and with a tolerability and efficacy profile consistent with that in other CHB patient populations.
Randomized controlled trials have demonstrated the efficacy and safety of tenofovir disoproxil fumarate (TDF) in patients with chronic hepatitis B (CHB). However, specific data in Asian patients are limited and Asian patients have been under-represented in clinical trials. CHB is diverse among different racial and ethnic groups in terms of mode of transmission and predominant hepatitis B virus (HBV) genotype, which may result in differences in natural history and disease progression, and also response to therapy.
Since a substantial proportion of the worldwide HBV-infected population is Asian, the comparison of data between Asian and non-Asian patients is important and can provide a better understanding of treatment outcome for Asian patients.
This study combined data from three clinical trials of TDF to achieve a large dataset of more than 500 patients, which allowed for comparison between Asians and non-Asians, hepatitis B e antigen (HBeAg)-negative and HBeAg-positive, and subgroups with differing baseline viral loads. Virologic response during the first 4 wk and 8 wk of treatment was specifically examined and compared between the subgroups. The study represents the first comparative analysis of large subgroups of CHB patients receiving TDF treatment, and as such provides valuable insight into the comparative safety and efficacy of TDF in Asian patients during the initial 48 wk of treatment.
The results of the present study show that TDF demonstrates similar efficacy in terms of viral suppression, normalization of alanine aminotransaminase (ALT) and improvements in liver fibrosis, with no detectable resistance in Asian and non-Asian patients regardless of baseline HBeAg status. The dynamics of viral load reduction during the first two months upon treatment initiation is similar for all subgroups of patients.
The Fibrotest score is calculated from a combination of serum markers (α-2-macroglobulin, haptoglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and total bilirubin) with the age and gender of the patient.
The authors compared the effectiveness of tenofovir Asian vs non-Asian patients with CHB regardless of baseline HBeAg status. Tenofovir showed similar rate of viral suppression, ALT normalization and improvement in liver fibrosis between the two groups. No drug resistance was noted in Asian and non-Asian patients.
P- Reviewer: Anand BS, Bozdayi AM, Vezali E S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Gilead Sciences. VIREAD® prescribing information. Accessed 2 June 2014. Available from: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/viread/viread_pi.pdf. |
| 2. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 3. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 4. | Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, Leduc TS. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 910] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 6. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 7. | Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Fung S, Kwan P, Fabri MJ, Horban A, Pelemis M, Husa P, Hann HW, Flaherty JF, Massetto B, Dinh P. Efficacy and safety of tenofovir DF in chronic hepatitis B virus infected patients with documented lamivudine resistance. Hepatology. 2012;56:S14. |
| 9. | Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, Caruntu FA, Flaherty JF, Massetto B. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Berg T, Zoulim F, Moeller B, Trinh H, Marcellin P, Chan S, Kitrinos KM, Dinh P, Flaherty JF, McHutchison JG. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014;60:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 11. | Pan CQ, Hu KQ, Tsai N. Long-term therapy with nucleoside/nucleotide analogues for chronic hepatitis B in Asian patients. Antivir Ther. 2013;18:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Pan CQ, Trinh H, Yao A, Bae H, Lou L, Chan S. Efficacy and safety of tenofovir disoproxil fumarate in Asian-Americans with chronic hepatitis B in community settings. PLoS One. 2014;9:e89789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol. 2008;32:22-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kim BK, Kim SU, Kim HS, Park JY, Ahn SH, Chon CY, Cho IR, Joh DH, Park YN, Han KH. Prospective validation of FibroTest in comparison with liver stiffness for predicting liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e35825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Snow-Lampart A, Chappell B, Curtis M, Zhu Y, Myrick F, Schawalder J, Kitrinos K, Svarovskaia ES, Miller MD, Sorbel J. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology. 2011;53:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P, Martins EB, Yee LJ, Flaherty JF, Kitrinos KM. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Gane EJ, Marcellin P, Sievert W, Trinh HN, Shiffman ML, Washington MK, Barnes CN, Bornstein JD, Flaherty JF, Heathcote EJ. Five years of treatment with tenofovir DF (TDF) for chronic hepatitis B infection in Asian patients is associated with sustained viral suppression and significant regression of histological fibrosis and cirrhosis. Hepatology. 2011;54:1038A. |
| 18. | Pan CQ, Zeng Z, Bae H, Trinh HN, Ma x, Mi LJ, LeducTS , Chan S, Hu KQ. Three-year outcomes of tenofovir disoproxil fumarate (TDF) treatment in Asian-American adults with chronic hepatitis B in real-life practice in the US: a prospective open label study. Hepatology. 2013;58:696A. |