Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4293
Peer-review started: November 12, 2014
First decision: December 11, 2014
Revised: January 11, 2015
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: April 14, 2015
Processing time: 155 Days and 12.5 Hours
AIM: To assess the impact of Arpin protein and tight junction (TJ) proteins in the intestinal mucosa on bacterial translocation in patients with severe acute pancreatitis (SAP).
METHODS: Fifty SAP patients were identified as study objects and then classified into two groups according to the presence of bacterial translocation (BT) in the blood [i.e., BT(+) and BT(-)]. Twenty healthy individuals were included in the control group. BT was analyzed by polymerase chain reaction, colonic mucosal tissue was obtained by endoscopy and the expression of TJ proteins and Arpin protein was determined using immunofluorescence and western blotting.
RESULTS: Bacterial DNA was detected in the peripheral blood of 62.0% of patients (31/50) with SAP. The expression of TJ proteins in SAP patients was lower than that in healthy controls. In contrast, Arpin protein expression in SAP patients was higher than in healthy controls (0.38 ± 0.19 vs 0.28 ± 0.16, P < 0.05). Among SAP patients, those positive for BT showed a higher level of claudin-2 expression (0.64 ± 0.27 vs 0.32 ± 0.21, P < 0.05) and a lower level of occludin (OC) (0.61 ± 0.28 vs 0.73 ± 0.32, P < 0.05) and zonula occludens-1 (0.42 ± 0.26 vs 0.58 ± 0.17, P = 0.038) expression in comparison with BT (-) patients. Moreover, the level of Arpin expression in BT (+) patients was higher than in BT (-) patients (0.61 ± 0.28 vs 0.31 ± 0.24, P < 0.05).
CONCLUSION: Arpin protein affects the expression of tight junction proteins and may have an impact on BT. These results contribute to a better understanding of the factors involved in bacterial translocation during acute pancreatitis.
Core tip: Tight junctions (TJs) are the structural basis for the intestinal epithelial barrier. Increased intestinal permeability caused by variations in TJ proteins may result in bacterial translocation (BT) and there is evidence that BT may contribute to infection and sepsis. However, the detailed mechanisms for BT remain unknown. Recent work has identified an Arp2/3 interacting protein called Arpin, which was shown to restrict the rate of actin polymerization and control cell migration. Our research shows that Arpin protein affects the expression of TJ proteins and may have an impact on BT.
- Citation: Deng WS, Zhang J, Ju H, Zheng HM, Wang J, Wang S, Zhang DL. Arpin contributes to bacterial translocation and development of severe acute pancreatitis. World J Gastroenterol 2015; 21(14): 4293-4301
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4293.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4293
Infection and sepsis are severe complications contributing to most late deaths in patients with severe acute pancreatitis (SAP)[1,2]. The organisms responsible for infection in cases of SAP are thought to be common enteric bacteria[3,4], which has generally been supported by animal experiments[5]. Bacterial translocation (BT) from the gut is the most widely accepted mechanism for the pathogenesis of infection and sepsis in SAP[4-6]. BT is defined as the passage of indigenous bacteria (or their products) colonizing the intestine through the intestinal mucosal barrier to the mesenteric lymph nodes and other distant sites[7]. The following three major factors have been proposed as promoters of BT: impairment of intestinal barrier function; alterations in gastrointestinal microflora; and deficiencies in host immunity[8]. During SAP, the structure and function of the intestinal mucosa are damaged, leading to gut barrier dysfunction[1,5]. The intestinal mucosal barrier is established by the intestinal epithelial barrier and distribution of microbial flora[9,10] and is composed of tight junctions (TJs) between intestinal epithelial cells. These TJ proteins form and regulate the paracellular pathway[11-13]; however, the detailed mechanism for this function remains unknown. Recent work has identified an Arp2/3 interacting protein called Arpin that restricts the rate of actin polymerization and is the latest component in the steadily expanding protein repertoire shown to control cell migration[14]. In the current study, we found that Arpin may contribute to BT occurrence and development through the regulation of TJs.
TJs, which are the structural basis of intestinal epithelial barrier, are comprised of the following 4 types of transmembrane proteins: occludin (OC), claudins (CLs), junctional adhesion molecules and tricellulin. CLs and OCs are the most prominent of these proteins[14]. Zonula occluden (ZO) proteins are linked with the main transmembrane proteins and form the CL-ZO protein interactions that are essential for TJ formation[15]. Extensive evidence has identified the Arp2/3 complex as an actin polymerizing complex localized to the tight junction[16]. Cell migration requires the generation of branched actin networks that power the protrusion of the plasma membrane in lamellipodia[17]. These structures are major sites of actin filament nucleation and typically display highly nonlinear kinetics, meaning that they are sharply defined in both space and time[18]. The Arp2/3 complex is the molecular machine that nucleates these branched actin networks by binding to the side of an existing filament and initiating branch formation[17,19]. The resulting two new filaments can then each be split again, creating a natural feed-forward mechanism, limited only by the supply of components, such as the Arp2/3 complex, actin monomers and activators, most of which diffuse from the cytoplasm[20]. Dang et al[14] reported that Arpin is a negative regulator of Arp2/3 activity and that cells utilize Arpin to fine-tune actin nucleation activity at the leading edge of the lamellipodium to steer the cell. These authors also performed a bioinformatics search for proteins containing a highly conserved carboxy-terminal Arp2/3 binding motif. The classical Arp2/3 binding domain consists of an amphipathic а-helix, sometimes referred to as the coil region, which binds to the barbed end groove of Arp2, followed by a short acidic motif with a highly conserved tryptophan residue at position-1 or -2 relative to the carboxyl terminus[21]. The acidic motif is thought to bind to Arp3[22]. The newly identified protein, Arpin, contains a prototypical acidic motif and through binding to the Arp2/3 complex, Arpin inhibits actin filament nucleation, thereby functioning as a competitive Arp2/3 inhibitor[14].
This prospective observational study included 50 patients with SAP who were admitted to the Affiliated Hospital of Medical College, Qingdao University and Jinlin Hospital, Nanjing University between January 2012 and September 2013. Patients were recruited if the onset of upper abdominal pain was within 48 h of admission. The diagnosis of SAP was made during the first 12 h of admission, based on the presence of acute upper abdominal pain, serum amylase and/or lipase levels greater than three times the upper limit of normal, and the results of contrast-enhanced CT[23]. SAP was defined according to the Atlanta clinical criteria[23]. The severity of the disease was assessed using Acute Physiology and Chronic Health Evaluation (APACHE)-II criteria[24]. Patients were treated on the basis of standardized protocols of interdisciplinary management, including gastrointestinal decompression, intravenous (iv) fluids, nutritional support and/or organ system support. The patients received antibiotic prophylactic treatment within 48 h after SAP onset that continued until unequivocal clinical improvement. Patients with one of the following clinical findings were included in the study: (1) local complications (pancreatic necrosis, pancreatic pseudocyst, pancreatic abscess); (2) organ failure; (3) APACHE-II score > 8; (4) Ranson criteria > 3; (5) Balthazar CT grading II or above; and (6) clinical course in the first 3 d showing colonic involvement, severe abdominal distention and colonic irrigation treated by endoscopic decompression. Patients with any of the following were excluded from the study: (1) concurrent sepsis or pancreatic infection or peripancreatic infection caused by a second disease; (2) patients with acute or chronic gastrointestinal diseases; (3) patients sent directly to the intensive care unit for multiorgan failure; (4) post-endoscopic retrograde cholangiopancreatography or traumatic or operative pancreatitis; and (5) pregnancy, malignancy, immunodeficiency or moribund patients regardless of cause within 48 h prior to enrollment. Twenty healthy volunteers were recruited and acted as controls. The controls were in good health and had no history of either pancreatic or gastrointestinal disease. This study was approved by the Human Subjects Institutional Committee of Affiliated Hospital of Medical College, Qingdao University. Written informed consent was obtained from the participants.
During the first 3 d, the SAP clinical course with colonic involvement presenting with severe abdominal distention was treated with colonic irrigation and decompression with endoscopy, during which colonic mucosal tissue was obtained. Isolation of colonic mucosal cells was performed as described previously[11]. The specimens were separated into two small pieces, placed in RIPA buffer (Tris, NaCl, deoxycholic acid, Triton-X-100, sodium dodecyl sulfate, complete proteinase inhibitor mixture; Roche, Mannheim, Germany) and homogenized. Meanwhile, peripheral venous blood samples were obtained and 2 mL of blood was used to detect bacterial DNA. Samples of colonic mucosal tissue and peripheral venous blood were obtained at the same time for the control group.
Bacterial DNA was detected as described previously[25]. Briefly, 200 μL plasma was incubated in lysozyme-proteinase K buffer for 2 h and placed into QIAamp Spin Columns (Qiagen, Hilden, Germany). A broad range polymerase chain reaction (PCR) for the amplification of a conserved region of the 16S ribosomal RNA prokaryotic gene was carried out using the following universal primers: 5’-AGAGTTTGATCATGGCTCAG-3’ and 5’-ACCGCGACTGCTGCTGGCAC-3’. The primers were located at positions 7-27 and 531-514 [Escherichia coli (E. coli) numbering]. The total PCR volume was filtered with QIAquick Spin Columns (Qiagen) to remove the remaining primers and analyzed by 2% agarose gel electrophoresis and ultraviolet visualization. The final product was purified by precipitation with ethanol acetate and analyzed with an ABIPRISM 310 Automated Sequencer (Applied Biosystems, Foster City, CA, United States). Sequences obtained were compared with the database of the National Center for Biotechnology Information (http://www.ncbi.nih.gov). DNA extracted from E. coli was used as a positive control and sterile water and PCR mixtures (without template) were used as negative controls. The limit of detection of the method was evaluated. One colony from a culture of E. coli was diluted up to 100000-fold in sterile water and DNA isolation from 200 mL of each dilution was performed. The yield and purity of DNA were measured by reading the optical densities at 260/280 nm, respectively. From each sample, 2 μL was used for PCR.
Immunofluorescent staining was carried out for Arpin, OC, CL-2 and ZO-1 in the obtained specimens. After blocking endogenous peroxidases activity to reduce nonspecific binding, slides were incubated in 1% bovine serum album for 30 min. After washing in phosphate-buffered saline, primary antibodies (rabbit anti-human Arpin antibody, rabbit anti-human CL-2 antibody: Zymed Laboratories, San Francisco; rabbit anti-OC antibody: Santa Cruz Biotechnology, Santa Cruz, CA, United States; mouse anti-ZO-2 antibody: BD, Heidelberg, Germany) were applied according to the dilutions advised by the manufacturers. Isotype staining assured specific staining results. Slides were then incubated with goat anti-rabbit Alexa546 or goat anti-mouse Alexa546 secondary antibody (Molecular Probes/Invitrogen, Karlsruhe, Germany). Nuclei were stained with 40, 6-diamidino-2-phenylindole in a mounting medium (Vectashield, Vector Laboratories). Immunofluorescent-stained sections (high-power fields) were visualized with a microscope at the indicated magnifications using fluorescent light (Axiovert, Zeiss, Goettingen, Germany).
Western blotting analyses were undertaken according to standard protocols using the following primary antibodies: rabbit anti-human Arpin antibody (1:250 dilution; Sinopharm Chemical Reagent Beijing Co.Ltd), rabbit anti-CL-2 (Zymed), mouse anti-OC (clone 19, 1:250 dilution; BD, San Diego, CA, United States) and mouse anti-ZO-2 (clone 1, 1:250 dilution; BD, San Diego, CA, United States). Membranes were blocked at room temperature for 1 h in Tris buffer saline containing 0.05% Tween 20 (TBS-T) and 5% non-fat dry milk. Nitrocellulose membranes were incubated with primary antibodies for 1 h at room temperature under slight agitation. The specific of staining was confirmed using the corresponding isotype controls. Equal loading was ensured by staining with mouse anti-b-actin antibody (clone C4, 1:3000 dilution; Chemicon, Temecula, CA, United States). After washing, the horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit IgG-HRP, 1:8,000; goat anti-mouse IgG-HRP, 1:3000 dilution; BD, San Diego, CA, United States) was added accordingly and the membrane was incubated for an additional hour under gentle shaking. Proteins were detected using the ECL-Plus Western Blotting Detection System (Amersham Life Science, Braunschweig, Germany). Protein bands were quantified by densitometry using Image-Pro Plus 6.0 (Media Cybernetics, MD, United States).
Continuous variables are expressed as the mean ± SD. Categorical variables are expressed as frequencies or percentages. Significant differences in basal characteristics between groups were analyzed using the χ2 test for categorical data and the 2-sample t test for quantitative data. The associations between Arpin and CL-2 expression in post-BT SAP, the BT ratio and APACHE-II scores were determined by linear regression analysis using Pearson’s test. One-way analysis of variance (ANOVA) was applied to compare parametric variables between the three aforementioned groups. P < 0.05 (two-sided) was considered significant. All analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL, United States).
The clinical features of the SAP patients and healthy controls are shown in Table 1. No significant difference was noted in gender or age between groups (P > 0.05). However, statistically significant differences were found in the Ranson score and APACHE-II score between SAP patients and healthy controls (P < 0.05).
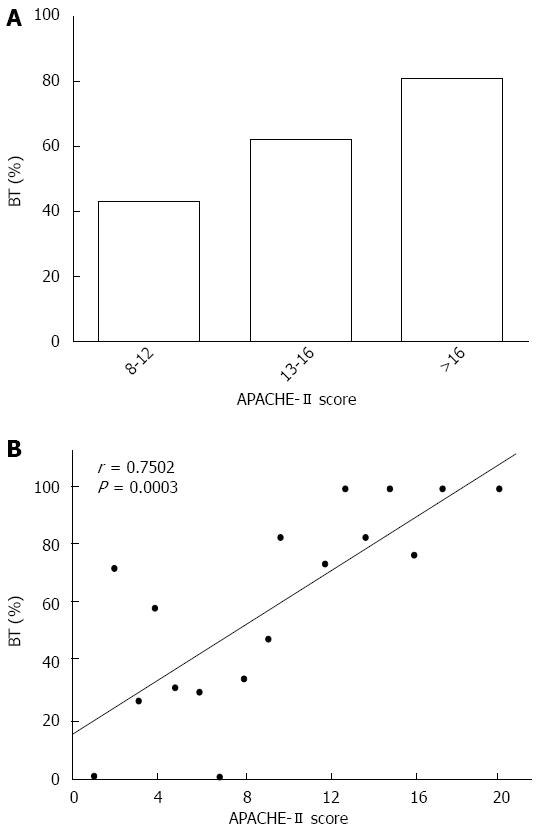
Fragments of bacterial DNA were detected in 31 of the 50 patients with SAP (62.0%) and in 1 of 20 healthy (5.0%) controls, showing significant differences between patients and healthy controls (P < 0.001). A representative photograph of a DNA agarose electrophoresis gel is shown in Figure 1. In patients with an APACHE-II score greater than 16 points, bacteria were detected in 15 patients (85.3%) (Figure 2A). For patients with an APACHE-II score of 12-14 points, DNA was identified in 68.5%. Only five of the patients (45.5%) with an APACHE-II of score 12-14 points showed evidence of bacterial DNA, which was significantly decreased compared to those with a score greater than 12 points (P < 0.01). The prevalence of bacteremia was positively correlated with the APACHE-II scores of patients with SAP (r = 0.7502; P < 0.05) (Figure 2B), suggesting a potential association between bacteremia and the severity of disease.
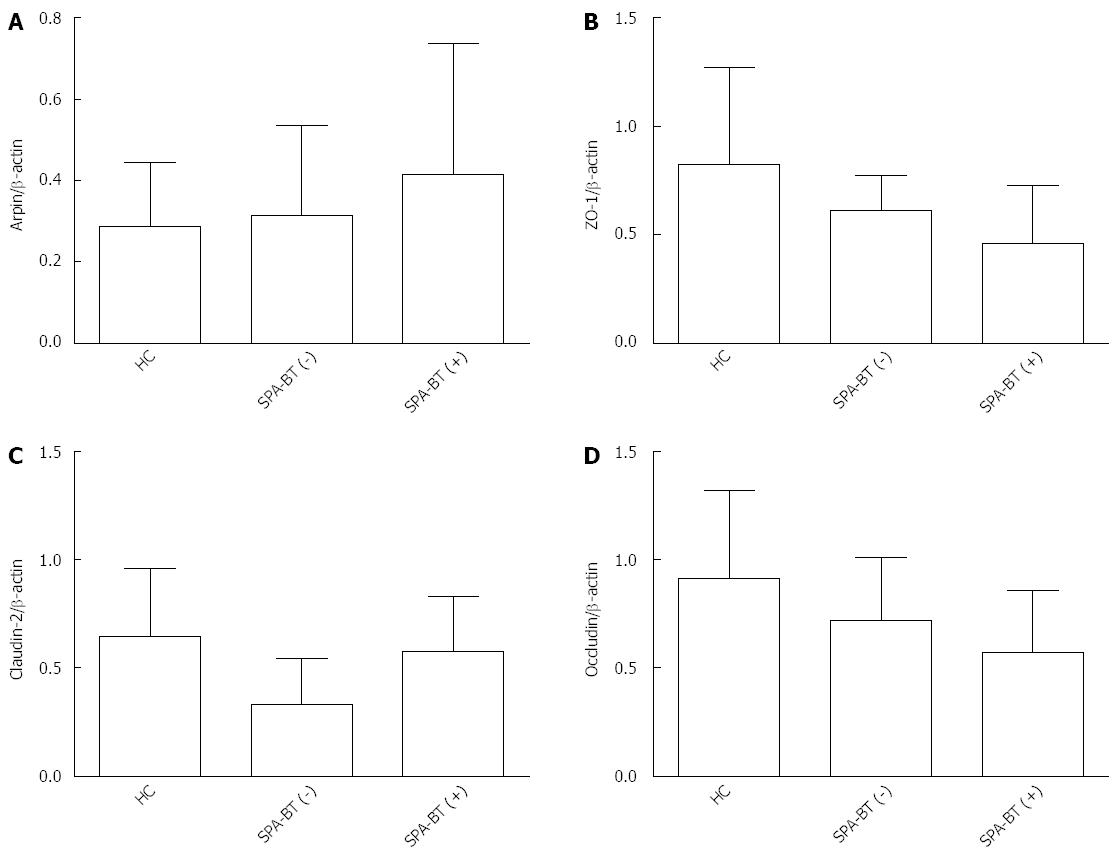
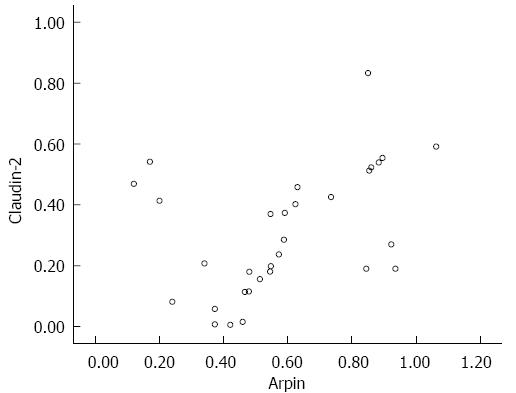
As shown in Figure 3, compared to BT (-) SAP patients, increased levels of Arpin expression were detected in tissue samples obtained from BT (+) SAP patients detected by immunofluorescence staining. Arpin protein was primarily detected between epithelial cells in the epithelium of BT (+) SAP patients. The TJ proteins, which were detected throughout the mucosa, were clearly decreased in BT (+) SAP patients, whereas more granular staining was observed in whole epithelial cells in samples from BT (+) SAP patients. Western blot analysis confirmed these differences between the groups and the details are shown in Table 2 and Figure 4. The expression levels of Arpin were 0.31 ± 0.24 in the SAP-BT (-) group and 0.61 ± 0.28 in the SAP-BT (+) group and this difference was statistically significant (P = 0.003). SAP-BT (+) patients also showed a higher expression level of CL-2 (0.64 ± 0.27 vs 0.32 ± 0.21, P = 0.005) and a lower expression level of OC (0.73 ± 0.32 vs 0.61 ± 0.28, P = 0.023) and ZO-1 (0.58 ± 0.17 vs 0.42 ± 0.26, P = 0.012) compared to SAP-BT(-) patients. Moreover, we found that the higher expression of Arpin protein in the colonic mucosa was significantly (r = 0.421, P = 0.032) associated with a higher level of CL-2 expression in SAP-BT (+) patients (Figure 5).
| HC (n = 20) | SAP | ||||
| Total (n = 50) | BT(-) (n = 19) | BT(+) (n = 31) | P value1 | ||
| Arpin | 0.28 ± 0.16 | 0.38 ± 0.19 | 0.31 ± 0.24 | 0.61 ± 0.28 | 0.003 |
| Zonula occludens-1 | 0.89 ± 0.46 | 0.48 ± 0.23 | 0.58 ± 0.17 | 0.42 ± 0.26 | 0.038 |
| Claudin-2 | 0.73 ± 0.32 | 0.49 ± 0.19 | 0.32 ± 0.21 | 0.64 ± 0.27 | 0.021 |
| Occludin | 0.85 ± 0.36 | 0.68 ± 0.21 | 0.73 ± 0.32 | 0.61 ± 0.28 | 0.010 |
In this study, SAP patients with colonic involvement were treated with endoscopy and an endoscopic biopsy was collected. In addition, peripheral blood was collected to detect bacterial DNA. Our results demonstrated a high prevalence of bacteremia in patients with SAP, indicating that the presence of circulating bacteria and their specific distribution are associated with the severity of pancreatitis. These conclusions are in accordance with the results of previous studies[26]. Bacterial translocation from the gut has been considered a central mechanism underlying the development of pancreatic infections and necrosis[27,28], although the detailed mechanism remains unknown. In this study, we also found that SAP-BT (+) patients showed higher levels of Arpin and Cl-2 expression than SAP-BT (-) patients, whereas ZO-1 and OC expression was lower in SAP-BT (+) patients. Moreover, we found that the expression of Arpin protein was also correlated with that of CL-2 protein in SAP-BT (+) patients. However, we could not determine the detailed mechanism of Arpin action and additional studies with improved methods are needed to explore this issue in the future.
Based on our results, we speculate that Arpin acts on cytoskeletal proteins of the TJ. TJs play a structural role in the regulation of intestinal permeability and may be important for BT. TJs are cell-cell adhesion structures in mucosal epithelial cell sheets and can migrate to maintain the function of the intestinal mucosal barrier[28,29]. In our research, we show that high expression of Arpin is correlated with low TJ expression in the mucosal epithelium, which suggests that Arpin acts as a competitive inhibitor of the Arp2/3 complex. To date, many proteins have been identified as components of the TJ and understanding their architectural organization and interactions is critical to understanding the biology of this barrier. CL-2, OC and ZO-1 are the most prominent proteins in TJs and their coupling to the TJ cytoskeleton is required for assembly of the junction and maintenance of the barrier[13]. In our study, the altered expression of these proteins provided important information about the function of Arpin protein. One common feature of physiological and pathological alterations of the barrier is changes to the junction-associated actin cytoskeleton. Unsurprisingly, the number of cytoskeletal proteins at the junction is large. Because Arpin was shown to act as an actin-polymerizing protein localized to TJs[14], this protein is a prime candidate for a local inhibitor, given that it functions as a competitive inhibitor of actin filament nucleation and has been shown experimentally to contribute to the collapse of lamellipodia. The absence or dysfunction of TJ protein is not sufficient to destroy the barrier and trigger pathogenesis, indicating a failure of the mechanism of the intestinal epithelial barrier and other processes. In cells subjected to ultraviolet irradiation, high temperature, osmotic pressure and a variety of stresses such as inflammation, the Rac signaling pathway is activated, which can activate downstream Arpin. Arpin then inhibits the Arp2/3 complex, leading to the formation of F-actin (produced by polymerization of inactive G-actin). When Arpin expression is elevated, inhibition of the Arp2/3 complex is enhanced, F-actin polymerization is reduced and the cytoskeleton rearranged. As our research shows, Arpin influences a variety of proteins such as CL-2, which may lead to TJ protein opening and barrier destruction. Thus, we can infer that Arpin is a negative regulator of Arp2/3 activity and that cells utilize Arpin to fine-tune actin nucleation activity at the leading edge of the lamellipodium to disrupt TJ proteins in intestinal permeability.
However, the present study had certain limitations. Due to a lack of technical assistance, we could not visualize the relationship between Arpin and TJ protein. Thus, more effective methods are needed to explore this topic more thoroughly in the future.
In summary, Arpin contributes to BT occurrence and development by disrupting the cytoskeletal proteins of TJs in patients with SAP.
Infection and sepsis are severe complications contributing to most late deaths in severe acute pancreatitis (SAP). In most patients, infection is caused by gram-negative bacteria, suggesting failure in the gut barrier function, and the intestinal epithelial barrier is composed of tight junctions (TJs) between intestinal epithelial cells. However, the detailed mechanism remains unknown. Recent work has identified an Arp2/3 interacting protein called Arpin, which restricts the rate of actin polymerization and is the latest component in the steadily expanding protein repertoire that controls cell migration. Extensive evidence has shown that protein regulates the Arp2/3 complex and TJs, promoting epidermal barrier formation. However, there were few reports about the research on intestinal mucosa. Thus, the authors undertook further investigations about the roles of the Arpin protein in the gut barrier of SAP patients.
TJs are considered among factors affecting the decision of the permeability of intestinal mucosa, with the intestinal epithelial barrier the first line of defense. Pure TJ protein expression is absent or dysfunctional but is not enough to destroy the barrier and disease, showing that there are other damage mechanisms of the intestinal epithelial barrier. In September 2013, the Duke University Medical Center reported the Arp2/3 complex in the role of the epidermal barrier, living and cell culture, and found that a lack of Arp two-thirds complex causes TJ protein expression level to be decreased and that zonula occludens-1 TJ form is flawed. For the first time, it was confirmed that the Arp2/3 complex maintains skin TJ formation and its function has important significance. The effect of TJs and the potential significance of the Arp2/3 complex in the intestinal epithelial barrier has not been further discussed in the literature.
SAP intestinal epithelial barrier damage, actin cytoskeleton and intestinal epithelium appear to be researched rarely and how skeletal actin changes in intestinal epithelial permeability increase the role of the mechanism is unclear. This is the first report about detecting Arpin proteins in the intestinal mucosa and the authors found that Arpin proteins relate to the function of intestinal mucosa, speculating that Arpin open TJs in intestinal mucosa, leading to bacterial translocation.
Arpin affects the intestinal epithelial barrier in SAP and opens TJs and TJ protein changes in the SAP regulatory mechanism of intestinal epithelial barrier damage, in order to provide a new way for revealing the pathogenesis and prevention of SAP associated intestinal epithelial barrier dysfunction. Further studies are needed to clarify the detailed mechanisms involved.
The newly discovered and reported proteins in 2013 are distributed in the intestine, pancreas and elsewhere. Arpin is implicated in cell movement and inflammation. The preliminary data suggest that the expressions of Arpin in SAP associated enterogenous infection changed but the mechanism needs to be further studied. Therefore, the authors put forward the hypothesis that the increase of Arpin opens the intestinal epithelial TJ, the reduced expression of TJ protein changes and Arpin affects the intestinal epithelial barrier function. To test this hypothesis, the roles of Arpin in SAP by means of SAP patients with the methods of Western blot and immunofluorescence were explored. The aim of the study was to clarify the mechanism of SAP associated intestinal epithelial barrier injury through open TJ and TJ protein changes caused by Arpin, in order to provide a new way for revealing the pathogenesis and prevention of SAP associated intestinal epithelial barrier dysfunction.
This report provides valuable insight into the pathophysiological mechanisms of bacterial translocation in the setting of acute pancreatitis. This is an area that remains in many ways a clinical and scientific unknown entity. The authors offer a comprehensive research method to answer the research question. Bacterial translocation is a main issue in the clinical management of severe acute pancreatitis. In this challenging scenario, molecular research could be an aid to clinicians and surgeons for a better management and in the staging of new severity predicting factors.
P- Reviewer: Boetto R, Haydock MD S- Editor: Yu J L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Schmid SW, Uhl W, Friess H, Malfertheiner P, Büchler MW. The role of infection in acute pancreatitis. Gut. 1999;45:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Nathens AB, Curtis JR, Beale RJ, Cook DJ, Moreno RP, Romand JA, Skerrett SJ, Stapleton RD, Ware LB, Waldmann CS. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [PubMed] |
| 4. | Lumsden A, Bradley EL. Secondary pancreatic infections. Surg Gynecol Obstet. 1990;170:459-467. [PubMed] |
| 5. | Tarpila E, Nyström PO, Franzén L, Ihse I. Bacterial translocation during acute pancreatitis in rats. Eur J Surg. 1993;159:109-113. [PubMed] |
| 6. | MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 290] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Gencay C, Kilicoglu SS, Kismet K, Kilicoglu B, Erel S, Muratoglu S, Sunay AE, Erdemli E, Akkus MA. Effect of honey on bacterial translocation and intestinal morphology in obstructive jaundice. World J Gastroenterol. 2008;14:3410-3415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 8. | Koh YY, Jeon WK, Cho YK, Kim HJ, Chung WG, Chon CU, Oh TY, Shin JH. The effect of intestinal permeability and endotoxemia on the prognosis of acute pancreatitis. Gut Liver. 2012;6:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Juvonen PO, Alhava EM, Takala JA. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol. 2000;35:1314-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, Rogler G, Grossmann J. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Morales Ciancio RA, Drain O, Rillardon L, Guigui P. Acute spontaneous spinal epidural hematoma: an important differential diagnosis in patients under clopidogrel therapy. Spine J. 2008;8:544-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 454] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 381] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 14. | Dang I, Gorelik R, Sousa-Blin C, Derivery E, Guérin C, Linkner J, Nemethova M, Dumortier JG, Giger FA, Chipysheva TA. Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature. 2013;503:281-284. [PubMed] |
| 15. | Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Zhou K, Muroyama A, Underwood J, Leylek R, Ray S, Soderling SH, Lechler T. Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation. Proc Natl Acad Sci USA. 2013;110:E3820-E3829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ardern H, Sandilands E, Machesky LM, Timpson P, Frame MC, Brunton VG. Src-dependent phosphorylation of Scar1 promotes its association with the Arp2/3 complex. Cell Motil Cytoskeleton. 2006;63:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197:239-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 19. | Kim DJ, Kim SH, Lim CS, Choi KY, Park CS, Sung BH, Yeo MG, Chang S, Kim JK, Song WK. Interaction of SPIN90 with the Arp2/3 complex mediates lamellipodia and actin comet tail formation. J Biol Chem. 2006;281:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Deligianni DD. MWCNTs enhance hBMSCs spreading but delay their proliferation in the direction of differentiation acceleration. Cell Adh Migr. 2014;8:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Boczkowska M, Rebowski G, Petoukhov MV, Hayes DB, Svergun DI, Dominguez R. X-ray scattering study of activated Arp2/3 complex with bound actin-WCA. Structure. 2008;16:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Ti SC, Jurgenson CT, Nolen BJ, Pollard TD. Structural and biochemical characterization of two binding sites for nucleation-promoting factor WASp-VCA on Arp2/3 complex. Proc Natl Acad Sci USA. 2011;108:E463-E471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Bradley EL. A clinically based classification system for acute pancreatitis. Ann Chir. 1993;47:537-541. [PubMed] |
| 24. | Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Li Q, Wang C, Tang C, He Q, Li N, Li J. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques*. Crit Care Med. 2013;41:1938-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127-1132. [PubMed] |
| 28. | Oláh A, Pardavi G, Belágyi T, Romics L. Preventive strategies for septic complications of acute pancreatitis. Chirurgia (Bucur). 2007;102:383-388. [PubMed] |
| 29. | Kojima T, Yamaguchi H, Ito T, Kyuno D, Kono T, Konno T, Sawada N. Tight junctions in human pancreatic duct epithelial cells. Tissue Barriers. 2013;1:e24894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |