Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4547
Peer-review started: September 21, 2014
First decision: October 19, 2014
Revised: December 7, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 21, 2015
Processing time: 211 Days and 16.3 Hours
AIM: To investigate the influence of lipopolysaccharide (LPS) through the p38/c-Jun N-terminal kinase (JNK) signalling pathway on aquaporin 3 (AQP3) expression in HT-29 human colon epithelial cells.
METHODS: HT-29 cells were treated with LPS, and then the membrane localisation of AQP3 was examined by immunofluorescence staining. The mRNA and protein expression of AQP3 with LPS exposure was measured by real-time reverse transcription-PCR and Western blot, respectively. Activation of p38 and JNK was evaluated by detection of phosphorylation of p38 and JNK using Western blot assay. AQP3 protein expression was determined by Western blot in cells after treatment with SB203580, a selective p38 MAPK inhibitor, or SP600125, a selective JNK inhibitor.
RESULTS: In HT-29 cells, the transcription and protein expression of AQP3 were decreased by LPS in a dose- and time-dependent manner, the expression of AQP3 was significantly decreased with the increased concentration of LPS, and at a dose of 100 μg/mL LPS, AQP3 mRNA and protein levels were decreased by a maximum (P < 0.05) of 1.51-fold and 1.49-fold, respectively. When cells were treated with 100 μg/mL LPS for 0, 3, 6, 12, and 24 h, the AQP3 mRNA level was significantly decreased at an early time point of 3 h, and reached about 10% of the control level at 24 h post-treatment (P < 0.05). Down-regulation of AQP3 expression was significantly inhibited by the p38 inhibitor (SB203580) and JNK inhibitor (SP600125).
CONCLUSION: p38 and JNK may be promising targets for the preservation of AQP3 expression and may be beneficial to the clinical management of diarrhoea.
Core tip: In this study we investigated the mRNA and protein expression levels of aquaporin 3 (AQP3) in HT-29 cells exposed to lipopolysaccharide. In addition, we examined the mechanism of regulation of AQP3 expression via the p38 and JNK signalling pathway in vitro.
-
Citation: Li FX, Huang LZ, Dong C, Wang JP, Wu HJ, Shuang SM. Down-regulation of aquaporin3 expression by lipopolysaccharide
via p38/c-Jun N-terminal kinase signalling pathway in HT-29 human colon epithelial cells. World J Gastroenterol 2015; 21(15): 4547-4554 - URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4547
Aquaporins (AQPs) are a family of small integral membrane proteins; in the plasma membranes of many human tissues, some members of this family are expressed, which serve as selective water transporters to regulate water homeostasis[1]. Thirteen different aquaporins have been identified in human tissues. Among these, Aquaporin3 (AQP3) is the most dominantly expressed AQP in the colon[2]. The role of AQP3 in the gastrointestinal tract has been considered to be more important with respect to constipation or diarrhoea[3], since AQP3 protein was found to be expressed not only in the basolateral but also in the apical membrane in the human colon[4]. AQP3 is a known aquaglyceroporin, which not only transports water, but can also transport glycerol and a few other small molecules. Multiple studies have suggested an association between the regulation of AQP3 expression function and diarrhoea. The vasodilation and muscle-relaxation action of vasoactive intestinal polypeptide (VIP, a gastrointestinal hormone) has been linked to increased mRNA and protein expression of AQP3 in human colonic epithelial cells (HT-29)[5]. Recently, results from a study by Okahira et al[6] and Ikarashi et al[7] suggested that the laxative effect of MgSO4 could be a response to the increased expression of AQP3, while bisacodyl decreased the expression of AQP3 in mucosal epithelial cells[8]. Furthermore, when they administered HgCl2 and CuSO4, which are known to inhibit AQP3 function, to rats, they found that the faecal water content increased significantly and serious diarrhoea occurred, despite the fact that no changes were observed in the level and distribution of AQP3 protein expression[9].
Diarrhoea is one of the most common intestinal symptoms caused by bacterial invasion and is pathologically characterised by imbalanced water transfer. Lipopolysaccharide (LPS) is the major constituent of the outer envelope of Gram-negative bacteria, which is thought to be a main cause of serious diarrhoea in many patients. Although previous studies have shown that LPS decreased lung aquaporin levels in a lung inflammation rat model[10-12] and salivary gland aquaporin in a human airway submucosal gland cell line[13], whether diarrhoea induced by the endotoxin LPS affects the expression of AQP3 in colon epithelial cells remains undetermined. In this study, we attempted to examine the modulation of AQP3 after LPS exposure in the human colonic epithelial cell line HT-29.
Regulating the expression or function of AQP3 in the colon may represent a promising therapeutic strategy for the clinical management of diarrhoea[14]. There is currently a profound interest in further understanding the regulatory mechanism of AQP3 expression in the colon. Mitogen-activated protein kinase (MAPK) plays an important role in the transduction of signals from the extracellular space to the nucleus[15]. MAPK modulates the expression of several transcriptional factors. There are three members of the MAPK signalling pathway: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38. ERK is activated in response to growth factors and intracellular calcium increases, while JNK and p38 are activated by a variety of cellular stresses, such as inflammatory cytokines and heat shock proteins. Some studies have shown that the expression of aquaporin was modulated via the p38/JNK signalling pathway by various stimulation factors, such as LPS, tissue ischaemia, and changes in osmolality[16]. In this study, we investigated the mRNA and protein expression levels of AQP3 in HT-29 cells exposed to LPS. In addition, we aimed to examine the mechanism responsible for the regulation of AQP3 expression via the p38/JNK signalling pathways in vitro.
The human colon adenocarcinoma cell line HT-29 and McCoy’s 5a medium were obtained from Boster Bioengineering Ltd. Bovine serum albumin (BSA) and TRI reagent were purchased from Boster Bioengineering Ltd., while LPS was from Sigma-Aldrich.
Various primers were purchased from Invitrogen Corp. Anti-human AQP3 antibody, horseradish peroxidase conjugated anti-rabbit IgG antibody, and antibodies against phospho-p38 (p-p38) and total p38, phospho-JNK and total JNK were all obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, United States). SB203580 (p38 MAPK inhibitor) and SP600125 (JNK inhibitor) were purchased from Selleck Chemicals Ltd.
HT-29 cells were cultured in McCoy’s 5a medium supplemented with 10% BSA, 100 U/mL penicillin G potassium, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere with 5% CO2. Cells were plated on a 100-mm dish at a density of 5 × 105 cells/cm2, cultured for 24 h, and antibiotics were then removed from culture 12 h before various time and dose treatments: 100 μg/mL LPS for 3, 6, 12 and 24 h; and 0, 10, 20, 50, and 100 μg/mL LPS for 12 h. To determine how AQP3 expression is modulated by MAPK activation, the cells were treated with p38 inhibitor SB203580 at 10 and 20 μg/mL, and JNK inhibitor SP600125 at 10 and 20 μg/mL. For inhibitor studies, cells were pre-treated with the inhibitor for 30 min before exposure to LPS stimulation, and then co-incubated with LPS and the inhibitor for 6 h.
The localisation of AQP3 protein in HT-29 cells was detected by immunofluorescence microscopy. After 12 h exposure to 20 μg/mL LPS, cells were fixed with 4% paraformaldehyde for 30 min, washed with phosphate-buffered saline (PBS) for 20 min and blocked with 2% BSA in PBS for 30 min at room temperature. Cells were incubated overnight at 4 °C with primary AQP3 antibody at a dilution of 1:50. Then, samples were washed with PBS three times and subsequently incubated in the FITC-labelled secondary antibody (1:100) for 60 min at room temperature. After washing with PBS three times, cells were visualised under a fluorescence microscope using Axio Vision software.
Total RNA was isolated from HT-29 cells using TRIzol reagent following the manufacturer’s instructions. Total RNA was reverse-transcribed using the following reaction conditions: 25 °C for 5 min, 42 °C for 1 h, 85 °C for 5 min, and then directly amplified using the PCR sense primer: 5’-AGACAGCCCCTTCAGGATTT-3’ and antisense primer 5’-TCCCTTGCCCTGAATATCTG-3’ for AQP3, and sense primer 5’-CGGGAAATCGTGCGTGAC-3’ and antisense primer 5’-TGGAAGGTGGACAGCGAGG-3’ for human β-actin. The mRNA expression levels were normalised using β-actin. Real-time PCR conditions for the amplification of target genes were as follows: pre-denaturing at 95 °C for 10 min, 40 cycles of denaturing at 95 °C for 15 s, and annealing and extension at 60 °C for 1 min. Amplification data measured by fluorescence were collected in real-time and analysed using the Rotor-Gene 6.0.14 software.
HT-29 cells were washed with ice-cold PBS and lysed with whole cell lysis buffer. The lysates were centrifuged at 3000 g for 10 min at 4 °C. Then, the protein concentration was tested using the Pierece Micro BCA protein assay, after which 50 μg protein was separated on a 12% SDS-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. The membrane was incubated with anti-human AQP3, p-p38, p38, phosphor-JNK (p-JNK), and JNK (1:10000) at 4 °C overnight. After washing with TBS buffer, the membrane was incubated with the corresponding horseradish peroxidase-conjugated secondary antibody for 90 min at room temperature. The immunoreactive bands were visualised by the enhanced chemiluminescence method.
Data were analysed with SPSS version 16.0 and are expressed as mean ± SD. Student’s t-test was employed to assess the statistical differences among multiple groups. A P-value < 0.05 was considered significant, and a P-value < 0.01 was considered highly significant.
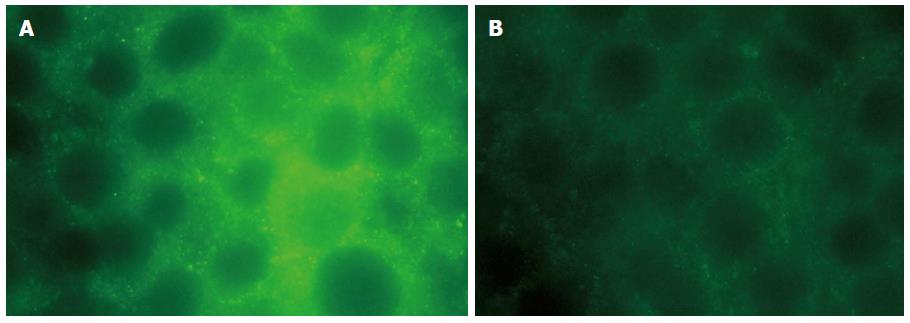
To determine whether LPS exposure affects the localisation of AQP3, HT-29 cells were treated with 20 μg/mL LPS for 6 h. Cells were stained and examined under an immunofluorescence microscope. In control cells, AQP3 was mainly located in intracellular punctate spots (Figure 1A) as previously reported. However, after LPS exposure, AQP3 was predominantly observed on the plasma membrane (Figure 1B).
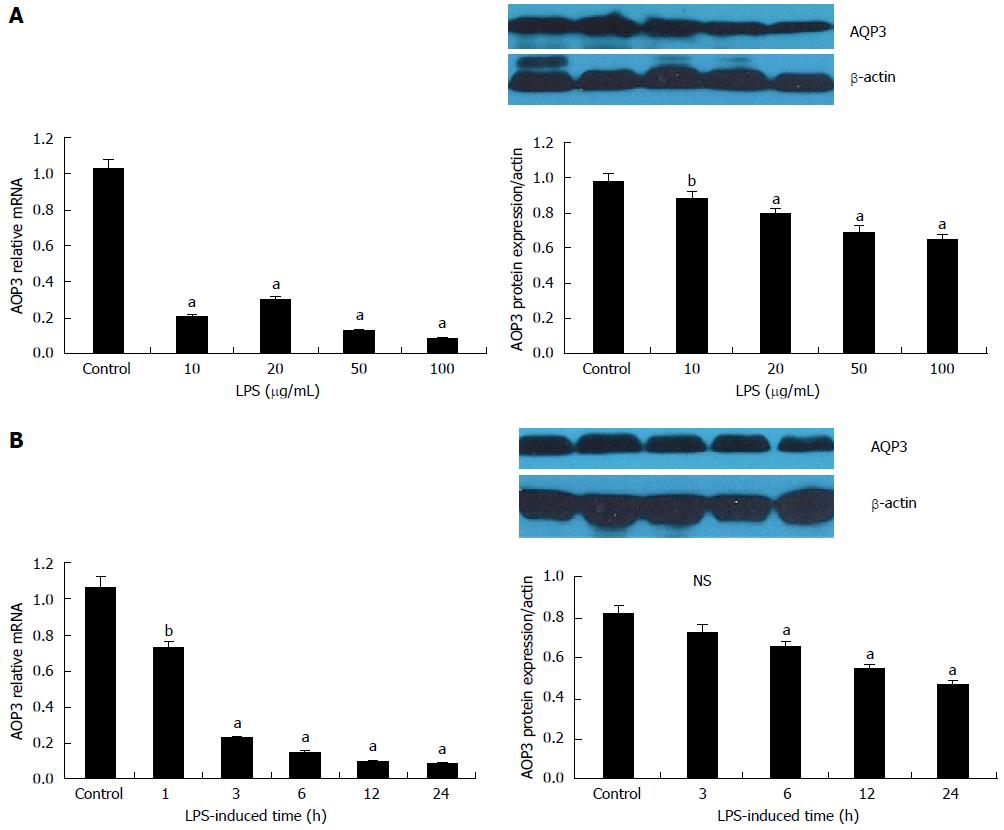
The cells were treated with 0, 10, 20, 50, and 100 μg/mL LPS for 12 h. The expression of AQP3 was quantified by reverse transcription-PCR or Western blot. As shown in Figure 2A, the expression of AQP3 mRNA and protein was significantly decreased in a dose dependent manner. At a dose of 100 μg/mL LPS, AQP3 mRNA and protein levels were decreased by a maximum (P < 0.05) of 1.51-fold and 1.49-fold, respectively, compared with the untreated control, thus 100 μg/mL was used for subsequent experiments.
To further examine the time course of LPS-mediated effects on AQP3 expression, cells were treated with 100 μg/mL LPS for 0, 3, 6, 12, and 24 h before mRNA and protein extraction. The AQP3 mRNA level was significantly decreased at the early time point of 3 h, and reached about 10% of the control level at 24 h post-treatment. A time-dependent decrease of AQP3 protein level was also observed, as shown in Figure 2B.
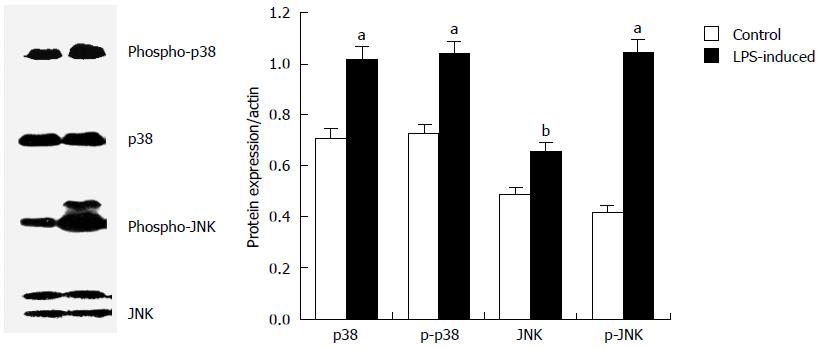
To determine whether the signal mediated by MAPK was involved in the down-regulation of AQP3 by LPS in HT-29 cells, cells were treated with 20 μg/mL LPS for 60 min and total cell extract was used to examine the phosphorylated and total forms of P38 and JNK. Western blot analysis showed that upon LPS stimulation, phosphor-p38 and phosphor-JNK levels were markedly elevated, in contrast to the relatively low basal expression, which indicated that the phosphorylation of MAPKs was activated by LPS in HT-29 cells (Figure 3).
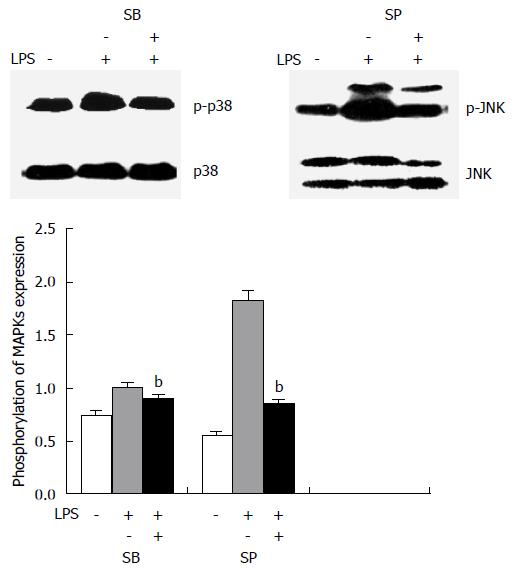
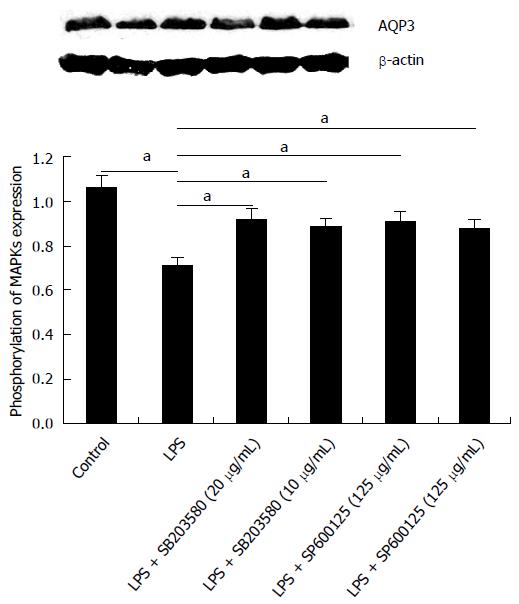
To specifically define the involvement of the activation of MAPK pathways in the down-regulation of AQP3 induced by LPS in HT-29 cells, SB203580, an inhibitor of p38 kinase, and SP600125, a specific inhibitor for JNK, were used. HT-29 cells were pre-treated with SB203580 at 10 μg/mL or SP600125 at 20 μg/mL for 30 min before exposure to LPS; total p38, JNK, p-p38 kinase, and p-JNK were analysed by immunoblotting. As shown in Figure 4, p38 and JNK inhibitors blocked the activation of p38 and JNK induced by LPS.
The effects of MAPK inhibitors on LPS-induced AQP3 protein expression were further investigated, along with whether activation of MAPK was involved in the down-regulation of AQP3 by LPS (Figure 5). Cells were pre-treated with MAPK inhibitors, followed by LPS stimulation. With SB203580 or SP600125, AQP3 protein levels were significantly increased compared with LPS alone, which suggested that the decrease of AQP3 expression was significantly inhibited by both p38 inhibitor and JNK inhibitor at a concentration of 10 μg/mL.
It is known that AQP3 is primarily expressed in mucosal epithelial cells in the human colon[17]. The gastrointestinal tract, particularly the colon, is an organ system that is responsible for an enormous amount of water transport; it is estimated that daily water absorption in the human colon is about 1.5 L[18]. It is widely thought that AQPs are involved in diseases that are characterised by alterations in fluid transport[19,20]. LPS is thought to be one of the main causes of serious diarrhoea in patients. In order to investigate whether there is altered expression of AQP3 in LPS-diarrhoea, we examined the mRNA and protein expression levels of AQP3 in HT-29 cells exposed to LPS and the mechanism of LPS down-regulating AQP3 expression in HT-29 cells. HT-29 cells have been widely used to study the mechanisms of diarrhoea and laxative effects because HT-29 cells represent the normal physiological conditions of the colon, despite the fact that they are cancer cell lines derived from human colon cancer.
AQP3 plays an important role in regulating faecal water content in the colon. The studies showed that the up-regulated expression of AQP3 was correlated with the changes in faecal water content in rats administered with MgSO4. The studies showed that VIP-diarrhoea up-regulated the expression of AQP3 mRNA and protein. For HgCl2 and CuSO4, which are known to inhibit AQP3 function, the results showed that the faecal water content in the HgCl2 administration group was increased significantly compared to the control group, and severe diarrhoea was observed[21]. Comparable results were observed in the CuSO4 administration group. These results indicate the possibility that a decrease in AQP3 causes an increase in faecal water content and diarrhoea. It has been confirmed that the down-regulation of AQP3 expression by LPS in HT-29 cells was in a dose- and time-dependent manner. The time-course experiments indicated that the level of the AQP3 mRNA expression started to decrease at 3 h after LPS addition and was rapidly decreased until 24 h. The AQP3 protein expression level was accompanied by a parallel decrease in mRNA expression level. Thus, the results of this study are consistent with previous results.
The review results show that the faecal water content in the colon is controlled by the transport of water from the luminal side to the vascular side, which is mediated by AQP3[22]. However, further studies are still needed to elucidate the mechanism of decrease of AQP3 in HT-29 cells after LPS exposure. After the oral administration of bisacodyl to rats, when diarrhoea occurred, a significant increase in PGE2 and decrease in AQP3 expression was observed[8]. This study showed that direct activation of colon macrophages by bisacodyl increased the secretion of PGE2, which acts as a paracrine factor and decreases AQP3 expression in colon mucosal epithelial cells. In LPS-induced sepsis-associated cholestasis the data suggest that LPS-induced the TNF-α-mediated posttranscriptional down-regulation of aquaporin functional expression in hepatocytes, a mechanism that is potentially relevant to the molecular pathogenesis of sepsis-associated cholestasis[23]. In mouse models of inflammatory bowel disease, the down-regulation of aquaporin could be a mechanism to defend against severe oxidative stress and may indicate that H2O2 is a universal mediator in the inflammatory process in the colon[24]. Studies have shown that with the knockdown of AQP3, the expression of claudin-1 and occludin was significantly decreased via an opening of the tight junction complex and increasing colonic epithelia permeability[25]. Therefore, AQP3 is involved in intestinal barrier integrity preservation. Epithelial damage usually leads to declined AQP3 expression, because AQP3 is mainly expressed in colonic epithelial cells. The preservation of AQP3 may promote colonic fluid clearance or maintain the integrity of the epithelial barrier. Therefore, regulation of AQP expression may have potential clinical applications. This requires further colonic studies in vivo to confirm.
Interactions between AQPs and cytokines and oxidative stress need further research. The MAPK signalling pathway consists of three members: ERK, JNK and p38. ERK is activated in response to growth factors, intracellular calcium increases, etc., while JNK and p38 are activated by a variety of cellular oxidative stresses, such as inflammatory cytokines and heat shock proteins[26-28]. Therefore, we examined the role of the JNK/p38 signalling pathway in regulating AQP3 expression in HT-29 cells exposed to LPS.
In this study, the JNK/p38 signalling pathway has been demonstrated to be involved in the down-regulation of AQP3 expression in HT-29 cells by LPS pre-treatment, although there was no sufficient detail regarding the regulatory mechanism. A large number of LPS signalling pathways have been elucidated before, such as the cAMP, PKC, NF-κB and p-c-Jun/c-Fos pathways. Therefore, the possibility of LPS down-regulating AQP3 levels through the cAMP, PKC, NF-κB and p-c-Jun/c-Fos pathways could not be excluded.
In conclusion, LPS exposure led to decreased AQP3 transcription and protein expression in HT-29 cells. We demonstrated that p38 and JNK are potential transcription factors for AQP3 expression in human HT-29 cells. A description of this pathway could provide a theoretical basis to study the membrane targets of AQP3. These studies may be beneficial for the clinical management of LPS-diarrhoea.
Aquaporins (AQPs) are a family of small integral membrane proteins; in the plasma membranes of many human tissues, some members of this family are expressed, which serve as selective water transporters to regulate water homeostasis. Diarrhoea is one of the most common intestinal symptoms caused by bacterial invasion and is pathologically characterised by imbalanced water transfer. Although previous studies have shown that lipopolysaccharide (LPS) decreased lung aquaporin levels in a lung inflammation rat model and salivary gland aquaporin in a human airway submucosal gland cell line, whether diarrhoea induced by the endotoxin LPS affects the expression of AQP3 in colon epithelial cells remains undetermined.
In this study, the authors investigated the mRNA and protein expression levels of AQP3 in HT-29 cells exposed to LPS. In addition, we aimed to examine the mechanism responsible for the regulation of AQP3 expression via the p38 and JNK signalling pathway in vitro.
In this study, the authors found that in HT-29 cells, the transcription and protein expression of AQP3 were decreased by LPS in a dose- and time-dependent manner; the expression of AQP3 was significantly decreased with the increased concentration of LPS. Down-regulation of AQP3 expression was significantly inhibited by the p38 inhibitor (SB203580) and JNK inhibitor (SP600125).
The results of the study suggest that p38 and JNK may be promising targets for the preservation of AQP3 expression and may be beneficial to the clinical management of diarrhoea.
This is an interesting study. In this study, the authors examined the influence of LPS on APQ3 expression and the possible role of p38/JNK signalling pathway in the regulation of AQP3 expression in human colon epithelial cells.
P- Reviewer: Elena A, Fondran JC S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Tradtrantip L, Tajima M, Li L, Verkman AS. Aquaporin water channels in transepithelial fluid transport. J Med Invest. 2009;56 Suppl:179-184. [PubMed] |
| 2. | Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med. 2012;33:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Guttman JA, Samji FN, Li Y, Deng W, Lin A, Finlay BB. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell Microbiol. 2007;9:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Yamamoto T, Kuramoto H, Kadowaki M. Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci. 2007;81:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol. 2003;18:203-210. [PubMed] |
| 6. | Okahira M, Kubota M, Iguchi K, Usui S, Hirano K. Regulation of aquaporin 3 expression by magnesium ion. Eur J Pharmacol. 2008;588:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ikarashi N, Ushiki T, Mochizuki T, Toda T, Kudo T, Baba K, Ishii M, Ito K, Ochiai W, Sugiyama K. Effects of magnesium sulphate administration on aquaporin 3 in rat gastrointestinal tract. Biol Pharm Bull. 2011;34:238-242. [PubMed] |
| 8. | Ikarashi N, Mochiduki T, Takasaki A, Ushiki T, Baba K, Ishii M, Kudo T, Ito K, Toda T, Ochiai W. A mechanism by which the osmotic laxative magnesium sulphate increases the intestinal aquaporin 3 expression in HT-29 cells. Life Sci. 2011;88:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ikarashi N, Kon R, Iizasa T, Suzuki N, Hiruma R, Suenaga K, Toda T, Ishii M, Hoshino M, Ochiai W. Inhibition of aquaporin-3 water channel in the colon induces diarrhea. Biol Pharm Bull. 2012;35:957-962. [PubMed] |
| 10. | Su X, Song Y, Jiang J, Bai C. The role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injury. Respir Physiol Neurobiol. 2004;142:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Ohinata A, Nagai K, Nomura J, Hashimoto K, Hisatsune A, Miyata T, Isohama Y. Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun. 2005;326:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Zhang W, Xu Y, Chen Z, Xu Z, Xu H. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett. 2011;585:3113-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Yao C, Purwanti N, Karabasil MR, Azlina A, Javkhlan P, Hasegawa T, Akamatsu T, Hosoi T, Ozawa K, Hosoi K. Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-kappaB and p-c-Jun/c-Fos. Am J Pathol. 2010;177:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Wang W, Zheng M. Role of cAMP-PKA/CREB pathway in regulation of AQP 5 production in rat nasal epithelium. Rhinology. 2011;49:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Liu L, Xie C. Effects of downregulation of aquaporin1 by peptidoglycan and lipopolysaccharide via MAPK pathways in MeT-5A cells. Lung. 2011;189:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Shen Y, Chen Z, Wang Y, Song Z, Zhang Z, Jin M, Wang X, Bai C. Aquaporin 5 expression inhibited by LPS via p38/JNK signaling pathways in SPC-A1 cells. Respir Physiol Neurobiol. 2010;171:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tsujikawa T, Itoh A, Fukunaga T, Satoh J, Yasuoka T, Fujiyama Y. Alteration of aquaporin mRNA expression after small bowel resection in the rat residual ileum and colon. J Gastroenterol Hepatol. 2003;18:803-808. [PubMed] |
| 18. | Conner MT, Conner AC, Bland CE, Taylor LH, Brown JE, Parri HR, Bill RM. Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel. J Biol Chem. 2012;287:11516-11525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Donowitz M, Kokke FT, Saidi R. Evaluation of patients with chronic diarrhea. N Engl J Med. 1995;332:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Zahn A, Moehle C, Langmann T, Ehehalt R, Autschbach F, Stremmel W, Schmitz G. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J Gastroenterol. 2007;13:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Ikarashi N, Baba K, Ushiki T, Kon R, Mimura A, Toda T, Ishii M, Ochiai W, Sugiyama K. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;301:G887-G895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Hardin JA, Wallace LE, Wong JF, O’Loughlin EV, Urbanski SJ, Gall DG, MacNaughton WK, Beck PL. Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn’s disease and infectious colitis. Cell Tissue Res. 2004;318:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Lehmann GL, Carreras FI, Soria LR, Gradilone SA, Marinelli RA. LPS induces the TNF-alpha-mediated downregulation of rat liver aquaporin-8: role in sepsis-associated cholestasis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G567-G575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Te Velde AA, Pronk I, de Kort F, Stokkers PC. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur J Gastroenterol Hepatol. 2008;20:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Zhang ZQ, Song YL, Chen ZH, Shen Y, Bai CX. Deletion of aquaporin 5 aggravates acute lung injury induced by Pseudomonas aeruginosa. J Trauma. 2011;71:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Bell CE, Larivière NM, Watson PH, Watson AJ. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating Aquaporin 3 and 9 expression and localization, as well as embryonic apoptosis. Hum Reprod. 2009;24:1373-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Suh HN, Lee SH, Lee MY, Heo JS, Lee YJ, Han HJ. High glucose induced translocation of Aquaporin8 to chicken hepatocyte plasma membrane: involvement of cAMP, PI3K/Akt, PKC, MAPKs, and microtubule. J Cell Biochem. 2008;103:1089-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Nito C, Kamada H, Endo H, Narasimhan P, Lee YS, Chan PH. Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. J Neurotrauma. 2012;29:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |