Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5496
Peer-review started: November 21, 2014
First decision: December 26, 2014
Revised: January 19, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 14, 2015
Processing time: 178 Days and 12.1 Hours
AIM: To identify predictors of sustained virological response in hemodialysed patients treated by PEGinterferon α for chronic hepatitis C, genotype 1.
METHODS: The sustained virological response (SVR) rate, IL28B genotype, IFNL4 genotype, initial viral load (IVL) and other pretreatment variables in 39 end-stage renal disease patients (ESRD) on maintenance haemodialysis (HD) infected with hepatitis C virus (HCV), genotype 1b, were compared with a control group of 109 patients with normal kidney function treated within the same period. All the patients were treatment naïve and had well compensated liver disease. The ESRD patients received 135 μg of PEGylated interferon α-2a (PegIFN-α) weekly and a reduced dose of ribavirin (RBV) was administered to 23/39 patients with an initial haemoglobin level > 10 g/dL. Control group patients were given standard doses of PegIFN-α and RBV. SVR was assessed as HCV RNA negativity 24 wk post-treatment. A t-test or ANOVA were used for comparisons of the means and a χ2 test compared the frequencies. Logistic regression was used to determine significant predictors of SVR. Cutoff values for continuous variables were obtained from Receiver Operating Characteristic analysis.
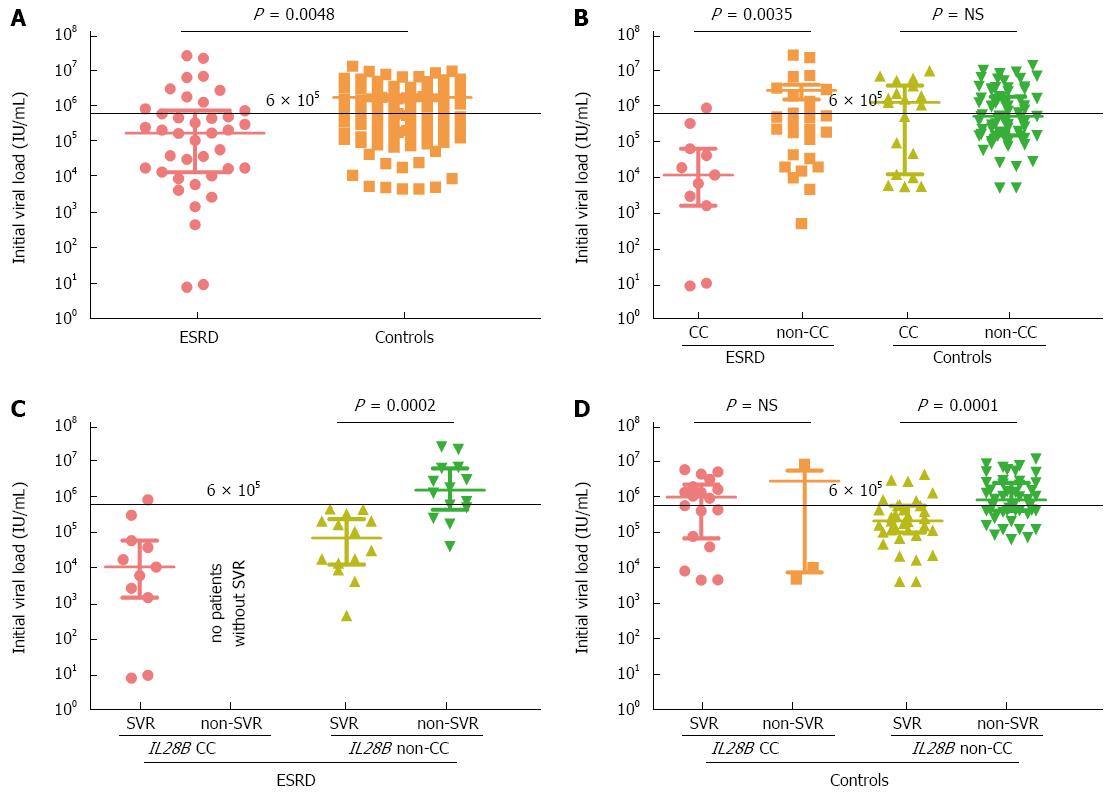
RESULTS: The distribution of IL28B rs12979860 CC, CT and TT genotypes in the ESRD group was 28.2%, 64.1% and 7.7%, respectively, and 19.3%, 62.4% and 18.3% in the controls. The IFNL4 genotype was in almost absolute linkage disequlibrium with IL28B. The proportion of patients with a low IVL (< 600000 IU/mL) was significantly higher in the ESRD group than in the controls (28/39, 71.8% vs 51/109, 46.8%, P = 0.009), as was the proportion of patients with low IVL in IL28B CC carriers compared with non-CC carriers in the ESRD group (10/11, 90.9% vs 18/28, 64.3%, P = 0.0035). This difference was not found in the controls (7/22, 31.8% vs 44/87, 50.6%, P = 0.9). The overall SVR rate was 64.1% (25/39) in the ESRD group and 50.5% (55/109) in the control group (P = 0.19). 11/11 (100%) and 19/22 (86.4%) IL28B CC patients achieved SVR in the ESRD and control groups, respectively. A statistically significant association between SVR and IL28B and IFNL4 variants was found in both groups. The ESRD patients who achieved SVR showed the lowest IVL [median 21000, interquartile range (IQR): 6000-23000 IU/mL], compared with ESRD individuals without SVR (1680000, IQR: 481000-6880000, P = 0.001), controls with SVR (387000, IQR: 111000-1253000) and controls without SVR (905000, IQR: 451000-3020000). In ESRD, an IVL < 600000 IU/mL was strongly associated with SVR: 24/28 (85.7%) patients who achieved SVR had viraemia below this threshold.
CONCLUSION: Haemodialysis decreases the viral load, especially in IL28B CC genotype carriers. A low IVL was the strongest predictor of SVR in ESRD patients identified in multivariate analysis.
Core tip: The proportion of haemodialysed patients infected with chronic hepatitis C virus (HCV) receiving antiviral treatment remains unsatisfactory and should increase. Patients should be selected with high probability of successful treatment. Therefore, this study evaluated predictors of sustained virological response (SVR) in haemodialysed patients treated with PEGylated interferon α for HCV, genotype 1. The results of the study indicated that there was a high number of individuals with a low initial viral load (< 600000 IU/mL) among haemodialysed patients, especially in IL28B CC genotype carriers. A low initial viral load was the strongest predictor of SVR in haemodialysed patients.
- Citation: Sperl J, Frankova S, Senkerikova R, Neroldova M, Hejda V, Volfova M, Merta D, Viklicky O, Spicak J, Jirsa M. Relevance of low viral load in haemodialysed patients with chronic hepatitis C virus infection. World J Gastroenterol 2015; 21(18): 5496-5504
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5496.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5496
The 10-year survival of kidney transplant recipients with hepatitis C (HCV) is significantly worse compared with non-infected patients[1]. Therefore, HCV eradication should be a standard procedure in HCV-infected patients considered for a kidney transplant. The reason to treat before kidney transplantation is supported by the fact that there is no effective and safe treatment in kidney transplant recipients. The use of interferon alpha in transplanted patients is considered controversial because of the high risk of interferon-induced kidney allograft dysfunction[2]. Furthermore, antiviral treatment should be also considered in all HCV-infected end-stage renal disease patients (ESRD) patients, because of their increased all-causes mortality when on maintenance haemodialysis[3,4]. Despite the negative impact of HCV infection on the life expectancy of patients on maintenance haemodialysis, most of the patients remain untreated. The epidemiological study published by Goodkin et al[5] showed that only 1% of HCV-infected patients were given antiviral therapy. The treatment rate was higher in the group of patients enlisted for kidney transplantation, but was still only 3.7%. The reason for treatment deferral is undoubtedly the burden of interferon alpha therapy, long treatment duration and postponement of kidney transplantation[6-10].
The proportion of haemodialysed patients receiving antiviral treatment is expected to increase and an accurate predictor of sustained virological response (SVR) would be helpful in the treatment decision algorithm. A treatment that significantly postpones patients’ enlistment should be proposed, especially to individuals who have a high probability of SVR, i.e., HCV eradication. The SVR rate is significantly better in non-genotype 1 infected patients than in genotype 1 patients, who are in general considered to be difficult-to-treat. Therefore, identification of the subset of easy-to-treat patients among those with genotype 1 is important to reliably select individuals with high probability of SVR.
In patients with normal kidney function, the IL28B rs12979860 genotype (a generally used marker of the functional IFNL4 ss469415590 genotype, which is responsible for genetic predisposition to SVR[11]), degree of liver fibrosis and low initial HCV RNA levels represent the most reliable pretreatment predictors of SVR in PEGylated interferon α (PegIFN-α) and RBV therapy[12-14]. Alterations of the innate immunity caused by haemodialysis could modify the eradication process of HCV and change the predictive value of the above-described factors. A low IVL as a predictive factor of SVR in haemodialysed patients has been described in the meta-analysis published by Gordon et al[15]. This meta-analysis included all HCV genotypes and was not specific for genotype 1. Furthermore, the predictive value of the IL28B genotype has not been evaluated so far in haemodialysed patients.
The aim of our study was to assess and validate reliability of the standard predictive factors in genotype 1 patients with ESRD on maintenance haemodialysis.
We evaluated 39 kidney transplant candidates with ESRD on maintenance haemodialysis, treated for chronic HCV infection in three outpatient speciality clinics in the Czech Republic, from January 2004 to October 2012. The cohort consisted of 24 males and 15 females of average age 52 years (range: 25-69). All patients were haemodialysed for at least 3 mo, three times per week. The mean duration of haemodialysis was 3 years (range: 1-19 years). Twenty-nine patients resumed maintenance haemodialysis after having undergone kidney transplant in the past with subsequent graft failure. All patients were Caucasian, HCV treatment-naïve, and infected with HCV genotype 1b.
Pretreatment liver biopsy was performed in 29 ESRD patients, nine of whom had fibrosis stage F3 or F4, according to the Metavir score, and 20 patients had stage F0-F2. All patients had compensated liver disease with no signs of proteosynthetic dysfunction (normal albumin, bilirubin and prothrombin time values), ascites or encephalopathy. Patients with a history of liver disease decompensation, HBV or HIV co-infection, and patients receiving any immunosuppressive or immunomodulation therapy at the time of treatment initiation, were excluded from the evaluation.
All patients were treated with PegIFN-α2a (40 kDa) at a reduced dose of 135 μg, administered subcutaneously once weekly after a haemodialysis session. Twenty-three patients (59%) with a haemoglobin level > 10 g/dL at baseline were concurrently treated with RBV at reduced dose, 200-400 mg weekly. Erythropoietin was used in patients with a haemoglobin level < 12 g/dL. The anticipated duration of treatment was 48 wk. SVR was assessed as HCV RNA negativity 24 wk post-treatment.
The control group consisted of 109 treatment-naïve Caucasian patients (54 males and 55 females) of average age 46 years, with chronic hepatitis C, genotype 1b. These patients were treated within the same period with once weekly subcutaneously administered PegIFN-α2a (40 kDa) at a dose of 180 μg, together with weight-adjusted RBV 1000-1200 mg daily. The anticipated treatment duration was 24 wk for patients with low pretreatment viraemia who achieved rapid virological response (RVR, i.e., negative HCV RNA at week 4 of treatment), and 48 wk for patients who had had high pretreatment viraemia or had not achieved RVR. SVR was assessed as HCV RNA negativity 24 wk post-treatment. All controls had normal renal function, estimated as glomerular filtration rate calculated using Cockcroft-Gault formula at baseline. Pretreatment liver biopsy was performed in 101 patients, of whom 49 had fibrosis F0-F2 and 52 had fibrosis score ≥ F3, according to the Metavir score.
HCV RNA was assessed accordingly to the period of treatment by the Roche AmpliPrep/COBAS® TaqMan® HCV Test v1.0 or v2.0 (Roche Molecular Systems, Branchburg, NJ, United States). Serum HCV RNA levels were determined at baseline, at weeks 4, 12, 24, 36, 48 of treatment and 12 and 24 wk after the end of therapy.
Patients were genotyped for IL28B rs12979860 C/T polymorphism by polymerase chain reaction, based on a restriction fragment length polymorphism assay, as described by Fabris et al[16]. To minimise genotyping errors, blank controls wells were left on the PCR plates and two operators, unaware of the status of the sample, performed the genotype assignment independently. Genotyping for the IFNL4 ss469415590 TT/ΔG polymorphism was performed by the custom TaqMan genotyping assay described in[11]. Written informed consent for DNA sampling was obtained from all patients and the study conformed to the declaration of Helsinki Ethical Guidelines.
Data are presented as means and standard deviations, medians and ranges or as frequencies, as appropriate. A t-test or ANOVA with Dunnet’s post hoc test were used for comparisons of the means and the χ2 test was used to compare frequencies. Logistic regression was used to determine significant predictors of SVR. Cutoff points for continuous variables were obtained from Receiver Operating Characteristic (ROC) analysis. A P value < 0.05 was considered statistically significant throughout the study. Statistical analysis was performed using the SPSS 13.0 software.
Compared with patients with maintained renal function, ESRD patients were significantly older, had lower baseline ALT activity, significantly lower initial HCV viral load (IVL) and achieved higher rate of early virological response (EVR). There were no statistically significant differences between the groups in terms of gender distribution, BMI, diabetes, Metavir fibrosis stage, and IL28B and IFNL4 genotypes (Table 1).
| ESRD | Controls | P value | |
| (n = 39) | (n = 109) | ||
| Age, median (range) | 52 (25-69) | 46 (17-67) | 0.013 |
| Gender, F/M | 15/24 (38.5/61.5) | 55/54 (50.5/49.5) | 0.262 |
| Fibrosis stage before treatment according to Metavir score | |||
| F0 | 2 (5.1) | 3 (2.8) | 0.072 |
| F1 | 12 (30.8) | 25 (22.9) | |
| F2 | 6 (15.4) | 21 (19.3) | |
| F3 | 1 (2.6) | 21 (19.3) | |
| F4 | 8 (20.5) | 31 (28.4) | |
| F unknown | 10 (25.6) | 8 (7.3) | |
| BMI, average (mean ± SD) | 24 ± 4.1 | 25 ± 4.0 | 0.071 |
| Type 2 diabetes | 5 (12.8) | 12 (11.0) | 0.773 |
| Initial ALT (IU/L), average (mean ± SD) | 57 ± 54 | 105 ± 87 | < 0.001 |
| Initial viral load (IU/mL x 1000), median (IQR) | 193 (16-810) | 541 (163-1853) | 0.003 |
| RVR | 20 (52.6) | 39 (36.1) | 0.074 |
| EVR | 29 (74.4) | 60 (55.0) | 0.038 |
| SVR | 25 (64.1) | 55 (50.5) | 0.190 |
| Premature termination of treatment | 14 (35.9) | 44 (40.4) | 0.704 |
| IL28B CC genotype | 11 (28.2) | 21 (19.3) | 0.262 |
| IFNL4 TT genotype | 11 (28.9) | 21 (19.3) | 0.370 |
| Initial viral load < 600000 IU/mL | 28 (71.8) | 51 (46.8) | 0.009 |
| History of kidney transplant | 29 (74.4) | NA | NA |
| Concurrent treatment with ribavirin | 23 (59.0) | 109 (100.0) | < 0.001 |
Of the 39 ESRD patients, 25 (64%) completed the entire course of treatment. In 8 patients (21%), the treatment was discontinued at week 12 or 24 because of lack of virological response, and in six patients (15%) because of severe adverse events (SAE). In the control group, 65 (60%) patients completed the entire course of treatment. The treatment was discontinued in 34 patients (31%) because of lack of virological response and in 10 patients (9%) because of SAE. The rate of treatment discontinuation did not differ significantly between groups. Six (15%) ESRD patients discontinued the treatment because of an SAE: non-functional renal allograft rejection (two patients), thrombocytopenia with bleeding complications (two patients), interferon-induced autoimmune hepatitis (one patient) and pneumonia (one patient). Nevertheless, five out of these six patients with SAE achieved SVR. Concerning further adverse events, nine patients presented with worsening of anaemia requiring erythropoietin therapy (eight patients) or transfusion (one patient). One patient developed pancytopaenia and one patient presented with respiratory infection requiring antibiotic therapy. In three patients, the dose of PegIFN-α had to be reduced to 90 μg because of adverse events.
ESRD group: The distribution of IL28B rs12979860 genotypes was CC 28.2%, CT 64.1%, TT 7.7%. The frequencies of the corresponding IFNL4 ss469415590 genotypes TT/TT, TT/ΔG and ΔG/ΔG were exactly the same, reflecting the strong linkage disequilibrium between the two loci. The percentage of patients with a low IVL (< 600000 IU/mL) was significantly higher in the ESRD group than in the controls (28/39, 71.8% vs 51/109, 46.8%, P = 0.009) as well as the percentage of patients with a low IVL in IL28B CC carriers compared with non-CC carriers in the ESRD group (10/11, 90.9% vs 18/28, 64.3%, P = 0.0035). This difference was not found in the controls (7/22, 31.8% vs 44/87, 50.6%, P = 0.9). The overall SVR rate was 64.1% (25/39). All CC patients, including one patient with a high IVL, achieved SVR. In contrast, only 50.0% (14/28) of non-CC patients achieved SVR (Table 2). All of them had a low IVL. The SVR rate in non-CC patients with a low IVL was 77.7% (14/18). In the subgroup of non-CC patients without SVR, there were only 4/14 (28.6%) with a low IVL. The CC genotype carriers, regardless of their IVL, and non-CC genotype carriers with a low IVL were easy-to-treat, with an overall SVR rate of 86.2% (25/29) (Table 2). The SVR rate in the ESRD patients treated with both PegIFN-α and RBV was 73.9% (17/23). Patients treated with PegIFN-α monotherapy achieved SVR only in 50.0% (8/16), but the difference was not significant (P = 0.126).
| Patients | SVR | IVL (IU/mL) | IL28B rs12979860 genotype | |
| CT or TT | CC | |||
| ESRD | Yes | < 600000 | 14 (58) | 10 (42) |
| > 600000 | 0 (0) | 1 (100) | ||
| No | < 600000 | 4 (100) | 0 (0) | |
| > 600000 | 10 (100) | 0 (0) | ||
| controls | Yes | < 600000 | 26 (84) | 5 (16) |
| > 600000 | 10 (42) | 14 (58)1 | ||
| No | < 600000 | 18 (90) | 2 (10) | |
| > 600000 | 33 (97) | 1 (3) | ||
The SVR rate was 60.0% (12/20) in ESRD patients with pretreatment liver fibrosis stage F0-F2, 66.7% (6/9) in patients with stage F3-4 and 70.0% (7/10) in patients without pretreatment liver biopsy. The difference between F0-2 and F3-4 subgroups was not significant (P = 1.0). The ESRD patients who achieved SVR did not significantly differ from the patients without SVR regarding the haemodialysis duration (2.4 ± 2.3 years vs 4.1 ± 5.9 years, P = 0.937).
Among 11 patients with high viraemia, only one achieved RVR and subsequently SVR (a CC genotype carrier). Among 28 patients with low viraemia, 19 (67.9%) achieved RVR and 18/19 then achieved SVR. Altogether 19/20 patients who had RVR also achieved SVR (95%).
The distribution of IL28B rs12979860 genotypes was CC (21/109) 19.3%, CT (68/109) 62.4%, TT (20/109) 18.3% and all but one control subjects carried the corresponding IFNL4 genotypes: TT/TT, TT/ΔG and ΔG/ΔG. The only exceptional control subject carried the combination of IL28B CT with IFNL4 TT/TT. A low IVL was observed in 7/22 (31.8%) “CC” patients (including the subject with the exceptional genotype combination) vs 44/87 (50.6%) of “non-CC” patients (P = 0.9). The overall SVR rate was 50.5% (55/109). Nineteen “CC” patients (19/22, 86.4%) achieved SVR (Table 2). Fourteen of these 19 patients (73.7%) had a high IVL. The SVR rate in the subgroup of “non-CC” patients was 41.4% (36/87), 10 SVR patients had a high IVL and 26 SVR patients had low IVL. In the subgroup of “non-CC” patients without SVR, there were 18/51 (35.3%) patients with a low IVL. The SVR rate in “non-CC” patients with a low IVL was 59.0% (26/44) (Table 2).
Among 58 patients with high viraemia in the control group, 15 patients achieved RVR (25.9%) and all of them subsequently achieved SVR. Among 51 patients with low viraemia, 25 (49%) achieved RVR and 25/25 then achieved SVR. In total, 40/40 patients with RVR also achieved SVR (100%).
The difference between the overall SVR rates in ESRD patients and controls was not statistically significant (P = 0.19). Consistent with the published data from the general population infected with HCV[6], we confirmed a significant association between SVR and genetic variants in the IL28B and IFNL4 loci in the controls, and we also found statistically significant association in the ESRD patients (Figure 1).
The ESRD patients who achieved SVR showed the lowest baseline IVL [median 21000, interquartile range (IQR) 6000-23000 IU/mL], compared with ESRD individuals without SVR (1680000, IQR: 481000-6880000, P < 0.001), and compared with control group with SVR (387000, IQR: 111000-1253000) and without SVR (905000, IQR: 451000-3020000). An IVL < 600000 IU/mL was strongly associated with SVR: 24/28 (85.7%) patients who achieved SVR had viraemia below this threshold.
In ESRD patients, RVR proved to be a very strong predictor of SVR (OR = 171, 95%CI: 26-490, P < 0.001), which reflected the fact that RVR and SVR are interdependent because they reflect the same biological phenomenon, i.e., clearance of the virus.
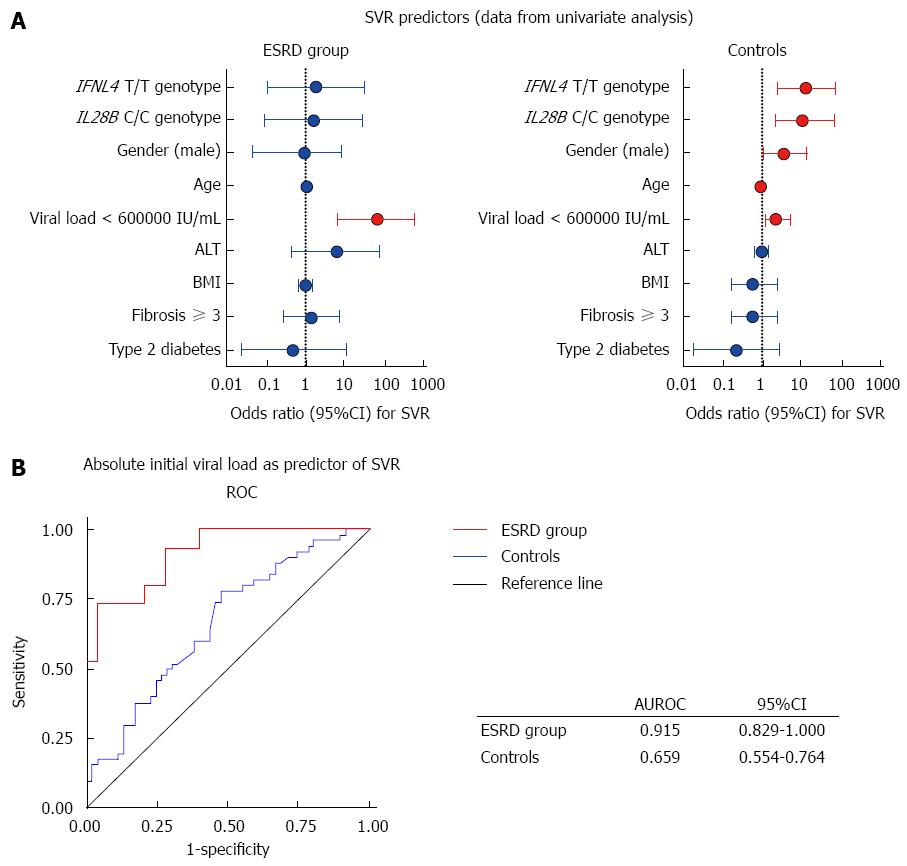
The potential role of pre-treatment viraemia as a predictor of SVR was evaluated using regression analysis. Age, male gender and IL28B/IFNL4 status were significant predictors of SVR in the general HCV population, whereas only pretreatment viral load proved to be significant predictor of SVR in patients with ESRD. Using Wald statistics to evaluate the relative contributions of these determinants to SVR, we found that the strongest determinant of SVR was age in controls and pretreatment viral load in ESRD patients. Notably, pretreatment viral load was not associated with SVR in the control group, and IL28B and IFNL4 did not prove to be significant determinants of SVR in ESRD patients (Figure 2).
The IFNL4 ss469415590 genotype, strongly linked with the IL28B rs12979860 variant, is the most reliable predictive host factor of SVR achievement with PegIFN-α and RBV therapy in patients with normal kidney function[11,12]. Since the IFNL4 variant ss469415590 ΔG converts the inactive IFNL4 pseudogene to an active gene producing interferon lambda 4, which likely counteracts signalling by other interferons involved in HCV clearance[11], the ΔG homozygotes have a low chance of achieving an SVR. To the best of our knowledge, the relevance of any of the above-described gene polymorphisms has not been described so far in a cohort of haemodialysed patients. Our data suggested that IL28B or IFNL4 genotypes play a similar role in HCV patients with ESRD as they do in HCV patients with normal kidney function. Moreover, despite the fact that all CC genotype carriers achieved SVR, we showed that low IVL is an even better predictor of SVR achievement in the ESRD group than the IL28B genotype.
RVR achievement turned out to be a very strong predictor of SVR, but we did not include it in the further statistical analysis of our cohort. Our aim was to validate pretreatment factors that allow selection of patients who have a high chance to achieve SVR. RVR, considered as an on-treatment predictive factor, may help to motivate patients to continue in poorly tolerated treatment, but the fact that the patient does not achieve RVR should not represent a reason to stop therapy.
A high percentage of individuals with low viraemia among HCV-infected patients on maintenance hemodialysis has already been reported, and two different hypotheses explaining low viraemia have been postulated. The first hypothesis is based on the adsorption of the virus on the haemodialysis membrane[17-19]. Accordingly, HCV RNA and HCV Ag decreases were observed during the hemodialysis session[20], but the adsorption activity was proved only when using the polysulphone membrane, not the cuprophan membrane[18]. The second hypothesis explains viraemia fluctuations by the immune mediated effect of increased levels of interferon-alpha during haemodialysis. Badalementi et al[21] described an HCV RNA decrease and the reciprocal interferon-alpha blood level increase during the haemodialysis sessions. Activation of the interferon-alpha pathway during haemodialysis procedure may represent the factor facilitating the mechanism of viral clearance in the patients on maintenance haemodialysis. This hypothesis is in accordance with our finding of significantly lower IVL in the IL28B CC carriers compared with IL28B non-CC carriers. IL28B CC genotype carriers are prone to a higher activation of interferon-sensitive genes compared with non-CC genotype carriers[14]. We speculated that in haemodialysed patients, the viral clearance mechanism is modified by the above-explained increase of interferon-alpha level together with the additional alterations in adaptive and innate immunity mechanisms described by Barbossa[22]. The low IVL, in our opinion, reflects the spontaneous effort of the immune system to clear the virus and the administration of PEG-IFN results in completion of the virus clearance process.
The efficacy of PegIFN-α monotherapy, as well as PegIFN-α and RBV combination described in previously published studies, varies widely. A recent review[23] analysed 13 original papers assessing the results of interferon-based anti-HCV therapy in hemodialysed patients. The analysis included patients treated by PegIFN-α monotherapy as well as patients to whom a reduced dose of RBV was administered. The SVR rate ranged from 27.3% to 78.8%, and was further increased by co-treatment with RBV. Similar conclusions were drawn in the review by Fabrizi[2]: the SVR rate ranged between 12.5% and 56% in nine studies with PegIFN-α monotherapy and between 29% and 97% in 7 studies assessing combined PegIFN-α and RBV therapy. The superiority of PegIFN-α and RBV combination to PegIFN-α monotherapy has recently been documented in two prospective comparative studies; however, other predictors of SVR have not been assessed[24,25]. Our ESRD patients, who were treated with the PegIFN-α and RBV combination, also achieved a better SVR rate than the patients treated with PegIFN-α monotherapy. However, there was no significant difference because of the small number of patients included in the PegIFN-α monotherapy group. The reason for the large variation in SVR rate may lie in variable ratios of genotype 1 to non-1 patients, and different percentages of patients with low viraemia in the published studies.
In our group, consisting solely of genotype 1b patients, we achieved a satisfactory SVR rate despite the fact that RBV was administered only to 23 out of the 39 treated patients. The high proportion of patients with low viral load in our group represented a factor that also increased the overall SVR rate in our ESRD cohort (IVL < 600000 IU/mL in 28/39, i.e., 71.8%). Our data are comparable with a recently published paper by Wang et al[26], in which the authors described 16 genotype 1b infected patients on maintenance hemodialysis treated with PegIFN-α monotherapy. Twelve of the 16 patients had low IVL and 11/12 achieved SVR.
We concluded that genotype 1 patients with ESRD should not be considered generally as difficult-to-treat, because in this group, patients with high probability of SVR achievement can be identified. In ESRD patients with genotype 1, SVR is predictable based on the same pretreatment variables as in patients with normal renal function. Patients with a high probability of SVR achievement can be identified according to low IVL and their IL28B genotype. Identically to patients with normal renal function, the prediction based on IFNL4 genotype testing in Europeans is not superior to IL28B genotype assessment in ESRD patients. The treatment-decision process, i.e., to treat immediately or to defer treatment and transplant with HCV infection waiting for the new treatment options, should also take into consideration overall life expectancy, comorbidities and the estimated risk of adverse events during therapy.
Hepatitis C virus (HCV) infection significantly decreases long-term survival after kidney transplantation. HCV infection should be eradicated in kidney transplant candidates within the period of hemodialysis. PEGylated interferon alpha and ribavirin therapy remains a treatment option, but should be offered only to patients with a high chance of a cure.
The study scrutinizes sustained virological response (SVR) predictors in patients with end-stage renal disease to permit selection of genotype 1 patients who are likely to respond to a combination of PEGylated interferon and ribavirin.
Low initial viral load was identified as the most accurate predictor of SVR. The SVR rate in patients with low initial viral load was 85.7%.
The proposed algorithm could be used in the treatment-decision process in HCV-infected patients with end-stage kidney disease on maintenance hemodialysis.
SVR is defined as undetectable HCV RNA 24 wk after treatment completion, and indicates the eradication of HCV infection. Low initial viral load was defined as HCV RNA < 600000 IU/mL before treatment.
The paper provides interesting and original data in this difficult to treat population. The main results of the paper are relevant, even in the upcoming era of direct antiviral agents, which have not yet been validated in end-stage renal disease patients with HCV-related hepatitis and are still not available in some countries.
P- Reviewer: Pompili M S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Ma S
| 1. | Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, Thibault V, Cadranel JF, Bernard B, Opolon P. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 405] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Fabrizi F, Lunghi G, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther. 2006;24:1413-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Butt AA, Skanderson M, McGinnis KA, Ahuja T, Bryce CL, Barnato AE, Chang CC. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. J Viral Hepat. 2007;14:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Espinosa M, Martin-Malo A, Alvarez de Lara MA, Aljama P. Risk of death and liver cirrhosis in anti-HCV-positive long-term haemodialysis patients. Nephrol Dial Transplant. 2001;16:1669-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol. 2013;38:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Martin P, Fabrizi F. Hepatitis C virus and kidney disease. J Hepatol. 2008;49:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, Carreno M, Esquenazi V, Miller J. De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation. 1995;59:1676-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Ozdemir BH, Ozdemir FN, Sezer S, Colak T, Haberal M. De novo glomerulonephritis in renal allografts with hepatitis C virus infection. Transplant Proc. 2006;38:492-495. [PubMed] |
| 9. | Morales JM, Pascual-Capdevila J, Campistol JM, Fernandez-Zatarain G, Muñoz MA, Andres A, Praga M, Martinez MA, Usera G, Fuertes A. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63:1634-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Mahmoud IM, Sobh MA, El-Habashi AF, Sally ST, El-Baz M, El-Sawy E, Ghoneim MA. Interferon therapy in hemodialysis patients with chronic hepatitis C: study of tolerance, efficacy and post-transplantation course. Nephron Clin Pract. 2005;100:c133-c139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 12. | Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120-9.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 13. | McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 886] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 14. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 15. | Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon for hepatitis C virus in hemodialysis--an individual patient meta-analysis of factors associated with sustained virological response. Clin J Am Soc Nephrol. 2009;4:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Fabris C, Falleti E, Cussigh A, Bitetto D, Fontanini E, Bignulin S, Cmet S, Fornasiere E, Fumolo E, Fangazio S. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol. 2011;54:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Furusyo N, Hayashi J, Ariyama I, Sawayama Y, Etoh Y, Shigematsu M, Kashiwagi S. Maintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infection. Am J Gastroenterol. 2000;95:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Mizuno M, Higuchi T, Yanai M, Kanmatsuse K, Esumi M. Dialysis-membrane-dependent reduction and adsorption of circulating hepatitis C virus during hemodialysis. Nephron. 2002;91:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Fabrizi F, Bunnapradist S, Lunghi G, Martin P. Kinetics of hepatitis C virus load during hemodialysis: novel perspectives. J Nephrol. 2003;16:467-475. [PubMed] |
| 20. | Kaiser T, Damerow HC, Tenckhoff S, Finger A, Böttcher I, Hafer C, Schwarz A, Lüth JB, Schmidt Gürtler H, Colucci G. Kinetics of hepatitis C viral RNA and HCV-antigen during dialysis sessions: evidence for differential viral load reduction on dialysis. J Med Virol. 2008;80:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Badalamenti S, Catania A, Lunghi G, Covini G, Bredi E, Brancaccio D, Salvadori M, Como G, Ponticelli C, Graziani G. Changes in viremia and circulating interferon-alpha during hemodialysis in hepatitis C virus-positive patients: only coincidental phenomena? Am J Kidney Dis. 2003;42:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Barbosa KV, Teixeira R, Bassetti-Soares E, de Souza AF, Penido JM, Teixeira-Carvalho A, Martins-Filho OA. Phenotypic features of innate and adaptive immunity in patients with chronic hepatitis C and end-stage renal disease. Liver Int. 2013;33:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Vallet-Pichard A, Pol S. Hepatitis C virus infection in hemodialysis patients. Clin Res Hepatol Gastroenterol. 2013;37:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, Chen SI. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Tseng PL, Chen TC, Chien YS, Hung CH, Yen YH, Chang KC, Tsai MC, Lin MT, Lee CT, Shen CH. Efficacy and safety of pegylated interferon alfa-2b and ribavirin combination therapy versus pegylated interferon monotherapy in hemodialysis patients: a comparison of 2 sequentially treated cohorts. Am J Kidney Dis. 2013;62:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Wang KL, Xing HQ, Zhao H, Liu JW, Gao DL, Zhang XH, Yao HY, Yan L, Zhao J. Efficacy and tolerability of low-dose interferon-α in hemodialysis patients with chronic hepatitis C virus infection. World J Gastroenterol. 2014;20:4071-4075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |