Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5465
Peer-review started: November 10, 2014
First decision: December 11, 2014
Revised: December 29, 2014
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: May 14, 2015
Processing time: 192 Days and 5.2 Hours
AIM: To investigate the hepatoprotective effects of phycocyanobilin (PCB) in reducing hepatic injury and accelerating hepatocyte proliferation following carbon tetrachloride (CCl4) treatment.
METHODS: C57BL/6 mice were orally administered PCB 100 mg/kg for 4 d after CCl4 injection, and then the serum and liver tissue of the mice were collected at days 1, 2, 3, 5 and 7 after CCl4 treatment. A series of evaluations were performed to identify the curative effects on liver injury and recovery. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin and superoxide dismutase (SOD) were detected to indirectly assess the anti-inflammatory effects of PCB. Meanwhile, we detected the expressions of hepatocyte growth factor, transforming growth factor alpha (TGF-α), TGF-β, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), the factors which are associated with inflammation and liver regeneration. The protein expressions of proliferating cell nuclear antigen (PCNA), TNF-α and cytochrome C were detected by western blot. Furthermore, the survival rates were analyzed of mice which were administered a lethal dose of CCl4 (2.6 mg/kg) with or without PCB.
RESULTS: In our research, PCB showed a strongly anti-inflammatory effect on CCl4-induced liver injury in mice. The ALT was significantly decreased after CCl4 treatment from day 1 (P < 0.01) and the AST was significantly decreased from day 2 (P < 0.001). Both albumin and liver SOD were increased from day 2 (P < 0.001 and P < 0.01), but serum SOD levels did not show a significant increase (P > 0.05). PCB protected the structure of liver from the injury by CCl4. TUNEL assay showed that PCB dramatically reduced the number of apoptotic cells after CCl4 treatment compared to the control (101.0 ± 25.4 vs 25.7 ± 6.4, P < 0.01). The result of western blotting showed that PCB could increase PCNA expression, decrease TNF-α and cytochrome C expression. Furthermore, data shows that PCB could improve the survival rate of acute liver failure (ALF) mice which were injected with a lethal dose of CCl4 (60.0% vs 20.0%).
CONCLUSION: Our study indicated that PCB could be an ideal candidate for reversing acute liver injury or ALF.
Core tip: Our research confirmed that phycocyanobilin (PCB) plays a hepatoprotective role on carbon tetrachloride-induced acute liver injury mice. It was shown that PCB has a strongly anti-inflammatory effect when the liver suffered oxidative damage. The results showed that PCB could accelerate liver regeneration, reduce apoptosis and necrosis of the hepatocytes by regulating the expression of hepatocyte growth factor, transforming growth factor (TGF)-α, TGF-β, tumor necrosis factor-α and interleukin-6. In addition, PCB could significantly improve the survival probability of the acute liver injury mice.
- Citation: Liu J, Zhang QY, Yu LM, Liu B, Li MY, Zhu RZ. Phycocyanobilin accelerates liver regeneration and reduces mortality rate in carbon tetrachloride-induced liver injury mice. World J Gastroenterol 2015; 21(18): 5465-5472
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5465
The liver is an important organ which plays a central role in metabolism, glycolysis and scavenging free radicals in the body[1,2]. Due to these essential functions, liver injuries need to be rapidly repaired[3]. Toxic substances including alcohol, acetaminophen and carbon tetrachloride (CCl4) can induce liver injury, which is associated with a large amount of cell apoptosis and necrosis[4,5].
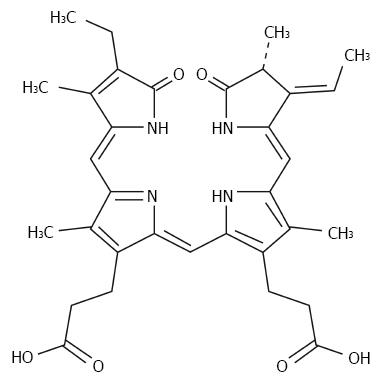
Although the pathogenesis of liver injury induced by chemical toxicity is not clear, reactive oxygen species (ROS) have been considered as a very important medium in liver pathological changes[6,7]. CCl4-induced acute liver injury is a well-known model, and CCl4 is transformed into trichloromethyl-free radical (CCl3• or CCl3OO•) by hepatic microsomal cytochrome P450[8,9]. Phycocyanobilin (PCB) [(2R,3E,4Z,10Z,15Z)-18-Ethyl-3-ethylidene-1,2,3,19,22,24-hexahydro-2,7,13,17- tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid; 3(E)-PCB; Figure 1] is a kind of chromophore extracted from the cyanobacterium Spirulina, which can be converted to phycocyanorubin by biliverdin reductase. Phycocyanorubin is a homolog of bilirubin, and is confirmed as a potent inhibitor of NADPH oxidase, which is the major intracellular oxidant stress producer[10,11]. We hypothesized that PCB could be a potential therapeutic for acute liver injury.
The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size (15-17 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Health of the People’s Republic of China. The protocol was approved by the Committee on the Ethics of Animal Experiments of Guangdong Medical College (Permit Number: SYXK 2008-0007). Male C57 BL/6 mice (8-wk-old, 25 ± 2 g in weight) were used in our experiment. The mice were purchased from Shanghai Slac Laboratory Animal Corporation, and kept in an SPF grade facility as specified by the National Animal Care and Use Committee. CCl4 and PCB were purchased from Sigma-Aldrich Biotechnology (St Louis, MO, United States). The kits for testing the level of serum ALT, AST, albumin and superoxide dismutase (SOD) were purchased from Jiancheng Biological Technology, Inc (Nanjing, China). Antibody against proliferating cell nuclear antigen (PCNA) of mouse and the SABC Staining Kit were from Boster Biological Technology (Wuhan, China). Antibodies of TNF-α, cytochrome C, PCNA and β-actin were obtained from Cell Signaling Technology (Beverly, MA). All other chemicals were of the highest grade commercially available.
Liver injury in mice was induced by intraperitoneal (i.p.) injection of CCl4 solution which was 1:3 diluted into corn oil, and the final concentration was 1 mL/kg body weight. A lethal dose was administered as described previously by injection of CCl4 solution which was 1:1 diluted into corn oil and the final concentration was 2.6 mL/kg body weight[12]. PCB was dissolved in sodium carboxymethylcellulose (CMC-Na) to a final concentration of 20 mg/mL, and intragastrically administered in mice at 100 mg/kg body weight 2 h after CCl4 treatment, once per day. Sixty liver injury mice were used in the experiment: 30 mice were treated with PCB and 30 mice were treated with CMC-Na only. At days 1, 2, 3, 5, 7 after CCl4 treatment, 6 mice were donated from the two groups respectively, and serum and liver tissue of each mouse was collected for the following tests. Another 60 mice which were injected with a lethal dose of CCl4 were used in this experiment; 30 of them were treated with PCB and the others treated with CMC-Na only, then the survival rates were recorded at intervals of 12 h for each group respectively.
Serum AST, ALT, albumin, SOD and liver SOD level were detected according to manufacturer’s instructions.
The paraffin-embedded liver sections were stained with hematoxylin-eosin to evaluate the degree of necrosis after liver injury by identifying the severity of necrotic lesions in the liver parenchyma.
Cell apoptosis rate was detected by the In Situ Cell Death Detection kit-POD (Roche, Basal, Switzerland) according to the manufacturer’s instructions. In brief, the process is as follows: Dewax and rehydrate tissue sections by using xylene and a graded series of ethanol, incubate tissue sections for 15 min at 37 °C, than incubate with 50 μL TUNEL reaction mixture in dark for 1 h (5 μL enzyme solution added into 45 μL label solution per sample) at 37 °C. After this step, sections were incubated with 50 μL converter-POD per sample for 30 min, hematoxylin was used to stain the nucleus, then the stained cells were analyzed under light microscope.
The total RNA was isolated by Trizol, then reverse transcripted into cDNA by the use of Primescript RT reagent Kit (Takara Biotechnology, Dalian, Liaoning, China), and the mRNA expression levels were detected by SYBR Premix Ex Taq II (Tli RNase H Plus) Kit (Takara Biotechnology, Dalian, Liaoning, China).
Liver tissues were homogenized in RIPA lysis buffer (Beyotime, Jiangsu, China); the concentration of each lysate was detected by Enhanced BCA Protein Assay kit (Beyotime, Jiangsu, China). Proteins were electrophoresed on a SDS-PAGE gel, and then transferred to PVDF membranes (Millipore, Bedford, United States). Membranes were incubated with primary antibodies at 4 °C overnight, then incubated with a second antibody for 1 h; proteins were exposed by Amersham ECL Select Western blotting detection reagent (GE Healthcare, Buckinghamshire, United Kingdom).
The data were obtained from at least six independent experiments and all results are presented as the mean ± SE. The differences between the groups were assessed using Student’s t-test. Comparisons were relative to untreated controls. The survival results were analyzed by log-rank test and presented as Kaplan-Meier survival curves. P < 0.05, P < 0.01 were considered to indicate a statistically significant difference.
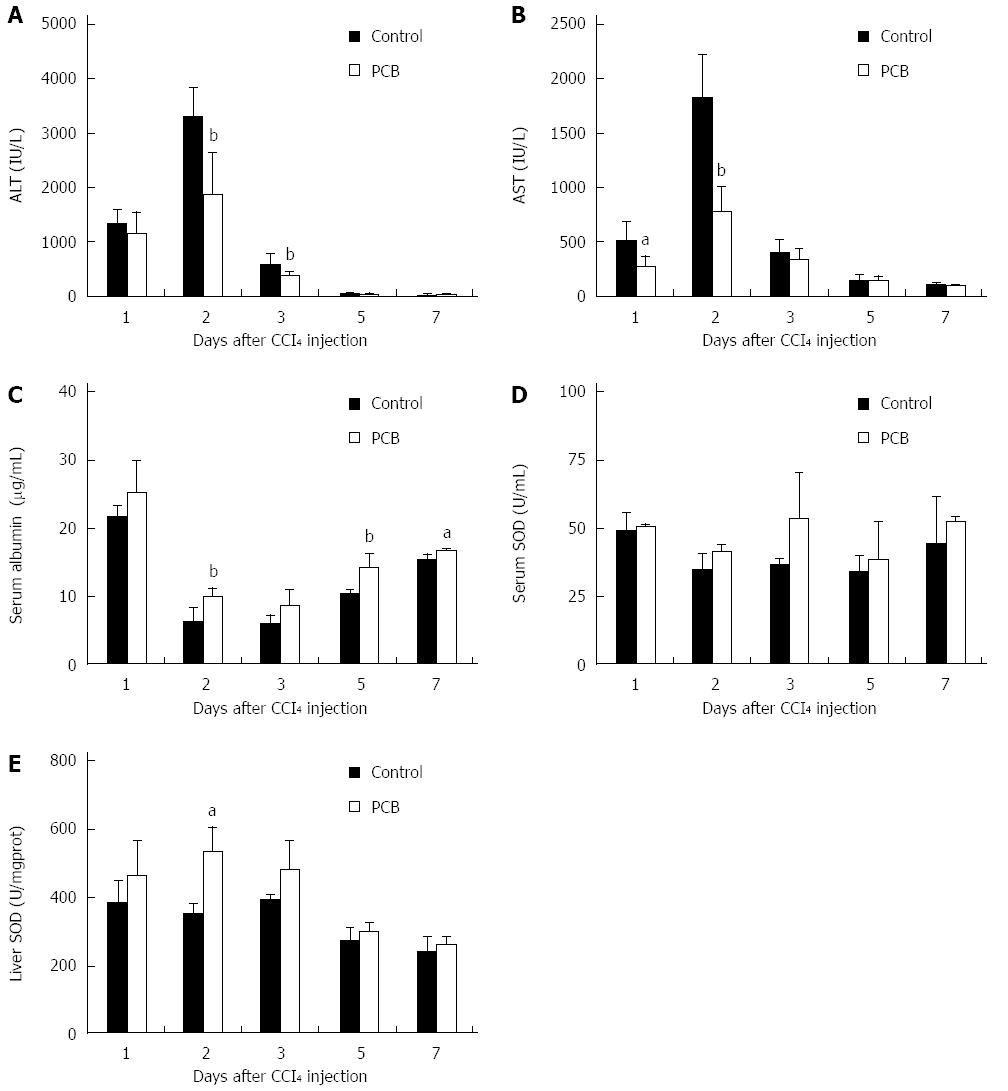
ALT and AST are considered as important indicators of liver function[13,14]. When liver is damaged, the levels of serum ALT and AST increase rapidly. A few days later, following the damage repair, serum ALT and AST fall back to normal levels. In this study, the data showed that serum ALT and AST levels were rapidly elevated to a peak level at day 2, and declined thereafter, while PCB could significantly down-regulate these elevations (Figure 2A and B).
Serum albumin is also considered as an indicator in liver injury[15]. Albumin decreases rapidly from an early phase during liver injury. Increasing serum albumin shows liver functional recovery. In this study, serum albumin was decreased sharply at day 2 after CCl4 treatment, and PCB could significantly improve the level in serum (Figure 2C).
SOD is a member of the active oxygen scavenging enzyme system, and is regarded as a marker to monitor the anti-oxidative ability of liver[16,17]. In this study, serum and liver SOD were detected; the results showed that PCB could significantly increase the level of SOD both in serum and liver (Figure 2D and E).
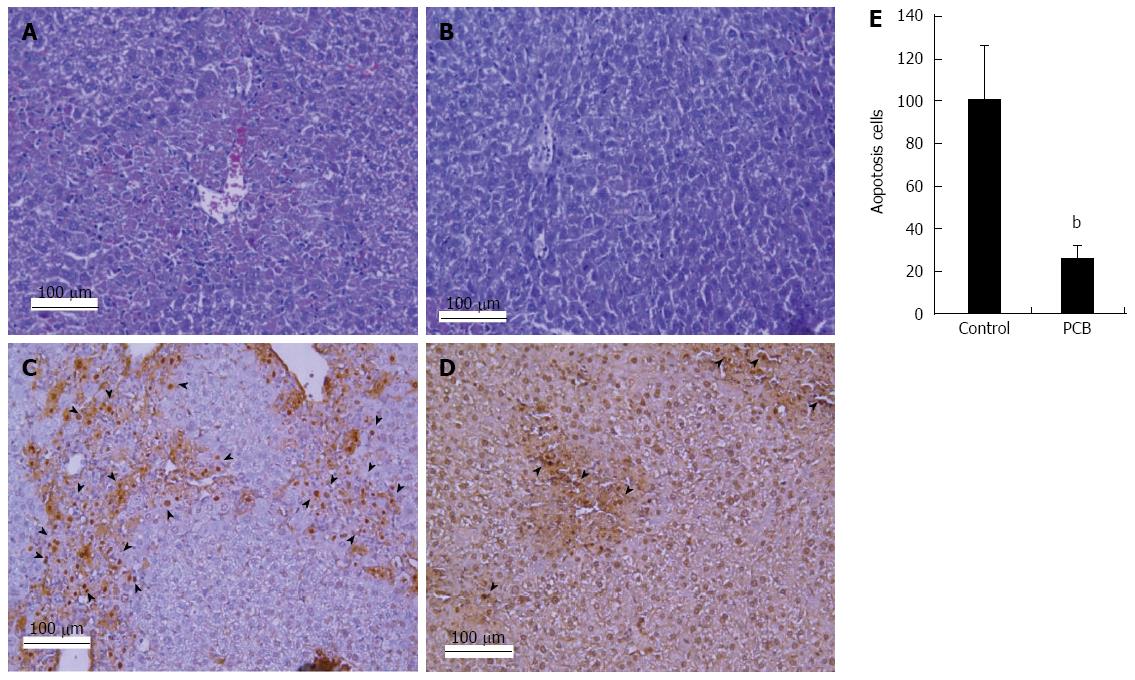
To confirm the role of PCB in the protection from hepatocellular injury, sections of liver tissue were stained by HE and TUNEL to observe the degree of necrosis and apoptosis. The results demonstrated that there was moderate necrosis around the centrilobular areas in PCB administered mice. On the contrary, a larger area of necrosis around the central vein was detected in the control (Figure 3A and B). The results of the TUNEL assay showed that PCB significantly decreased the number of apoptosis cells in the section compared with the control (Figure 3C-E).
To evaluate the molecular mechanism of PCB’s hepatoprotection, important cytokines related to liver regeneration, such as hepatocyte growth factor (HGF), transforming growth factor alpha (TGF-α), transforming growth factor beta (TGF-β), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were detected[18]. In this study, the results of real-time quantitative PCR demonstrated that PCB could significantly increase the expression of HGF and TGF-β from an early time after CCl4 treatment (Figure 4A and C), meanwhile PCB could decrease TGF-α, TNF-α and IL-6 expressions (Figure 4B, D and E). It is very interesting that the expression of TNF-β in the PCB group increased more dramatically compared to the control (Figure 4D). In this study, proteins of PCNA, TNF-α, and cytochrome C in liver tissue from days 1-3 and day 5 were detected by western blotting assay. The results indicated that PCNA expressions in the PCB group were higher than the control at days 1, 2, 3. TNF-α protein expression in the PCB group was up-regulated at day 1 compared to the control, but then significantly dropped down after day 2. Cytochrome C was lower in the PCB group compared to the control at day 3 and day 5 (Figure 4F).
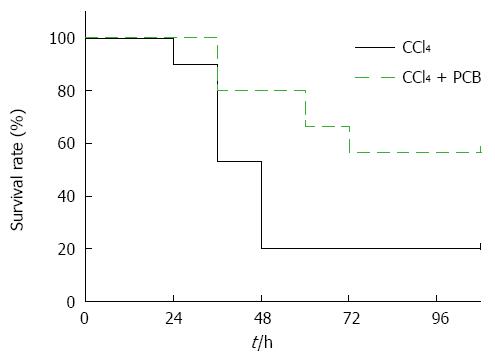
Mice were administered a lethal dose of CCl4 with or without PCB; data indicate that PCB improved the survival rate of mice dramatically after CCl4 injection over 108 h (Figure 5).
PCB is found in blue-green algae and cyanobacteria such as Spirulina, and it has been indicated that PCB could protect DNA from oxidative damage by scavenging of intracellular peroxynitrite (ONOO-)[19]. Previous studies confirmed that PCB is a potent inhibitor of NADPH oxidase activity in mammals, because it can be converted into phycocyanorubin (a homolog of bilirubin) by biliverdin reductase[20]. CCl4-induced acute liver injury has been used as an ideal model for the research of human liver diseases[12,21-23]. Previous study confirmed that the pathological lesion of CCl4-induced damage is restricted to the liver[12]. Serum ALT and AST were utilized in this study as indicators of liver damage; when mice were administered with 1 mg/kg CCl4, serum ALT and AST levels elevated rapidly, then declined from day 2. However, in the mice which were treated with PCB after CCl4 intraperitoneal injection, the elevation of serum ALT and AST was slower than in the control. Furthermore, serum albumin was improved significantly by PCB, which demonstrated that PCB could promote the recovery of liver function. SOD was detected to evaluate the antioxidant capacity of the liver. The results confirmed that PCB could markedly enhance the activity of SOD both in serum and in liver, which implied it is an effective antioxidant.
The results of HE staining showed less inflammation and necrosis in the sections of the PCB treatment group, which indicated that PCB could significantly suppress inflammation and necrosis of liver structure. TUNEL assay results demonstrated that PCB could reduce apoptosis in hepatocytes, and further experiments proved that the molecular mechanism by which PCB decreased the number of apoptotic cells may be related to the reduced release of cytochrome c. The results of PCNA detection showed that PCB could accelerate hepatocyte proliferation.
Cytokines play important roles in liver regeneration, such as HGF, TGF-α and TNF-α[24-26]. HGF is one of the most important cytokines in the repair of tissue injury; it could rapidly be elevated by 10 to 20-fold at the early stage of liver injury[24]. TGF-α is a direct mitogen which induces a strong mitogenic response in hepatocytes[27]. In our study, compared with the control, PCB significantly up-regulated the expression of HGF and TGF-α at the early stage of liver damage (days 1-2), but these cytokines rapidly declined a few days later (day 5), which implied that PCB could accelerate liver regeneration from the early stage and terminate the process ahead of time. TNF-α is a multifunctional factor implicated in both starting liver regeneration and acting as a pro-inflammatory mediator in hepatocyte apoptosis[28,29]. The expression of TNF-α rapidly elevated to a 3-fold higher level in the PCB group compared to the control at day 1 after CCl4 treatment, and then declined significantly after day 2. IL-6 has been considered to be a biomarker that reflects inflammatory activity, and TGF-β is a key factor to induce epithelial to mesenchymal transition and inhibit hepatocyte proliferation[30]. PCB could down-regulate the expression of IL-6 and TGF-β during the liver regeneration process through reducing inflammatory reactions and accelerating hepatocyte proliferation.
Survival rate is always considered to be strong evidence to evaluate the hepatoprotective potential in acute liver failure (ALF). In this study, ALF was induced by intraperitoneal injection of 2.6 mg/kg CCl4, and 100 mg/kg PCB was orally administrated once per day. The survival rate of the control group rapidly declined to 20% at 48 h after CCl4 treatment, but the survival rate of the PCB group was more than 60%. The results positively confirmed that PCB could protect mice against ALF.
In conclusion, we demonstrated that PCB confers a strong protective effect on acute liver injury. This study suggests to us that PCB is a novel therapeutic candidate for acute liver injury and ALF.
Liver is an important organ which plays a central role in metabolism, glycolysis and scavenging free radicals in the body. Due to these essential functions, liver injuries need to be rapidly repaired.
The authors’ team has demonstrated that phycocyanobilin (PCB) has a dramatic antioxidant and hepatoprotective effect. However, whether PCB protects liver from acute liver injury and accelerates liver regeneration is not yet known.
This is the first study to demonstrate that PCB can accelerate liver regeneration and protect the liver from causing death from acute liver failure.
PCB may serve as a potential effective candidate agent for the therapy of chemical liver injury and acute liver failure.
In this study, the authors found that PCB reduces the necrosis and apoptosis of hepatocytes, accelerates liver regeneration and protects mice from death from carbon tetrachloride-induced acute liver failure. It is very interesting research work.
P- Reviewer: Zocco MA S- Editor: Qi Y L- Editor: Logan S E- Editor: Ma S
| 1. | Chen XG, Xu CS. Proteomic analysis of the regenerating liver following 2/3 partial hepatectomy in rats. Biol Res. 2014;47:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1156] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 3. | Ganaie MA, Khan TH, Siddiqui NA, Ansari MN. Ameliorative effect of methanol extract of Rumex vesicarius on CCl4-induced liver damage in Wistar albino rats. Pharm Biol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Yeh YH, Hsieh YL, Lee YT, Hu CC. Protective effects of Geloina eros extract against carbon tetrachloride-induced hepatotoxicity in rats. Food Res Int. 2012;48:551-558. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Alkreathy HM, Khan RA, Khan MR, Sahreen S. CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern Med. 2014;14:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 347] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Mahmud Z, Bachar S, Qais N. Antioxidant and Hepatoprotective Activities of Ethanolic Extracts of Leaves of Premna esculenta Roxb. against Carbon Tetrachloride-Induced Liver Damage in Rats. J Young Pharm. 2012;4:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Alavian SM, Banihabib N, Es Haghi M, Panahi F. Protective Effect of Cornus mas Fruits Extract on Serum Biomarkers in CCl4-Induced Hepatotoxicity in Male Rats. Hepat Mon. 2014;14:e10330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | AlSaid M, Mothana R, Raish M, Al-Sohaibani M, Al-Yahya M, Ahmad A, Al-Dosari M, Rafatullah S. Evaluation of the effectiveness of Piper cubeba extract in the amelioration of CCl4-induced liver injuries and oxidative damage in the rodent model. Biomed Res Int. 2015;2015:359358. [PubMed] |
| 10. | Terry MJ, Maines MD, Lagarias JC. Inactivation of phytochrome- and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J Biol Chem. 1993;268:26099-26106. [PubMed] |
| 11. | McCarty MF. Clinical potential of Spirulina as a source of phycocyanobilin. J Med Food. 2007;10:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 12. | Zhu R, Zeng G, Chen Y, Zhang Q, Liu B, Liu J, Chen H, Li M. Oroxylin A accelerates liver regeneration in CCl4-induced acute liver injury mice. PLoS One. 2013;8:e71612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 13. | Liu G, Liu X, Zhang Y, Zhang F, Wei T, Yang M, Wang K, Wang Y, Liu N, Cheng H. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int J Biol Macromol. 2015;76:169-175. [PubMed] |
| 14. | Niu C, Sheng Y, Yang R, Lu B, Bai Q, Ji L, Wang Z. Scutellarin protects against the liver injury induced by diosbulbin B in mice and its mechanism. J Ethnopharmacol. 2015;164:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Ware BR, Berger DR, Khetani SR. Prediction of Drug-Induced Liver Injury in Micropatterned Co-cultures Containing iPSC-Derived Human Hepatocytes. Toxicol Sci. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Sila A, Kamoun Z, Ghlissi Z, Makni M, Nasri M, Sahnoun Z, Nedjar-Arroume N, Bougatef A. Ability of natural astaxanthin from shrimp by-products to attenuate liver oxidative stress in diabetic rats. Pharmacol Rep. 2015;67:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Li G, Wang XY, Suo YR, Wang HL. Protective effect of seed oil of Herpetospermum pedunculosum against carbon tetrachloride-induced liver injury in rats. Saudi Med J. 2014;35:981-987. [PubMed] |
| 18. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 19. | Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001;285:262-266. [PubMed] |
| 20. | McCarty MF, Barroso-Aranda J, Contreras F. Genistein and phycocyanobilin may prevent hepatic fibrosis by suppressing proliferation and activation of hepatic stellate cells. Med Hypotheses. 2009;72:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tanioka N, Shimizu H, Takahashi T, Omori E, Kuroda K, Shibata M, Yamaoka M, Toda Y, Matsusaki T, Morimatsu H. Induction of hepatic Bach1 mRNA expression by carbon tetrachloride-induced acute liver injury in rats. Biomed Rep. 2014;2:359-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Jaswal A, Shukla S. Therapeutic efficacy of Nigella sativa Linn. seed extract against CCl4 induced hepatic injury in Wistar rats. Indian J Exp Biol. 2015;53:44-50. [PubMed] |
| 23. | Vetvicka V, Garcia-Mina JM, Proctor M, Yvin JC. Humic Acid and Glucan: Protection Against Liver Injury Induced by Carbon Tetrachloride. J Med Food. 2015;Epub ahead of print. [PubMed] |
| 24. | Pediaditakis P, Lopez-Talavera JC, Petersen B, Monga SP, Michalopoulos GK. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34:688-693. [PubMed] |
| 25. | Zhang J, Zhou S, Zhou Y, Feng F, Wang Q, Zhu X, Ai H, Huang X, Zhang X. Hepatocyte growth factor gene-modified adipose-derived mesenchymal stem cells ameliorate radiation induced liver damage in a rat model. PLoS One. 2014;9:e114670. [PubMed] |
| 26. | Cienfuegos JA, Rotellar F, Baixauli J, Martínez-Regueira F, Pardo F, Hernández-Lizoáin JL. Liver regeneration--the best kept secret. A model of tissue injury response. Rev Esp Enferm Dig. 2014;106:171-194. [PubMed] |
| 27. | Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133-1149. [PubMed] |
| 28. | An J, Harms C, Lättig-Tünnemann G, Sellge G, Mandić AD, Malato Y, Heuser A, Endres M, Trautwein C, Donath S. TAT-apoptosis repressor with caspase recruitment domain protein transduction rescues mice from fulminant liver failure. Hepatology. 2012;56:715-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Wollborn J, Wunder C, Stix J, Neuhaus W, Bruno RR, Baar W, Flemming S, Roewer N, Schlegel N, Schick MA. Phosphodiesterase-4 inhibition with rolipram attenuates hepatocellular injury in hyperinflammation in vivo and in vitro without influencing inflammation and HO-1 expression. J Pharmacol Pharmacother. 2015;6:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Xue ZF, Wu XM, Liu M. Hepatic regeneration and the epithelial to mesenchymal transition. World J Gastroenterol. 2013;19:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |