Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5303
Peer-review started: October 28, 2014
First decision: November 14, 2014
Revised: December 5, 2014
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
Processing time: 199 Days and 13.1 Hours
AIM: To investigate the prognostic value of preoperative platelet count (PLT) in patients with primary gallbladder cancer (GBC).
METHODS: The clinical data of 223 GBC patients after surgery was retrospectively reviewed. A receiver operating characteristic (ROC) curve was plotted to verify the optimum cutoff point for PLT. Univariate and multivariate survival analyses were performed to identify the factors associated with the prognosis.
RESULTS: The ROC curve showed that the optimum cutoff point for PLT was 178 × 109/L, and the entire cohort was stratified into group A with PLT > 178 × 109/L and group B with PLT ≤ 178 × 109/L. Group A had a better survival than group B (P < 0.001). There was an obvious difference between the two groups in terms of the differentiation degree, advanced tumor stage, lymph node metastasis (P < 0.001) and pathological type (P < 0.05). The univariate analysis demonstrated that tumor location, differentiation degree, TNM stage, Nevin stage, lymph node metastasis and PLT were associated with overall survival (P < 0.001). In the multivariate analysis, PLT (P = 0.032), lymph node metastasis (P = 0.007), tumor location (P < 0.001) and TNM stage (P = 0.005) were independent prognostic factors.
CONCLUSION: PLT is closely correlated with GBC prognosis and could be used to identify the population with a poorer prognosis after surgery.
Core tip: Platelet count (PLT) is implicated with a poor prognosis in many types of malignancies. Its prognostic value has not been reported in gallbladder carcinoma (GBC). The most important finding in this study was that PLT was correlated with GBC prognosis, and was an independent prognostic factor after surgery.
- Citation: Wang RT, Zhang LQ, Mu YP, Li JB, Xu XS, Pang Q, Sun LK, Zhang X, Dong SB, Wang L, Liu C. Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J Gastroenterol 2015; 21(17): 5303-5310
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5303
Primary gallbladder carcinoma (GBC) is the most common malignancy of the biliary tract[1] and the seventh most common gastrointestinal cancer[2]. The Surveillance, Epidemiology, and End Results program estimated the incidence of GBC at 2.5 per 100000 persons[3]. GBC has a poor prognosis because of early metastasis via the lymphatic, perineural, and hematogenous routes, as well as by direct invasion into the liver[3,4]. GBC is asymptomatic until aggressive disease progresses to an advanced and noncurative stage. The overall survival (OS) for GBC is 6 mo, with a 5-year survival rate of 5%[5,6]. Although the TNM staging system is widely used in clinical practice, there is no global consensus on the preoperative markers to predict the prognosis of GBC patients[3].
Numerous studies have revealed that elevated platelet count (PLT) is typically related to poor cancer prognosis[7-11]. Hernandez et al[12] showed that thrombocytosis is an independent indicator of poor prognosis in cervical cancer. Recently, Stone et al[13] confirmed that thrombocytosis was significantly associated with advanced disease and shortened survival in ovarian cancer. Numerous clinical data have shown that increasing PLT is associated with poor survival in patients with tumors including pancreatic adenocarcinomas[9], esophageal squamous cell carcinomas[7], and gastrointestinal cancers[14] as well as colorectal cancer[15]. Whether PLT plays important roles in the prognosis of GBC has not been reported.
GBC is a relatively rare disease with high mortality. Improving the survival rate after surgery is an enormous challenge. Based on the advances in PLT research and tumor prognosis, we hypothesized that PLT is a possible prognostic factor for GBC patients and aimed to find a novel prognostic marker for this malignancy.
From January 2006 to December 2012, a retrospective analysis was conducted on 223 GBC patients after surgery in the Department of Hepatobiliary Surgery at the First Affiliated Hospital of the Xi’an Jiaotong University College of Medicine. The patients included in the analysis fit the following criteria: (1) GBC diagnosis confirmed by histopathology; and (2) gallbladder resection was neither preceded nor followed by adjuvant chemotherapy and/or radiotherapy. The patients with the following characteristics were excluded: (1) coexisting or previous cancers other than GBC; (2) concomitant diseases suspected of increasing the serum platelet concentration, including severe hypertension, splenic disease and blood coagulation disorders; and (3) the use of aspirin or other acetylsalicylic acid drugs one month before the surgery. Based on the medical records, the following data were collected for each patient: age, gender, PLT, complications, tumor location, gallstone history, tumor differentiation, TNM stage, Nevin stage, lymph node metastasis, pathological type and other miscellaneous characteristics. All subjects provided their written informed consent, and the study was approved by the Ethical Committees of the First Affiliated Hospital of the Xi’an Jiaotong University College of Medicine.
A blood sample was obtained before breakfast 3 d prior to the surgery by a peripheral venous puncture. A complete blood count was performed regularly for each patient.
All of the patients were followed by telephone interviews. The date of surgery marked the beginning of the follow-up period, which ended at the last follow-up visit (October 2014) or death.
The statistical evaluation was conducted with SPSS 19.0 (SPSS Inc., Chicago, IL, United States). The mean values are presented as the mean ± SD. An independent t-test was used to compare the groups of continuous, normally distributed variables. Pearson’s χ2 test was used to determine the significance of the differences for the dichotomous variables. A receiver receiver operating characteristic (ROC) curve was plotted to verify the optimum cutoff point for PLT. OS was calculated as the time from the curative surgery to the time of mortality or censoring. The OS was calculated by the Kaplan-Meier method, and the difference was assessed by the log-rank test. Univariate analysis and multivariate analysis using the Cox regression proportional hazard model were performed to evaluate the prognostic parameters for survival. A P-value less than 0.05 was considered statistically significant.
The characteristics of the patients are summarized in Table 1. Among the 223 patients, there were 156 (70.0%) women and 67 (30.0%) men. Ninety-nine (44.4%) of the patients were > 65 years, and 124 (55.6%) were ≤ 65 years. The mean age was 59.1 ± 8.1 years. There were 119 (53.36%) patients with a history of gallstones before the surgery. The entire cohort was comprised of 183 adenocarcinoma carcinomas, 40 carcinomas of other pathology types, including squamous cell carcinomas, adeno-squamous cell carcinomas and undifferentiated carcinomas (21, 13, and 6, respectively). The majority of the patients had relatively poor differentiation [17 (7.6%) with good differentiation, 90 (40.36%) with moderate differentiation, 115 (51.57%) with poor differentiation and 1 (0.45%) undifferentiated].
| Parameter | Cases | PLT | ||||
| mean ± SD | P value | ≤178 | >178 | P value | ||
| Gender | 0.921 | 0.985 | ||||
| Men | 67 (30.0) | 219 ± 91 | 25% | 42% | ||
| Women | 156 (70.0) | 224 ± 91 | 58% | 98% | ||
| Age | 0.573 | 0.379 | ||||
| > 65 | 99 (44.4) | 226 ± 90 | 40% | 59% | ||
| ≤ 65 | 124 (55.6) | 217 ± 92 | 43% | 81% | ||
| Comorbidity | 0.250 | 0.101 | ||||
| Yes | 84 (37.7) | 214 ± 97 | 37% | 47% | ||
| No | 139 (62.3) | 227 ± 87 | 46% | 93% | ||
| Gallstone history | 0.358 | 0.361 | ||||
| Yes | 119 (53.4) | 231 ± 95 | 41% | 78% | ||
| No | 104 (46.6) | 212 ± 85 | 42% | 62% | ||
| ABO blood group | 0.713 | 0.189 | ||||
| A | 59 (26.4) | 211 ± 95 | 26% | 33% | ||
| B | 82 (36.8) | 228 ± 84 | 23% | 59% | ||
| O | 27 (12.1) | 224 ± 98 | 11% | 16% | ||
| AB | 55 (24.7) | 224 ± 93 | 23% | 32% | ||
| Tumor location | 0.422 | < 0.001 | ||||
| Neck | 90 (40.4) | 279 ± 83 | 9% | 81% | ||
| Other | 133 (59.6) | 184 ± 75 | 74% | 59% | ||
| TNM stage | 0.006 | < 0.001 | ||||
| 0-II | 49 (22.0) | 161 ± 72 | 34% | 15% | ||
| III-IV | 174 (78.0) | 239 ± 88 | 49% | 125% | ||
| Nevin stage | 0.011 | < 0.001 | ||||
| I-III | 70 (31.4) | 160 ± 69 | 49% | 21% | ||
| IV-V | 153 (68.6) | 251 ± 85 | 35% | 119% | ||
| Tumor differentiation | 0.771 | < 0.001 | ||||
| Well and moderately | 108 (48.4) | 187 ± 84 | 60% | 48% | ||
| Poorly and undifferentiated | 115 (51.6) | 255 ± 85 | 23% | 92% | ||
| Lymph node metastasis | 0.013 | < 0.001 | ||||
| Yes | 149 (66.8) | 251 ± 86 | 33% | 116% | ||
| No | 74 (33.2) | 163 ± 69 | 50% | 24% | ||
| Pathological type | 0.049 | 0.027 | ||||
| Adenocarcinoma | 183 (82.1) | 226 ± 88 | 62% | 121% | ||
| Other types | 40 (17.9) | 205 ± 104 | 21% | 19% | ||
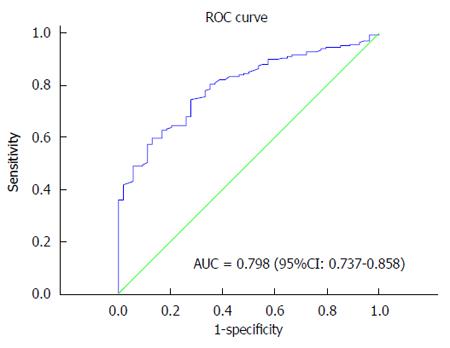
The median PLT was 222 × 109/L ± 91 × 109/L. The optimum cutoff point for PLT according to a ROC curve was 178 × 109/L (Figure 1). The entire cohort was divided into 2 groups for further analysis, group A with PLT > 178 × 109/L and group B with PLT ≤ 178 × 109/L. There was an obvious difference between the groups in the degree of differentiation, advanced tumor stage, lymph node metastasis (P < 0.001) and pathology type (P = 0.027); there was no significant difference in the gender, age, comorbidity, gallstone history or ABO blood group (P > 0.05) (Table 1).
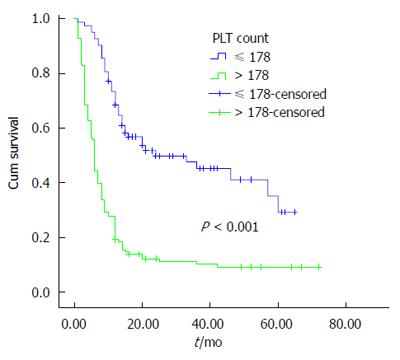
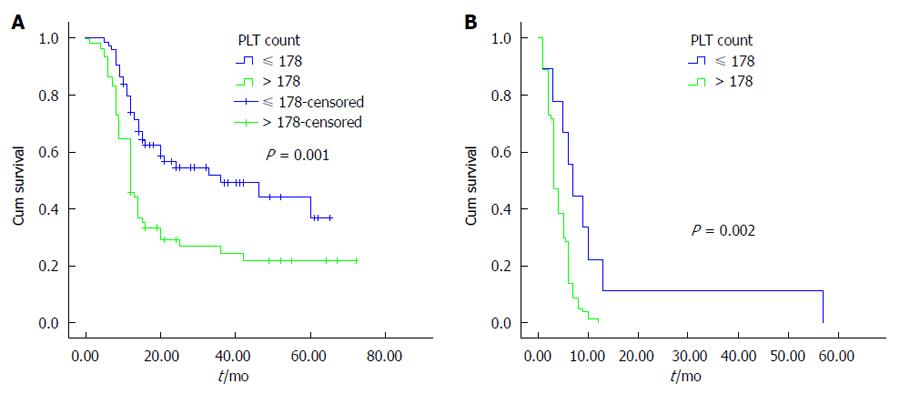
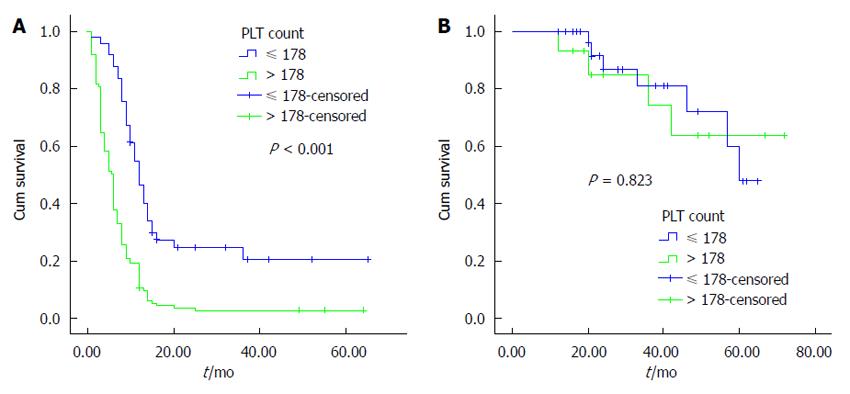
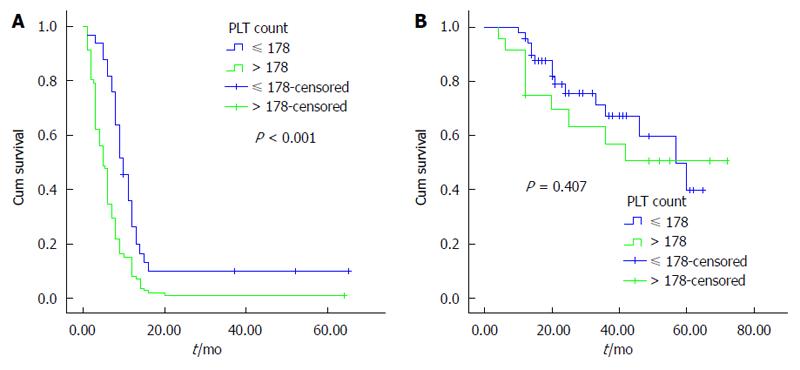
The univariate analysis was performed using the Kaplan-Meier method to assess the predictive capability of each variable. Our results showed that tumor location, tumor differentiation, TNM stage, Nevin stage, lymph node metastasis and PLT were predictive factors of OS (P < 0.001) (Table 2). Regarding OS, group B was superior to group A (P < 0.001) (Figure 2). As shown in Figures 3, 4 and 5, different PLR levels play important roles in the prognosis of a subgroup, and group A exhibited a worse prognosis than group B (P < 0.05). The Cox proportional hazards model demonstrated that lymph node metastasis (P = 0.007), TNM stage (P = 0.005), PLT (P = 0.032) and tumor location (P < 0.001) were independent prognostic factors (Table 3).
| Variable | HR (95%CI) | P value |
| Gender | 0.940 (0.677-1.306) | 0.712 |
| Male | ||
| Female | ||
| Age (yr) | 1.137 (0.840-1.539) | 0.408 |
| ≤ 65 | ||
| > 65 | ||
| Gallstone history | 1.066 (0.923-1.232) | 0.383 |
| Yes | ||
| No | ||
| Comorbidity | 0.912 (0.665-1.251) | 0.567 |
| Yes | ||
| No | ||
| Tumor location | 8.910 (6.236-12.730) | < 0.001 |
| Neck | ||
| Other (body, bottom) | ||
| Tumor differentiation | 3.209 (2.325-4.427) | < 0.001 |
| Well and Moderately | ||
| Poorly and undifferentiated | ||
| TNM stage | 11.003 (5.896-20.535) | < 0.001 |
| 0-II | ||
| III-IV | ||
| Nevin stage | 10.642 (6.612-17.127) | < 0.001 |
| I-III | ||
| IV-V | ||
| Lymph node metastasis | 9.775 (6.224-15.352) | < 0.001 |
| Yes | ||
| No | ||
| Pathological type | 0.708 (0.469-1.070) | 0.101 |
| Adenocarcinoma | ||
| Other types | ||
| PLT | 3.333 (2.351-4.726) | < 0.001 |
| ≤ 178 | ||
| > 178 |
| Variable | Characteristic | HR (95%CI) | P value |
| Lymph node metastasis | Yes | 1.795 (1.170-2.755) | 0.007 |
| No | |||
| TNM stage | 0-II | 3.349 (1.436-7.814) | 0.005 |
| III-IV | |||
| PLT | ≤ 178 | 1.541 (1.038-2.287) | 0.032 |
| > 178 | |||
| Tumor location | Neck | 6.200 (4.120-9.329) | < 0.001 |
| Other (body, bottom) |
The incidence of GBC appears to be increasing worldwide, creating an enormous public health and economic burden. In this study, our results demonstrated that PLT is an important prognostic factor for OS in GBC, and group B showed a better survival than group A. Additionally, we found that similar results exist in different subgroups (tumor location, lymph node metastasis, and TNM staging system). The multivariate analysis showed that tumor location, lymph node metastasis, TNM stage and PLT were independent prognostic factors. To the best of our knowledge, this study is the first to investigate the association between PLT and the prognosis of GBC.
Although PLT is associated with many types of cancers, little is known regarding PLT in GBC. Ong et al[16] hypothesized that GBC patients with a PLT > 345 × 109/L should not undergo surgical exploration. This hypothesis should be confirmed by investigations with large samples. In this study, a PLT > 178 × 109/L was the optimal cutoff value to identify GBC patients with a poorer prognosis. To ensure the credibility of this research, patients without neoadjuvant or adjuvant treatment were selected because systemic chemotherapy or radiation inevitably affects systemic inflammation, which is strongly linked with cancer[7].
The location of GBC was an independent prognostic factor in this study. Shindoh et al[17] hypothesized that tumor location was a strong predictor of tumor progression and survival in GBC in the T2 category and that the density of the vascular structures and the length of the drainage route from the tumor to first-echelon lymph nodes or the liver affect the incidence of vascular invasion and metastasis. Because classical studies using staining methods have reported that the hepatic side of the gallbladder is drained by short cystic veins (2-20 in number) directly connecting to intrahepatic portal veins, whereas the peritoneal side is typically drained by 1 or 2 cystic veins terminating into the adjacent liver parenchyma or the venous plexus at the hepatic hilum. We proposed that the anatomical regions adjacent to the neck of the gallbladder bile duct, portal vein, liver, duodenum and colon are vulnerable to damage, and the early radical resection rate is greatly reduced. A cystic tumor in the neck greatly increases the difficulty of surgery and reduces the probability of radical resection.
Platelets are involved in the physiological process of coagulation and in the growth and metastasis of tumors although the mechanism has not been determined. Platelets could adhere to, aggregate and locally release their angiogenic contents in tumors, which was hypothesized to interact with tumor cells and vascular endothelial cells in physiological as well as pathological angiogenesis[9,18]. Platelets are the source of platelet-derived endothelial cell growth factor (TP/PD-ECGF), which has the potential to promote mitogenesis and angiogenesis[19]. They could endocytose and store TP/PD-ECGF in their α-granules, and this molecule is secreted immediately after platelet activation[20]. Yamamoto et al[21] found that TP/PD-ECGF, which stimulates the chemotaxis of endothelial cells in vitro and possesses angiogenic activity in vivo, is produced by cancer cells and infiltrating cells associated with tumor progression in human GBC. Additionally, platelets endocytose and concentrate the plasma protein vascular endothelial growth factor secreted from tumor cells and later transport them into their granules[22-25]. A recent study reported that the interactions between platelets and tumor cells augmented metastasis by promoting epithelial mesenchymal transition through the TGFB/SMAD and NFKB pathways and that inhibition of these two pathways solely in platelets could suppress metastasis in vivo[26]. Platelets enhance tumor metastasis by expressing immunoregulatory proteins including the glucocorticoid-induced TNF-related protein to protect tumor cells from the host’s immune system[7,27,28]. Intratumoral platelet activation and the subsequent release of thrombopoietin could lead to increased platelets[18]. The thrombopoietic cytokine interleukin-6 has been found to be produced by tumor tissues and was correlated with platelets[29-31]. The interaction between platelets and tumor cells promotes tumor progression.
This study has some limitations. First, this study was a retrospective investigation. Second, the data were obtained from a single institution. Our results should be validated by prospective research and multiple center data.
PLT is an independent prognostic factor for GBC, which facilitates the identification of patients with poorer survival by subgroups (tumor location, lymph node metastasis, and TNM staging system) after surgery. As an inexpensive, simple, reliable and reproducible method, we hypothesize that PLT could be used in clinical practice to determine the GBC prognosis.
Numerous studies have indicated that platelet count (PLT) is correlated with a variety of cancers. In the clinic, the overall survival (OS) of gallbladder cancer (GBC) is poor, and there are no effective markers that identify the patients with a poorer prognosis.
In recent decades, the OS of GBC has been far from satisfactory despite rapid technological developments, which might be attributed to the following reasons: (1) although sufficient molecular investigations have been conducted, the specific mechanism is unclear; and (2) effective clinical prognostic markers are lacking. Exploring the novel markers associated with GBC is necessary to improve the OS.
These data show that PLT is an independent factor and can be used to identify the patients with poorer OS.
GBC, originating in the biliary tract system, is characterized by a very poor prognosis. The risk factors for GBC include gallstones, aging, and female gender. The common mechanism of GBC has not been determined. Platelets are bioactive small cytoplasmic cells that originate in the bone marrow of mature megakaryocyte cytoplasmic cleavage and play an important role in hemostasis, wound healing, inflammation, thrombosis, organ transplant rejection, and other physiological and pathological processes.
The purport of this article is to study the relationship between PLT and the prognosis of patients with primary gallbladder, and the results showed that PLT count is an independent prognostic factor of primary gallbladder, and can be used in the clinical evaluation of the prognosis of primary gallbladder.
P- Reviewer: Pan HC, Qiao T, Tuncyurek O S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu WG, Cao Y, Bao RF, Shu YJ, Ding QC. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol. 2014;21:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, Liu C, Shen B, Wang XA, Wu W. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 5. | Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Fourteen year surgical experience of gallbladder cancer: validity of curative resection affecting survival. Hepatogastroenterology. 2012;59:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Wang RT, Xu XS, Liu J, Liu C. Gallbladder carcinoma: analysis of prognostic factors in 132 cases. Asian Pac J Cancer Prev. 2012;13:2511-2514. [PubMed] |
| 7. | Feng JF, Huang Y, Lu WS, Chen QX. Preoperative platelet count in esophageal squamous cell carcinoma: is it a prognostic factor? Langenbecks Arch Surg. 2013;398:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Wan S, Lai Y, Myers RE, Li B, Hyslop T, London J, Chatterjee D, Palazzo JP, Burkart AL, Zhang K. Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J Gastrointest Cancer. 2013;44:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Brown KM, Domin C, Aranha GV, Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005;189:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Patnaik MM, Caramazza D, Gangat N, Hanson CA, Pardanani A, Tefferi A. Age and platelet count are IPSS-independent prognostic factors in young patients with primary myelofibrosis and complement IPSS in predicting very long or very short survival. Eur J Haematol. 2010;84:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992;69:2975-2977. [PubMed] |
| 13. | Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 14. | Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol. 2014;6:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Ong SL, Garcea G, Thomasset SC, Neal CP, Lloyd DM, Berry DP, Dennison AR. Ten-year experience in the management of gallbladder cancer from a single hepatobiliary and pancreatic centre with review of the literature. HPB (Oxford). 2008;10:446-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, Javle M, Conrad C, Maru DM, Aoki T. Tumor location is a strong predictor of tumor progression and survival in t2 gallbladder cancer: an international multicenter study. Ann Surg. 2015;261:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, Kim YH, Kim JS. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol. 2010;40:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W, Heldin CH. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 515] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Harrison P, Wilbourn B, Debili N, Vainchenker W, Breton-Gorius J, Lawrie AS, Masse JM, Savidge GF, Cramer EM. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J Clin Invest. 1989;84:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Yamamoto S, Kitadai Y, Tsuchida A, Sasaki T, Matsubara K, Kajiyama G. Expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human gallbladder lesions. Eur J Cancer. 2000;36:257-263. [PubMed] |
| 22. | Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663-668. [PubMed] |
| 23. | Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187-2190. [PubMed] |
| 24. | Kim SJ, Choi IK, Park KH, Yoon SY, Oh SC, Seo JH, Choi CW, Kim BS, Shin SW, Kim YH. Serum vascular endothelial growth factor per platelet count in hepatocellular carcinoma: correlations with clinical parameters and survival. Jpn J Clin Oncol. 2004;34:184-190. [PubMed] |
| 25. | Handagama PJ, George JN, Shuman MA, McEver RP, Bainton DF. Incorporation of a circulating protein into megakaryocyte and platelet granules. Proc Natl Acad Sci USA. 1987;84:861-865. [PubMed] |
| 26. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1396] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 27. | Müller BG, De Aretxabala X, González Domingo M. A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Am Soc Clin Oncol Educ Book. 2014;e165-e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Placke T, Kopp HG, Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Nakano T, Chahinian AP, Shinjo M, Tonomura A, Miyake M, Togawa N, Ninomiya K, Higashino K. Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br J Cancer. 1998;77:907-912. [PubMed] |
| 31. | Degeorges A, Tatoud R, Fauvel-Lafeve F, Podgorniak MP, Millot G, de Cremoux P, Calvo F. Stromal cells from human benign prostate hyperplasia produce a growth-inhibitory factor for LNCaP prostate cancer cells, identified as interleukin-6. Int J Cancer. 1996;68:207-214. [PubMed] |