Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3711
Peer-review started: August 17, 2014
First decision: September 27, 2014
Revised: October 7, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: March 28, 2015
Processing time: 226 Days and 3.1 Hours
AIM: To evaluate the effect of dietary cholesterol and serum total cholesterol (TC) on the risk of pancreatic cancer.
METHODS: A literature search was performed up to June 2014 in PubMed, EMBASE, China National Knowledge Infrastructure and China Biology Medical literature database for relevant articles published in English or Chinese. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated with a random-effects model.
RESULTS: We included 14 published articles with 439355 participants for dietary cholesterol, and 6 published articles with 1805697 participants for serum TC. For the highest vs lowest category of dietary cholesterol, the pooled RR (95%CI) of pancreatic cancer was 1.308 (1.097-1.559). After excluding two studies (RR > 3.0), the pooled RR (95%CI) was 1.204 (1.050-1.380). In subgroup analysis stratified by study design, the pooled RRs (95%CIs) were 1.523 (1.226-1.893) for case-control studies and 1.023 (0.871-1.200) for cohort studies. The association of dietary cholesterol with the risk of pancreatic cancer was significant for studies conducted in North America [1.275 (1.058-1.537)] and others [2.495 (1.565-3.977)], but not in Europe [1.149 (0.863-1.531)]. No significant association [1.003 (0.859-1.171)] was found between the risk of pancreatic cancer and serum TC.
CONCLUSION: Dietary cholesterol may be associated with an increased risk of pancreatic cancer in worldwide populations, except for Europeans. The results need to be confirmed further.
Core tip: Many epidemiological studies have explored the association of cholesterol with the risk of pancreatic cancer, but the results of these studies are conflicting. We conducted the current meta-analysis to evaluate the effect of dietary cholesterol and serum total cholesterol on the risk of pancreatic cancer. The results suggested that dietary cholesterol may be associated with an increased risk of pancreatic cancer. However, the finding needs to be confirmed further.
- Citation: Wang J, Wang WJ, Zhai L, Zhang DF. Association of cholesterol with risk of pancreatic cancer: A meta-analysis. World J Gastroenterol 2015; 21(12): 3711-3719
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3711.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3711
Pancreatic cancer is an uncommon but fatal malignant tumor. The overall 5-year survival rate of pancreatic cancer is less than 4%[1]. Worldwide, the estimated numbers of cases and deaths for pancreatic cancer are 277000 and 266000 in 2008[2], respectively. In the United States, the estimated numbers of new pancreatic cancer cases and deaths are 46420 and 39590 in 2014[3], respectively. Several factors have been associated with the risk of pancreatic cancer, such as age[4], body mass index (BMI)[5], smoking[6], coffee drinking[7], hepatitis B virus (HBV) and hepatitis C virus (HCV) infection[8], type 2 diabetes mellitus[9] and family history[10]. In addition, many nutritional factors, such as folate[11], fat[12] and cholesterol[13-16], might also have an influence on the risk of pancreatic cancer.
Several epidemiologic studies have been performed to evaluate the relationship between cholesterol and the risk of pancreatic cancer. Although some studies found that dietary cholesterol was associated with an increased risk of pancreatic cancer[13-15], others demonstrated no association between dietary cholesterol and the risk of pancreatic cancer[17-19]. The association between serum total cholesterol (TC) and the risk of pancreatic cancer also remains controversial[16,20,21]. So far, there is no sufficient epidemiological evidence to establish an association between the risk of pancreatic cancer and dietary cholesterol or serum TC level.
Therefore, we conducted a meta-analysis to evaluate the effect of dietary cholesterol and serum TC on the risk of pancreatic cancer.
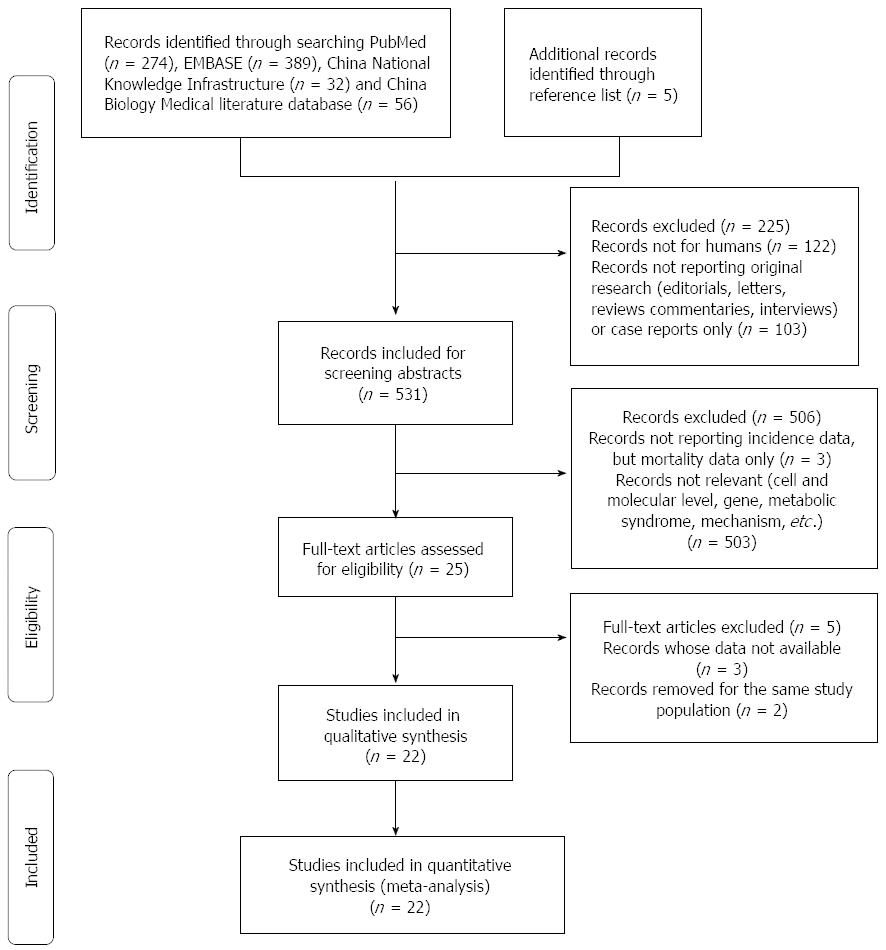
A literature search was performed up to June 2014 for relevant available articles published in English or Chinese from the following databases: (1) PubMed; (2) EMBASE; (3) China National Knowledge Infrastructure (CNKI); and (4) China Biology Medical literature database (CBM). The following search terms were used: “pancreatic cancer OR pancreatic neoplasm OR pancreatic carcinoma OR pancreatic tumour” and “cholesterol OR hypercholesterolemia”. Moreover, we reviewed the bibliographies of included articles to search additional studies not captured by our databases. The detailed steps of the literature search are shown in Figure 1.
The inclusion criteria were as follows: (1) an observational study published as an original study to evaluate the association between the risk of pancreatic cancer and dietary cholesterol and serum TC; (2) the exposure of interest was cholesterol; (3) the outcome of interest was pancreatic cancer; and (4) relative risk (RR) and 95% confidence interval (CI) (or data to calculate these) were provided. The most recent and complete study was included if data from the same population had been published repeatedly.
Two investigators (JW and LZ) searched and reviewed all identified studies independently. If the two investigators cannot reach an agreement, it was resolved by consensus with a third reviewer.
The following data were extracted from each study by two investigators (JW and LZ) independently: the first author’s name, publication year, country where the study was performed, study design, sample size and number of cases, mean age, male percentage in case (exposed) and control (unexposed) groups, RRs (we presented all results as RR for simplicity) with corresponding 95%CIs for highest vs lowest categories of cholesterol, the cut-points for cholesterol exposure and variables adjusted for in the analysis. We extracted the RRs that were adjusted for the most confounders.
Pooled measure was calculated as the inverse variance-weighted mean of the logarithm of RR with 95%CI to assess the strength of association between cholesterol and the risk of pancreatic cancer. The I2 was adopted to assess the heterogeneity between studies (I2 values of 0%, 25%, 50% and 75% represent no, low, moderate and high heterogeneity[22], respectively). The random-effects model (REM) was used as the pooling method. Meta-regression was performed to evaluate the potentially important covariates that might exert substantial impacts on between-study heterogeneity[23]. Influence analysis was performed with one study removed at a time to assess whether the results could have been affected markedly by a single study[24]. The Egger et al[25] regression asymmetry test and the funnel plot were adopted to evaluate publication bias. Subgroup analysis was performed by study design (case-control or cohort study) and continent (North America, Europe or others).
All statistical analyses were performed with STATA version 10.0 (Stata Corporation, College Station, TX, United States). All reported probabilities (P-values) were two-sided with a statistical significance level of 0.05.
For dietary cholesterol, 14 articles[13-15,17-19,26-33] with 14 studies (4 cohort studies and 10 case-control studies) were included, involving 439355 participants. For serum TC, 6 articles[16,20,21,34-36] with 8 studies (6 cohort studies and 2 case-control studies) were included, involving 1805697 participants. The detailed characteristics of the included studies are shown in Tables 1 and 2.
| Ref. | Country (year) | Study design | Mean age (case/control)Percentage of males (case/control) | Sample size (cases) | Cut-points for cholesterol exposure RR (95%CI) | Adjustment for covariates |
| Lin et al[13] | Japan | Case-control | 64.7/65.1 | 327 | Dietary cholesterol exposure (mg), < 206 (referent), 206-330, | Age and pack-years of smoking |
| -2005 | NA | -109 | > 330 [2.06 (1.11-3.85)] | |||
| Chan et al[14] | United States | Case-control | NA | 2233 | Dietary cholesterol exposure (g/d) median, 122.8 (referent), 192.6, 257.6, 368.9 [1.5 (1.1-2.0)] | Age, sex, BMI, race, education, smoking, history of diabetes and energy intake |
| -2007 | 54.7/51.9 | -532 | ||||
| Hu J et al[15] | Canada | Case-control | 61.6/57.1 | 5667 | Dietary cholesterol cut-point | Age, sex, BMI, province, education, alcohol drinking, pack year smoking, total of vegetable and fruit intake, saturated fat and total energy intake |
| -2012 | 56.2/50.5 | -628 | (mg/wk) < 966.261 (referent), 966.262-1412.753, 1412.754-1880.265, | |||
| > 1880.266 [1.57 (1.09-2.26)] | ||||||
| Howe et al[17] | Metropolitan Toronto | Case-control | 64.6/64.8 | 754 | Mean difference per day | Caloric and fibre intake, lifetime cigarette consumption |
| -1990 | 56.6/53.5 | -249 | quartile 4-quartile 1 (569 mg) [0.95 (0.51-1.75)] | |||
| Bueno de Mesquita et al[18] | Netherlands | Case-control | NA | 644 | Dietary cholesterol | Age, sex, response status, total smoking and dietary intake of energy |
| -1991 | 54.9/48.3 | -164 | [1.33 (0.72-2.45)] | |||
| Lucenteforte et al[19] | Italy | Case-control | NA | 978 | First quintile of cholesterol exposure (referent), second vs first, | Year of interview, education, tobacco smoking, history of diabetes and total energy intake |
| -2010 | 53.4/53.4 | -326 | third vs first, fourth vs first, | |||
| fifth vs first [1.10 (0.68-1.77)] | ||||||
| Baghurst et al[26] | Australia | Case-control | NA | 357 | First quintile of cholesterol exposure (referent), second vs first, third vs first, fourth vs first [3.19 (1.58-6.47)] | Age and pack-years of smoking |
| -1991 | 50.0/56.1 | -104 | ||||
| Ghadirian et al[27] | Canada | Case-control | 63.9/62.1 | 418 | First quintile of cholesterol exposure (referent), second vs first, third vs first, fourth vs first [2.24 (0.83-6.05)] | Age, sex, lifetime cigarette consumption, response status and total energy intake |
| -1995 | 54.2/51.5 | -179 | ||||
| Heinen et al[28] | The Netherlands | Case-cohort | NA | 120852 | Dietary cholesterol (mg/d), first quintile of cholesterol exposure (referent), second vs first, third vs first, fourth vs first, fifth vs first [0.78 (0.52-1.18)] | Age, sex, BMI, energy, smoking, alcohol, history of diabetes mellitus, history of hypertension, vegetables and fruits intake |
| -2009 | 52.9/49.1 | -350 | ||||
| Kalapothaki et al[29] | Greece | Case-control | NA | 362 | Dietary cholesterol (mg), an increment of about one standard deviation of the energy-adjusted residual of the corresponding nutritional variable [1.19 (0.96-1.47)] | Age, sex, hospital, past residence, years of schooling, smoking, diabetes mellitus and energy intake |
| -1993 | NA | -181 | ||||
| Michaud et al[30] | United States | Cohort | NA | 88802 | Median of cholesterol exposure (g/d) 212 (referent), 275, 322, 371, 466 [1.11 (0.67-1.83)] | Pack-years of smoking, BMI, history of diabetes mellitus, caloric intake, height, physical activity, menopausal status and glycemic load intake |
| -2003 | NA | -178 | ||||
| Nöthlings et al[31] | Hawaii and Los Angeles | Cohort | 65/60 | 190545 | Cholesterol density (mg/1000 kcal per day) median intake 56.8 (referent), 81.6, 100.4, 120.8, 156.8 [1.09 (0.89-1.32)] | Age, ethnicity, history of diabetes mellitus, familial history of pancreatic cancer, smoking status and energy intake |
| -2005 | 51.2/45.3 | -482 | ||||
| Stolzenberg-Solomon et al[32] | Finland | Cohort | 58/57 | 27111 | First quintile of cholesterol exposure (referent), second vs first, | Energy intake, age, years of smoking and energy-adjusted saturated fat intake |
| -2002 | NA | -163 | third vs first, fourth vs first, | |||
| fifth vs first [0.92 (0.53-1.59)] | ||||||
| Zatonski et al[33] | Poland | Case-control | 62.2/63.2 | 305 | First quintile of cholesterol exposure (referent), second vs first, third vs first, fourth vs first [4.31 (1.60-11.59)] | Cigarette lifetime consumption and calories |
| -1991 | 61.8/45.6 | -110 |
| Ref. | Country (year) | Study design | Mean age (case/control) Percentage of males (case/control) | Sample size (cases) | Cut-points for cholesterol Exposure RR (95%CI) | Adjustment for covariates |
| Wu et al[16] | China (2012) | Case-control | 59.3/59.358.6/58.6 | 840(210) | Serum TC < 5.70 mmol/L (referent),≥ 5.70 mmol/L [1.793 (1.067-3.013)] | Age, sex, hypertension, HBV markers, the levels of HDL, LDL, Tri and Apo B |
| Stolzenberg-Solomon et al[20] | Finland (2002) | Cohort | NA | 29048(172) | Serum TC < 5.18 mmol/L (referent),≥ 5.18 mmol/L [0.88 (0.60-1.28)] | Age, years smoked, cigarettes smoked per day, self-reported history of diabetes andbronchial asthma, occupational activity and measured high blood pressure |
| Johansen et al[21] | Austria, Norway, and Sweden (2010) | Cohort | NA | 289866(543) | Serum TC mean level (mmol/L) 4.5 (referent), 5.3, 5.8, 6.4, 7.6 [0.70 (0.53-0.93)] | Age, BMI and smoking status |
| Johansen et al[21] | Austria, Norway, and Sweden (2010) | Cohort | NA | 288834(314) | Serum TC mean level (mmol/L) 4.4 (referent), 5.1, 5.7, 6.3, 1.11 [0.75 (0.53-1.64)] | Age, BMI and smoking status |
| Kitahara et al[34] | South Korea (2011) | Cohort | NA | 756604(1799) | Serum TC (mg/dL) < 160 (referent), 160-179, 180-199, 200-239, ≥ 240 [0.88 (0.74-1.05)] | Smoking, drinking, fasting serum glucose,BMI, hypertension and physical activity |
| Kitahara et al[34] | South Korea (2011) | Cohort | NA | 433115(776) | Serum TC (mg/dL) < 160 (referent), 160-179, 180-199, 200-239, ≥ 240 [0.96 (0.74-1.24)] | Smoking, drinking, fasting serum glucose,BMI, hypertension and physical activity |
| Kuzmickiene et al[35] | Lithuania (2013) | Cohort | NA | 6788(73) | Serum TC (mmol/L) < 5.20 (referent), 5.20-5.89, 5.90-6.62, ≥ 6.63 [1.76 (0.87-3.55)] | Age, BMI, smoking status, alcohol consumption and education |
| Xu et al[36] | China (2011) | Case-control | 61.4/60.7459.3/60.5 | 602(290) | Serum TC (mmol/L) < 5.72 (referent), ≥ 5.72 [1.01 (0.88-1.17)] | Diabetes mellitus, smoking, hypertension, family history of cancer, history of gastrointestinal surgery, history of biliary disease, history of chronic pancreatitis and triglyceride |
The main results are summarized in Table 3.
| Cholesterol source | Subgroup | No. of studies | Pooled RR (95%CI) REM | I2 | Pheterogeneity |
| Dietary cholesterol | All studies | 14 | 1.308 (1.097-1.559) | 55.3% | 0.006 |
| After excluding two studies[24,31] (RR > 3.0) | 12 | 1.204 (1.050-1.380) | 29.4% | 0.158 | |
| Study design | |||||
| Case-control | 10 | 1.523 (1.226-1.893) | 49.7% | 0.037 | |
| Cohort | 4 | 1.023 (0.871-1.200) | 0.0% | 0.508 | |
| Continent | |||||
| North America | 6 | 1.275 (1.058-1.537) | 29.3% | 0.215 | |
| Europe | 6 | 1.149 (0.863-1.531) | 55.4% | 0.047 | |
| Others | 2 | 2.495 (1.565-3.977) | 0.0% | 0.362 | |
| Serum TC | All studies | 8 | 1.003 (0.859-1.171) | 55.5% | 0.028 |
| Continent | |||||
| Europe | 4 | 1.034 (0.722-1.481) | 65.1% | 0.035 | |
| Asia | 4 | 1.005 (0.847-1.192) | 56.2% | 0.077 |
Dietary cholesterol and the risk of pancreatic cancer: For the highest vs lowest category of dietary cholesterol, the pooled RR of pancreatic cancer was 1.308 (95%CI: 1.097-1.559, I2 = 55.3%, Pheterogeneity = 0.006). The pooled RRs for case-control and cohort studies were 1.523 (95%CI: 1.226-1.893, I2 = 49.7%, Pheterogeneity = 0.037) and 1.023 (95%CI: 0.871-1.200, I2 = 0.0%, Pheterogeneity = 0.508), respectively. The pooled RRs for studies conducted in North America, Europe and others were 1.275 (95%CI: 1.058-1.537, I2 = 29.3%, Pheterogeneity = 0.215), 1.149 (95%CI: 0.863-1.531, I2 = 55.4%, Pheterogeneity = 0.047) and 2.495 (95%CI: 1.565-3.977, I2 = 0.0%, Pheterogeneity = 0.362), respectively (Figure 2).
Serum TC and the risk of pancreatic cancer: Serum TC level (highest vs lowest) was not significantly associated with the risk of pancreatic cancer (RR = 1.003, 95%CI: 0.859-1.171, I2 = 55.5%, Pheterogeneity = 0.028). The pooled RRs for European and Asian populations were 1.034 (95%CI: 0.722-1.481, I2 = 65.1%, Pheterogeneity = 0.035) and 1.005 (95%CI: 0.847-1.192, I2 = 56.2%, Pheterogeneity = 0.077), respectively.
In order to explore the between-study heterogeneity, we performed univariate meta-regression with the covariates of sex, age, publication year, sample size, continent where the study was conducted and study design. For the analysis between the risk of pancreatic cancer and dietary cholesterol, study design was found to contribute significantly to the between-study heterogeneity (P = 0.037). After excluding two studies[26,33] (RR > 3.0), the heterogeneity was reduced to 29.4% (Pheterogeneity =0.158), and the pooled RR was 1.204 (95%CI: 1.050-1.380). For the analysis between the risk of pancreatic cancer and serum TC, no covariate contributed significantly to the between-study heterogeneity.
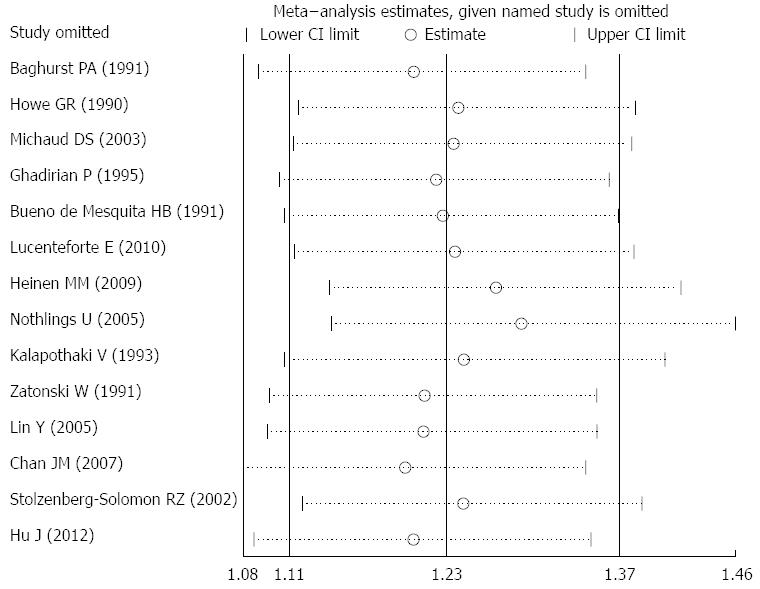
For the relationship between dietary cholesterol and the risk of pancreatic cancer, the summary RR (95%CI) ranged from 1.203 (95%CI: 1.079-1.341) to 1.291 (95%CI: 1.146-1.455) in influence analysis (Figure 3). For the relationship between serum TC and the risk of pancreatic cancer, the range was from 0.941 (95%CI: 0.840-1.054) to 1.003 (95%CI: 0.913-1.101).
Egger test and funnel plot showed no evidence of significant publication bias for the analysis between the risk of pancreatic cancer and dietary cholesterol (P = 0.107) (Figure 4) or serum TC (P = 0.204).
Recently, many studies have been performed to evaluate the association between cholesterol and the risk of pancreatic cancer. However, the results are conflicting. Generally, individual study has a relatively small sample size with insufficient power to detect the effect. Therefore, we conducted a meta-analysis to get a more reasonable conclusion. This meta-analysis, containing 439355 participants for dietary cholesterol and 1805697 participants for serum TC, can effectively assess the association of cholesterol and the risk of pancreatic cancer. Findings from this meta-analysis suggested that dietary cholesterol may be associated with an increased risk of pancreatic cancer. The association of dietary cholesterol with the risk of pancreatic cancer was significant in case-control studies, and for studies conducted in North America and others but not in Europe. No significant association between the risk of pancreatic cancer and serum TC was found in this meta-analysis.
The exact mechanism whereby high total cholesterol levels could lead to an increased risk of pancreatic cancer is unclear. There are several theories explaining the possible role of cholesterol in pancreatic cancer. Increased level of serum TC is related to increased levels of proinflammatory cytokines[37-39]. Longstanding pre-existing chronic pancreatitis is a strong risk factor for pancreatic cancer[40]. Moreover, dietary cholesterol may affect bile excretion. This may cause bile reflux into the head of the pancreas via the common duct, where most tumors occur[26,41].
Between-study heterogeneity is common in meta-analysis. It is essential to explore the potential sources of between-study heterogeneity. Diversity in a number of indeterminate characteristics such as sex, age, publication year, sample size, the continent where the study was performed or study design might be the source of between-study heterogeneity. Therefore, we explored the potential sources of the between-study heterogeneity with meta-regression. However, only study design was found to contribute to the between-study heterogeneity significantly in the analysis for dietary cholesterol. In subgroup analysis by study design, the between-study heterogeneities for case-control studies and cohort studies were reduced to 49.7% and 0.0%, respectively. After excluding two studies[26,33] (RR > 3.0) in the analysis for dietary cholesterol, the between-study heterogeneity was reduced to 29.4%, and the result did not change substantially, suggesting that the result was stable.
This meta-analysis has several strengths. First, a large number of participants were included, allowing a much greater possibility of reaching a reasonable conclusion. Second, almost all studies included in this meta-analysis were adjusted for major risk factors, such as age, sex, smoking, BMI, energy intake, making the results more credible. Third, influence analysis showed that no individual study had an excessive influence on the pooled effects of dietary cholesterol and serum TC on the risk of pancreatic cancer. Fourth, after excluding two studies[26,33] (RR > 3.0) in dietary cholesterol analysis, the between-study heterogeneity was reduced to 29.4%, but the result did not change substantially.
However, the present study has several limitations. First, unknown confounders might result in exaggerating or underestimating the risk. Second, disparate results were found between the association of dietary cholesterol and serum TC with the risk of pancreatic cancer. Third, in subgroup analysis by continent, a significant association between dietary cholesterol and the risk of pancreatic cancer was found for studies conducted in North America and others, but no association was found for those in Europe. However, the discrepancy might also be caused by the relatively small number of studies in each subgroup analysis. Fourth, results from case-control studies are susceptible to recall bias, thus prospective cohort studies that do not suffer from recall bias are believed to provide better evidence. However, only 4 cohort studies were included in this meta-analysis. Therefore, further cohort studies are warranted to confirm this association. In addition, patients might change their dietary habits after the diagnosis of pancreatic cancer; however, in most case-control studies included in this meta-analysis, the investigators collected the dietary information of participants at least 1 year before the interview. Finally, although serum TC was not found to be associated with the risk of pancreatic cancer, the blood of patients was collected after the diagnosis of pancreatic cancer in case-control studies and at the start of the study in cohort studies.
In summary, this meta-analysis suggested that dietary cholesterol may be associated with the risk of pancreatic cancer in worldwide populations, except for Europeans. The finding needs to be confirmed further.
Pancreatic cancer is an uncommon but fatal malignant tumor. Several factors have been associated with the risk of pancreatic cancer, but the association between cholesterol and the risk of pancreatic cancer is still unclear.
Until now, many epidemiological studies have explored the association of cholesterol with the risk of pancreatic cancer, but the results of these studies are conflicting.
This is the first meta-analysis to investigate the association of cholesterol with the risk of pancreatic cancer. Dietary cholesterol may be associated with an increased risk of pancreatic cancer in worldwide populations, except for Europeans.
The results of our study may give people instructions to prevent pancreatic cancer by limiting cholesterol intake.
This manuscript presents a well-designed meta-analysis that assessed the association between cholesterol and the risk of pancreatic cancer. The results suggest that dietary cholesterol may be associated with an increased risk of pancreatic cancer in worldwide populations, except for Europeans.
P- Reviewer: Huang J S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Welsch T, Kleeff J, Seitz HK, Büchler P, Friess H, Büchler MW. Update on pancreatic cancer and alcohol-associated risk. J Gastroenterol Hepatol. 2006;21 Suppl 3:S69-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 3. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 4. | Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, Fuchs CS, Gross MD, Jacobs EJ, Lacroix AZ. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med. 2010;170:791-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (2)] |
| 7. | Dong J, Zou J, Yu XF. Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2011;17:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:4234-4241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Lin HL, An QZ, Wang QZ, Liu CX. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health. 2013;127:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin ME. Nutrition and pancreatic cancer. Anticancer Res. 2014;34:9-21. [PubMed] |
| 13. | Lin Y, Tamakoshi A, Hayakawa T, Naruse S, Kitagawa M, Ohno Y. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J Gastroenterol. 2005;40:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Chan JM, Wang F, Holly EA. Pancreatic cancer, animal protein and dietary fat in a population-based study, San Francisco Bay Area, California. Cancer Causes Control. 2007;18:1153-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Wu Q, Chen G, Wu WM, Zhou L, You L, Zhang TP, Zhao YP. Metabolic syndrome components and risk factors for pancreatic adenocarcinoma: a case-control study in China. Digestion. 2012;86:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Howe GR, Jain M, Miller AB. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int J Cancer. 1990;45:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Bueno de Mesquita HB, Maisonneuve P, Runia S, Moerman CJ. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1991;48:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Lucenteforte E, Talamini R, Bosetti C, Polesel J, Franceschi S, Serraino D, Negri E, La Vecchia C. Macronutrients, fatty acids, cholesterol and pancreatic cancer. Eur J Cancer. 2010;46:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control. 2002;13:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Johansen D, Stocks T, Jonsson H, Lindkvist B, Björge T, Concin H, Almquist M, Häggström C, Engeland A, Ulmer H. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev. 2010;19:2307-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46535] [Article Influence: 2115.2] [Reference Citation Analysis (3)] |
| 23. | Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 876] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 24. | Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15-17. |
| 25. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40546] [Article Influence: 1448.1] [Reference Citation Analysis (2)] |
| 26. | Baghurst PA, McMichael AJ, Slavotinek AH, Baghurst KI, Boyle P, Walker AM. A case-control study of diet and cancer of the pancreas. Am J Epidemiol. 1991;134:167-179. [PubMed] |
| 27. | Ghadirian P, Baillargeon J, Simard A, Perret C. Food habits and pancreatic cancer: a case-control study of the Francophone community in Montreal, Canada. Cancer Epidemiol Biomarkers Prev. 1995;4:895-899. [PubMed] |
| 28. | Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer. 2009;125:1118-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Kalapothaki V, Tzonou A, Hsieh CC, Karakatsani A, Trichopoulou A, Toupadaki N, Trichopoulos D. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control. 1993;4:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Dietary meat, dairy products, fat, and cholesterol and pancreatic cancer risk in a prospective study. Am J Epidemiol. 2003;157:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst. 2005;97:1458-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Zatonski W, Przewozniak K, Howe GR, Maisonneuve P, Walker AM, Boyle P. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int J Cancer. 1991;48:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 306] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Kuzmickiene I, Everatt R, Virviciute D, Tamosiunas A, Radisauskas R, Reklaitiene R, Milinaviciene E. Smoking and other risk factors for pancreatic cancer: a cohort study in men in Lithuania. Cancer Epidemiol. 2013;37:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Xu P, Huang Q, Liu CH, Xie F, Shao F, Zhu CL, Liu L. Risk factors for pancreatic cancer: a case-control study. Tumor. 2011;31:653-657. |
| 37. | Feingold KR, Soued M, Adi S, Staprans I, Shigenaga J, Doerrler W, Moser A, Grunfeld C. Tumor necrosis factor-increased hepatic very-low-density lipoprotein production and increased serum triglyceride levels in diabetic rats. Diabetes. 1990;39:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, Lambert D, Visvikis S. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Hardardóttir I, Grünfeld C, Feingold KR. Effects of endotoxin and cytokines on lipid metabolism. Curr Opin Lipidol. 1994;5:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 41. | Mack TM, Yu MC, Hanisch R, Henderson BE. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst. 1986;76:49-60. [PubMed] |