Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5336
Peer-review started: October 15, 2014
First decision: December 11, 2014
Revised: December 30, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: May 7, 2015
Processing time: 210 Days and 18.2 Hours
AIM: To investigate the expression and prognostic role of pyruvate dehydrogenase (PDH) in gastric cancer (GC).
METHODS: This study included 265 patients (194 male, 71 female, mean age 59 years (range, 29-81 years) with GC who underwent curative surgery at the First Affiliated Hospital of China Medical University from January 2006 to May 2007. All patients were followed up for more than 5 years. Patient-derived paraffin embedded GC specimens were collected for tissue microarrays (TMAs). We examined PDH expression by immunohistochemistry in TMAs containing tumor tissue and matched non-neoplastic mucosa. Immunoreactivity was evaluated independently by two researchers. Overall survival (OS) rates were determined using the Kaplan-Meier estimator. Correlations with other clinicopathologic factors were evaluated by two-tailed χ2 tests or a two-tailed t-test. The Cox proportional-hazard model was used in univariate analysis and multivariate analysis to identify factors significantly correlated with prognosis.
RESULTS: Immunohistochemistry showed that 35.47% of total cancer tissue specimens had cytoplasmic PDH staining. PDH expression was much higher in normal mucosa specimens (75.09%; P = 0.001). PDH expression was correlated with Lauren grade (70.77% in intestinal type vs 40.0% in diffuse type; P = 0.001), lymph node metastasis (65.43% with no metastasis vs 51.09% with metastasis; P = 0.033), lymphatic invasion (61.62% with no invasion vs 38.81% with invasion; P = 0.002), histologic subtypes (70.77% in intestinal type vs 40.0% in diffuse type; P = 0.001) and tumor-node-metastasis (TNM) stage (39% in poorly differentiated vs 65.91% in well differentiated and 67.11% in moderately differentiated; P = 0.001) in GC. PDH expression in cancer tissue was significantly associated with higher OS (P < 0.001). The multivariate analysis adjusted for age, Lauren classification, TNM stage, lymph node metastasis, histological type, tumor size, depth of invasion and lymphatic invasion showed that the PDH expression in GC was an independent prognostic factor for higher OS (HR = 0.608, 95%CI: 0.504-0.734, P < 0.001).
CONCLUSION: Our study indicated that PDH expression is an independent prognostic factor in GC patients and that positive expression of PDH may be predictive of favorable outcomes.
Core tip: This is the first reported study to evaluate the prognostic role of pyruvate dehydrogenase (PDH) expression in gastric cancer (GC). This study showed that reduced PDH expression was correlated with Lauren grade, lymph node metastasis, lymphatic invasion, histologic subtype and TNM stage in GC. We propose that increased PDH expression may contribute to a decrease in the proliferation and development of GC. In particular, PDH protein expression was found to positively correlate with survival in GC patients, and a high level of PDH expression was found to be associated with better overall survival in patients with resected GC. In conclusion, this study showed that PDH expression is an independent prognostic factor in gastric carcinomas.
- Citation: Sun XR, Sun Z, Zhu Z, Guan HX, Li CY, Zhang JY, Zhang YN, Zhou H, Zhang HJ, Xu HM, Sun MJ. Expression of pyruvate dehydrogenase is an independent prognostic marker in gastric cancer. World J Gastroenterol 2015; 21(17): 5336-5344
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5336
Although the incidence of gastric cancer (GC) is declining, it is still ranked as the fourth most common cancer and the second leading cause of cancer-related mortality worldwide[1,2]. Despite advances in early diagnosis and therapy, metastases are a common cause of death[3,4]. Among the prognostic factors for GC that are now available, the most important is the tumor-node-metastasis (TNM) stage. However, the prognosis varies among patients of the same stage[5]. Therefore, novel molecular markers need to be defined to better identify different subsets of the disease and assist in the implementation of individualized therapeutic regimens.
The Warburg effect, also known as aerobic glycolysis, is one of the characteristics of tumor cells in which a high rate of glycolysis occurs, even in the presence of adequate oxygen[6-8]. The surprisingly high rate of glucose uptake and lactate production in tumors in the presence of oxygen led Warburg to speculate that an aberrant metabolism could be the cause of many cancers[9]. It has been a belief that the glycolytic phenotype of cancer cells is attributable to defects in mitochondrial oxidative phosphorylation (OXPHOS). However, recent results have revealed that most tumor cells have a substantial reserve capacity to produce adenosine triphosphate by OXPHOS when glycolysis is suppressed, showing that the high rate of glycolysis exhibited by most tumors is required to support cell growth rather than to compensate for defects in mitochondrial function[10]. Because reactive oxygen species are natural by-products of mitochondrial respiration, it has been proposed that the conversion of glucose to lactate may protect cancer cells from oxidative stress[11]. Therefore, taking advantage of the glycolytic characteristics of cancer cells and the activating function of mitochondrial OXPHOS may serve as a significant strategy for cancer therapy.
Pyruvate dehydrogenase (PDH) is a mitochondrial enzyme that plays a central role in aerobic energy metabolism by catalyzing the irreversible oxidation of glucose-derived pyruvate to acetyl-CoA. Acetyl-CoA then enters the tricarboxylic acid cycle, where it reacts with oxaloacetate to form citrate. In cancerous cells the inhibition of PDH activity via the over-expression of pyruvate dehydrogenase kinase (PDK) leads to the energetic switch from mitochondrial glucose oxidation to cytoplasmic glycolysis[12]. Therefore, PDH serves as a gate-keeper enzyme link between glycolysis and the mitochondrial citric acid cycle[13,14]. Several studies have found that the activation of PDH shifts cancer cell metabolism from glycolysis to glucose oxidation and thus decreases the mitochondrial membrane potential and lactate production, augments reactive oxygen species, and is associated with the induction of apoptosis and reduction in tumor cell proliferation without any harmful effects in normal cells[12-17]. Recent research showed that the normalization of glucose metabolism by stimulating PDH in cancer cells restored their susceptibility to anoikis and impaired their metastatic potential[18]. However, the expression status in GC, the relation of PDH expression with progression, and the prognosis of patients remains unknown. In this study, we first examined the expression of PDH in GC and then correlated its expression with clinical pathological parameters and overall survival (OS). Our results demonstrate that the loss of PDH expression is a marker of tumor aggressiveness and that a high expression of PDH in GC may be predictive of favorable outcomes.
The present study included 265 patients with GC who received curative surgery from January 2006 to May 2007 at the First Affiliated Hospital of China Medical University. There were 194 males and 71 females, with a mean age of 59 years (range, 29-81 years). None of the patients underwent chemotherapy or radiotherapy before surgery. Follow-up information was collected from all patients. The Institutional Review Board at the First Affiliated Hospital of China Medical University approved this study.
Ethical approval for this research was obtained from the Research Ethics Committee of China Medical University, China. All patients providing tumor tissue as well as normal gastric tissue samples signed a consent form prior to surgical removal of the gastric carcinoma to allow this research to be undertaken.
All patient-derived formalin-fixed and paraffin-embedded GC specimens and matched non-neoplastic mucosa (NNM) specimens (from at least 2 cm away from the carcinoma) were collected during surgical resection and archived under protocols that were approved by the Institutional Review Board of the University. The histologic diagnosis and other microscopic characteristics were confirmed by pathologists, and the TNM stage of each gastric carcinoma was evaluated according to the Union for International Cancer Control system for the extent of tumor spread[19]. The histologic architecture of the gastric carcinoma was expressed using Lauren’s classification[20,21] and the World Health Organization (WHO) classification[22]. Tumor size, depth of invasion, and lymphatic invasion were also determined.
Representative areas of solid tumors and adjacent NNM were identified in hematoxylin and eosin (HE)-stained sections of the selected cases. A 1.5 mm diameter tissue core per donor block was punched out using a 1.5 mm diameter punch and then transferred to a recipient block with a maximum of 200 cores. The sections (4 μm thick) were consecutively cut from each tissue microarray block, and HE staining was performed on the tissue microarrays (TMAs) to confirm tumor and NNM tissue. Immunohistochemical analysis was performed on the TMA sections, and pressure cooker-mediated antigen retrieval was performed in citrate buffer (pH 6.0) for 10 min. The sections were incubated with a 1:100 dilution of pyruvate dehydrogenase (C54G1) in Rabbit mAb (Cell Signaling) overnight at 4 °C, and then incubated with goat anti-mouse or anti-rabbit Envision System Plus-HRP (Dako Cytomation) for 30 min at room temperature. After rinsing three times in PBS for 10 min each, the sections were incubated with DAB for 1 min, counterstained with Mayer’s hematoxylin, dehydrated, rinsed and mounted. The same protocol with the omission of the primary antibody was used as a negative control.
The patients underwent close clinical observation, including chest/abdominal/pelvic computed tomographic imaging, measurement of carcinoembryonic antigen level, blood testing at 2- to 3-mo intervals, and a yearly gastroscopy. Follow-up was in accordance with National Comprehensive Cancer Network Practice Guidelines for Gastric Cancer. OS rate was defined as the interval from the initial surgery to death. The end date of follow-up for conducting the analysis was June 29, 2012.
Immunoreactivity was evaluated independently by two researchers who were blinded to the patient outcomes. The evaluation was based on the staining intensity for PDH and was scored as 0 (-) negative; 1 (+) weak; 2 (++) moderate; or 3 (+++) strong. If heterogeneity was detected by cell immunohistochemical staining, the proportion of positive cells was considered as follows: 0, negative; 1, positive in ≤ 10% of cells; 2, positive > 10% and ≤ 50%; 3, positive > 50% and ≤ 80%; and 4, positive in > 80% of cells. The two scores were then multiplied, and the expression was graded as follows: negative, score = 0 (-); weak expression, score = 1-4 (+); moderate expression, score = 5-8 (++); or strong expression, score = 9-12 (+++). These specimens were divided into two groups according to their scores: 0-4 was the negative group, and 5-12 was the positive group. In the event of a discrepancy in scoring, both pathologists re-examined the slides under a microscope.
All statistical analyses were performed using SPSS 17.0 software (SPSS Inc, Chicago, IL, United States). OS rates were determined using Kaplan-Meier curves, and an event was defined as death from cancer-related causes. The log-rank test was used to identify the differences between survival curves. In univariate analysis, a two-tailed χ2 test or a two-tailed t-test was used for statistical comparisons. The Cox proportional-hazard model was used in univariate analysis and multivariate analysis to identify the significant factors that were correlated with prognosis. For all analyses, P values < 0.05 were considered significant.
The statistical methods of this study were reviewed by Wu Wei from the Statistics Teaching and Research section of China Medical University.
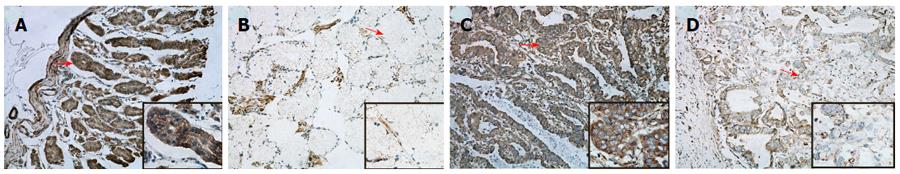
We graded stained sections of the TMAs of GC and NNM tissue cores according to their cytoplasmic immunohistochemical staining intensity against PDH protein, and the percentage of positive cells was determined when HE staining had heterogeneity. The readable samples included 265 GC and matched NNM samples. The typical diffuse cytoplasmic staining of the protein can be found in many GC and NNM tissues, as shown in Figure 1.
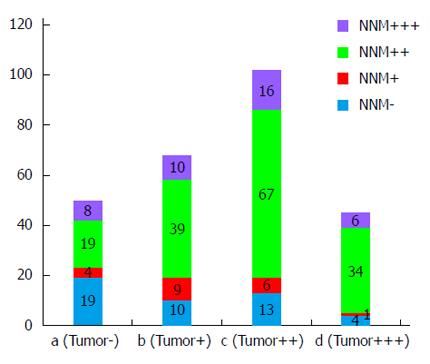
Of the 265 GC tissue specimens, 147 (55.47%) showed positive cytoplasmic PDH staining (102 moderate positive and 45 strong positive), and 118 (44.53%) displayed negative staining (68 weak staining and 50 negative staining). In contrast, PDH expression was much higher in NNM specimens with positive staining in 199 (75.09%) specimens (40 strong positive and 159 moderate positive) and negative staining in 66 (24.91%) specimens (20 weak staining and 46 negative staining). A comparative analysis of the immunohistochemistry results of the TMAs indicated that PDH was differentially downregulated in the GC specimens compared with the NNM tissues (Table 1; P = 0001). Figure 2 shows the PDH expression level in GC and NNM tissues from the same patient. The results showed that 123 patients had PDH co-expression (GC+/NNM+), 24 patients had GC+/NNM- single expression, 76 patients had GC-/NNM+ single expression, and 42 patients had double-PDH negative expression.
| Tissue sample | n | PDH | P value1 | ||||||
| - | +- | Total | + | ++ | Total | PR (%) | χ2test | ||
| Adjacent normal mucosa | 265 | 46 | 20 | 66 | 159 | 40 | 199 | 75.09 | 0.001 |
| Primary cancer tissue | 265 | 50 | 68 | 118 | 102 | 45 | 147 | 55.47 | |
As summarized in Table 2, we found that decreased expression of PDH was significantly associated with GC Lauren grade, lymph node metastasis, lymphatic invasion, and TNM stage (P < 0.05), but not with age, sex, tumor size or depth of invasion (P > 0.05). Among the WHO histologic subtypes, the poorly differentiated subtype displayed a lower PDH expression than did the well differentiated, moderately differentiated and mucinous carcinoma subtypes (P < 0.05; Table 3). In addition, PDH expression was significantly inversely correlated with TNM stage and the depth of invasion of differentiated carcinomas (P < 0.05; data not shown) and was significantly inversely correlated with the depth of invasion, Lauren grade and TNM stage of undifferentiated carcinomas (P < 0.05; data not shown).
| Clinicopathologic variable | n | PDH expression | |||
| - | + | PR (%) | P value1 | ||
| 265 | 118 | 147 | 55.47 | ||
| Sex | 0.577 | ||||
| Female | 71 | 34 | 37 | 52.11 | |
| Male | 194 | 84 | 110 | 56.7 | |
| Age (yr) | 0.902 | ||||
| < 60 | 133 | 60 | 73 | 54.89 | |
| ≥ 60 | 132 | 58 | 74 | 56.06 | |
| Tumor size (cm) | 0.347 | ||||
| < 4 | 80 | 32 | 48 | 60.00 | |
| ≥ 4 | 185 | 87 | 98 | 52.97 | |
| Depth of invasion | 0.093 | ||||
| Tis-1 | 25 | 7 | 18 | 72.00 | |
| T2-4 | 240 | 111 | 129 | 53.75 | |
| Lauren grade | 0.001 | ||||
| Intestinal type | 130 | 38 | 92 | 70.77 | |
| Diffuse type | 135 | 81 | 54 | 40.00 | |
| TNM Stage | 0.010 | ||||
| 0-I | 38 | 8 | 30 | 78.95 | |
| II-IV | 227 | 110 | 117 | 51.54 | |
| Lymph node metastases | 0.033 | ||||
| Negative | 81 | 28 | 53 | 65.43 | |
| Positive | 184 | 90 | 94 | 51.09 | |
| Lymphatic invasion | 0.002 | ||||
| Absent | 198 | 76 | 122 | 61.62 | |
| Present | 67 | 41 | 26 | 38.81 | |
| Tissue Sample | n | PDH | PR (%) | |||
| - | +- | + | ++ | |||
| Well-differentiated | 44 | 6 | 9 | 12 | 17 | 65.91 |
| Moderately differentiated | 76 | 9 | 16 | 36 | 15 | 67.11 |
| Poorly differentiated | 100 | 30 | 31 | 31 | 8 | 391 |
| Mucinous | 30 | 2 | 6 | 17 | 5 | 73.33 |
| Signet ring cell | 15 | 3 | 6 | 6 | 0 | 40 |
| Total | 265 | 50 | 68 | 102 | 45 | 55.50 |
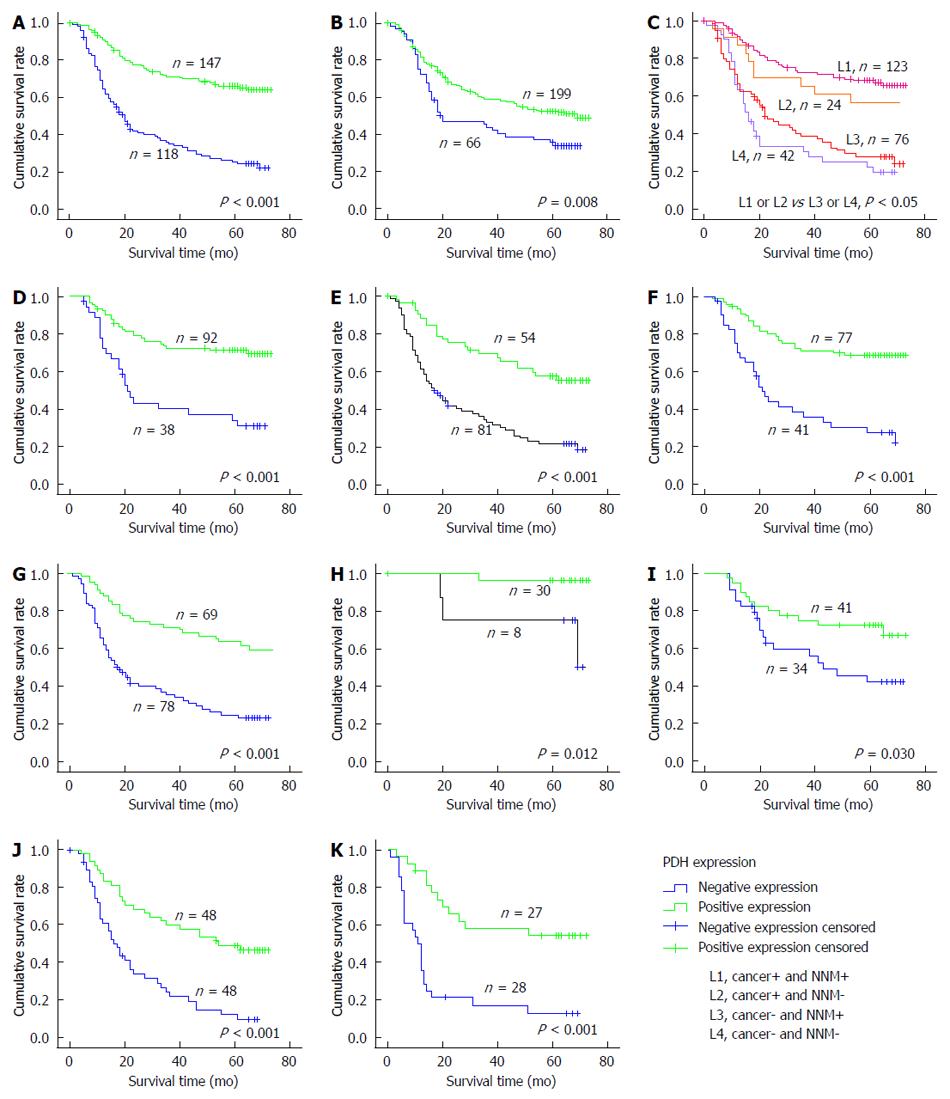
The 5-year OS rate of the 265 patients with primary GC was 46% (122/265), with 143 deaths observed during the follow-up period. The median duration of follow-up was 50 mo (range, 9-78 mo). Kaplan-Meier survival curves and the log-rank test demonstrated that patients with positive expression of PDH in GC tissue had better OS than did patients with negative PDH expression in the tumor (P < 0.001; Figure 3A). The 5-year survival rate of patients with positive expression was significantly higher than that of patients with negative expression (65.8% vs 28.0%, respectively). In addition, the expression of PDH in NNM had a predictive prognostic role (P = 0.008; Figure 3B). We combined the expression of PDH in GC and in NNM and then divided the patients into a co-expression group (GC+/NNM+), two single-expression groups (GC+/NNM- and GC-/NNM+), and a double-PDH negative group. The co-expression group had a significantly longer survival time than did the double-PDH negative group (P < 0.001; Figure 3C). Because GC can be classified into different subtypes, we also analyzed the prognostic value of PDH in different subtypes stratified by Lauren grade (intestinal-type and diffuse-type), histological classification (differentiated type and undifferentiated type), and TNM stage (I-IV). The results showed that stronger PDH staining was significantly associated with better OS in each subtype of GC (P < 0.05; Figure 3D-K).
Multivariate analysis was performed using the Cox proportional hazards model for all of the significant variables in the univariate analysis. Table 4 shows the analysis, adjusted for age, Lauren classification, TNM stage, lymph node metastasis, histological type, tumor size, depth of invasion and lymphatic invasion covariates, and that PDH expression in GC was an independent prognostic factor for higher OS (HR = 0.608, 95%CI 0.504-0.734, P < 0.001).
| Variable | Univariate analysis | Multivariate analysis | ||
| RR (95%CI) | P value | RR (95%CI) | P value | |
| PDH expression in tumor (positive vs negative) | 0.552 (0.462-0.658) | < 0.0011 | 0.608 (0.504-0.734) | < 0.0011 |
| PDH expression in NNM (positive vs negative) | 0.830 (0.699-0.987) | 0.035 | 0.869 (0.727-1.039) | 0.123 |
| Age (≥ 60 yr vs < 60 yr) | 1.446 (1.030-2.032) | 0.033 | 1.447 (1.010-2.073) | 0.044 |
| Tumor size (< 4 cm vs≥ 4 cm) | 2.297 (1.505-3.504) | < 0.0011 | 1.492 (0.956-2.329) | 0.078 |
| Depth of invasion (Tis-1 vs T2-4) | 5.114 (1.889-13.843) | 0.0011 | 1.683 (0.567-4.995) | 0.348 |
| Lauren grade (intestinal-type vs diffuse-type) | 1.978 (1.396-2.803) | < 0.0011 | 1.574 (1.081-2.292) | 0.0181 |
| TNM Stage (0-I vs II-IV) | 1.809 (1.504-2.176) | < 0.0011 | 1.512 (1.191-1.920) | 0.0011 |
| Lymph node metastasis (positive vs negative) | 3.146 (1.989-4.977) | < 0.0011 | 1.733 (1.061-2.830) | 0.0281 |
| Lymphatic invasion (positive vs negative) | 2.041 (1.424-2.924) | < 0.0011 | 1.271 (0.875-1.847) | 0.208 |
| Histological type (differentiated vs undifferentiated) | 1.460 (1.033-2.063) | 0.032 | 1.058 (0.667-1.678) | 0.811 |
A striking discovery was made in a tumor metabolism study in the 1920s by Warburg. The study demonstrated that, as a result of multiple adaptive mechanisms, cancer cells take up glucose at higher rates than do normal cells and produce energy primarily by aerobic glycolysis rather than by the mitochondrial oxidation of pyruvate for their energy requirements. The important function of aerobic glycolysis in tumor progression has been recognized[23,24], though the molecular mechanisms leading to this phenotype and its functional significance in cancer development remain unknown. PDH is a mitochondrial enzyme that catalyzes the conversion of pyruvate into acetyl-CoA. In tumor cells, the inhibition of PDH activity can inhibit mitochondrial OXPHOS and promote tumor aerobic glycolysis. Nevertheless, the expression of PDH in GC remains unknown. Therefore, we hypothesized that tumor cell inhibition of the activity of PDH and the resultant altered expression of PDH in cancer cells may represent a potential prognostic biomarker in patients who are at risk of developing metastasis or recurrence with gastric carcinoma.
To our knowledge, this is the first reported study to investigate PDH expression in vitro in a large series of human GC specimens. An important finding was that PDH protein expression significantly correlated with survival in OS patients and that increased expression of PDH was found to be associated with good survival in GC patients.
We examined the expression of PDH in tumor tissue from 265 GC patients, and presented its correlation with clinicopathological parameters and patients’ prognosis. Our study revealed that immunohistochemical staining of PDH was detected in GC and NNM, and it was interesting to find that PDH expression was significantly downregulated in GC specimens compared with adjacent NNM tissues. These results may be consistent with a previous study that found that aberrant metabolism could be the cause of many cancers[9]. Our study also showed that tumor tissue PDH expression was strongly inversely correlated with the following GC clinicopathologic characteristics: Lauren grade, lymph node metastasis, lymphatic invasion, histologic subtypes and TNM stage. Therefore, we propose that the loss of PDH expression may contribute to the proliferation and development of GCs. In addition, we analyzed the prognostic role of PDH on OS of patients with GC and found a significant association between PDH expression (in GC or NNM) and OS of patients, and patients with stronger PDH staining had a longer survival time. When we combined the expression of PDH in GC and NNM and divided patients into a co-expression group, two single-expression groups and a double-PDH negative group, the co-expression group patients had a much longer survival time than did the double-PDH negative group. We also analyzed the prognostic value of PDH in different subtypes stratified by TNM stage, Lauren classification and histological classification separately. The results showed that stronger PDH staining was significantly associated with better OS in each subtypes of GC. Our results suggest that the expression of PDH is an independent prognostic factor of OS and indicate that PDH might have an anticancer role in tumor development.
PDH is widely expressed in the mitochondrial matrix of mammalian cells and provides a link between glycolysis and the tri-carboxylic acid cycle by catalyzing the conversion of pyruvate into acetyl-CoA. The activity of PDH depends on the integrity of a multienzyme complex, which is comprised of PDH(E1), dihydrolipoamide acetyltransferase (E2) and dihydrolipoamide dehydrogenase (E3), and two regulatory components, PDK and PDH phosphatase[25]. PDK-1 is a Ser/Thr kinase that negatively regulates PDH activity by phosphorylating the PDHA1 subunit[26]. A study demonstrated that the mitochondrial metabolism of tumor cells is increased by the pharmacologic inhibition of PDK-1[27]. In cancer cells, lactate dehydrogenase A has been shown to play a critical role in glycolysis by converting pyruvate to lactate, and a high level of lactate is associated with a poor prognosis in a number of tumors[28]. Maintaining the glycolysis pathway may be a more important aspect of the inhibition of PDH[9,29]. Ozden et al[30] reported that SIRT3 interacts with PDH(A1) and directs its enzymatic activity via changes in protein acetylation and links glycolysis to respiration. Kikuchi et al[31] revealed that prolyl-hydroxylase regulates PDH activity in cells by physically interacting with the PDH complex. The above studies and our research suggested that a decrease in the quantity and quality of PDH may occur during the development of tumorigenesis. Recent research showed that stimulating PDH in cancer cells restored their susceptibility to anoikis and impaired their metastatic potential[18]. In our study, the correlation between the expression of PDH and OS in GC patients may provide important evidence that a decrease in the quantity and quality of PDH is implicated in cancer metabolism in the process of GC development. However, the mechanism remains obscure and is a subject for further study.
In conclusion, these results indicate that the expression of PDH is significantly inversely correlated with clinical pathological characteristics of GC. Moreover, the expression of PDH may be useful in predicting outcomes as a strong molecular marker in GC patients, and may be associated with a negative carcinogenic regulatory role. Nevertheless, it remains to be determined how the expression of PDH is regulated and what the relationship is between the expression of PDH and the activity of PDH in tumor cells. In addition, our results could not explicitly explain why a certain number of patients expressed PDH in tumor tissue but not in their NNM tissue. The hypothesis that we proposed to account for this phenomenon is that normal tissues could have a lower expression of PDH when they have an abundant oxygen supply, which could not be detected by immunohistochemistry. We also do not know whether the PDH expression in NNM inhibits the growth and metastasis of the tumor cells. These biological roles of PDH in the progression of these tumors need to be further elucidated.
Pyruvate dehydrogenase (PDH), a mitochondrial enzyme that catalyzes the conversion of pyruvate into acetyl-CoA, serves as a gate-keeper enzyme link between glycolysis and the citric acid cycle. The inhibition of PDH activity is linked to cancer aerobic glycolysis. However, its expression in cancer has not been characterized. The purpose of this study was to clarify the expression of PDH in gastric cancer and its potential impact on the development and prognosis in gastric cancer (GC).
Aerobic glycolysis is a hallmark of cancer progression. Pyruvate kinase M2 (PKM2) and lactate dehydrogenase A (LDHA) are the hotspots of aerobic glycolysis. Some studies note that the high expression of PKM2 and LDHA could promote glycolysis. The aerobic glycolysis process showed that high rates of glucose uptake and lactate production in tumors facilitated cancer proliferation. The inhibition of PDH activity is linked to the promotion of cancer aerobic glycolysis.
This is the first reported study to evaluate the prognostic role of PDH expression in cancer. The purpose of this study was to clarify the expression of PDH in GC and its potential impact on the development and prognosis in GC.
The expression of PDH may be useful in predicting outcomes in GC patients as a strong molecular marker associated with a negative carcinogenic regulatory role.
The Warburg effect, also known as aerobic glycolysis, is one of the characteristics of tumor cells in which a high rate of glycolysis occurs even in the presence of adequate oxygen. PDH is a mitochondrial enzyme that plays a central role in aerobic energy metabolism by catalyzing the irreversible oxidation of glucose-derived pyruvate to acetyl-CoA. Acetyl-CoA then goes into the tricarboxylic acid cycle where it reacts with oxaloacetate to form citrate.
This is a study in which the authors analyzed the expression of PDH in gastric cancers. The results suggest that positive expression of PDH was independent prognostic factors in cancer tissue, and PDH expression was significantly associated with higher overall survival.
P- Reviewer: Li H, Mohammadi M, Yuan Y S- Editor: Yu J L- Editor: Cant MR E- Editor: Ma S
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8224] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 2. | Goggins WB, Wong GK. Poor survival for US Pacific Islander cancer patients: evidence from the Surveillance, Epidemiology, and End Results database: 1991 to 2004. J Clin Oncol. 2007;25:5738-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1254] [Article Influence: 66.0] [Reference Citation Analysis (8)] |
| 4. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 505] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 871] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 7. | Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927-8930. [PubMed] |
| 8. | Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9914] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 10. | Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425-434. [PubMed] |
| 11. | Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388-395. [PubMed] |
| 12. | Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 13. | Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1170] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 14. | Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 15. | Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br J Cancer. 2010;102:1746-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Sun RC, Board PG, Blackburn AC. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol Cancer. 2011;10:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol. 2008;109:394-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, Lu J. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Sobin LH. TNM classification of malignant tumours. 6th ed. Hoboken (NJ): John Wiley & Sons 2002; . |
| 20. | Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol. 2007;60:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Hamilton SR. WHO classification of tumors: pathology and genetics of tumors of the digestive system. Lyon, France: IARC press; . |
| 23. | Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3472] [Cited by in RCA: 3608] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 24. | Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S-42S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 463] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 25. | Hiromasa Y, Fujisawa T, Aso Y, Roche TE. Organization of the cores of the mammalian pyruvate dehydrogenase complex formed by E2 and E2 plus the E3-binding protein and their capacities to bind the E1 and E3 components. J Biol Chem. 2004;279:6921-6933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Roche TE, Baker JC, Yan X, Hiromasa Y, Gong X, Peng T, Dong J, Turkan A, Kasten SA. Distinct regulatory properties of pyruvate dehydrogenase kinase and phosphatase isoforms. Prog Nucleic Acid Res Mol Biol. 2001;70:33-75. [PubMed] |
| 27. | Cairns RA, Papandreou I, Sutphin PD, Denko NC. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci USA. 2007;104:9445-9450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409-415. [PubMed] |
| 29. | Sun X, Sun Z, Zhu Z, Guan H, Zhang J, Zhang Y, Xu H, Sun M. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS One. 2014;9:e91068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Ozden O, Park SH, Wagner BA, Yong Song H, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, Gius D. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med. 2014;76:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Kikuchi D, Minamishima YA, Nakayama K. Prolyl-hydroxylase PHD3 interacts with pyruvate dehydrogenase (PDH)-E1β and regulates the cellular PDH activity. Biochem Biophys Res Commun. 2014;451:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |