Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3607
Peer-review started: September 18, 2014
First decision: October 29, 2014
Revised: December 3, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: March 28, 2015
Processing time: 194 Days and 14.8 Hours
AIM: To assess the diagnostic yield and safety of a deep and large biopsy technique under the guidance of endoscopic ultrasound (EUS) for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies.
METHODS: From January 2009 to March 2014, 36 patients in whom gastric infiltrating tumors had been diagnosed by EUS received negative results for malignancy after endoscopic biopsies. The deep and large biopsy technique combined bite-on-bite technique with or without endoscopic mucosal resection (EMR) to obtain submucosal tissue from lesions. EUS was used to select the appropriate biopsy sites. If the lesion protruded into the cavity, EMR was performed for removal of the overlying mucosa and then bite-on-bite technique was conducted in the resected area to obtain submucosal tissue. If the lesion appeared to be flat or was difficult to lift by injection, the bite-on-bite technique was directly used.
RESULTS: Twenty-eight of the 36 patients were treated by EMR followed by bite-on-bite technique, while 8 patients only underwent bite-on-bite technique. Histological results showed 23 of the 36 lesions were poorly differentiated adenocarcinomas, 2 diffuse large B cell lymphomas, 4 mucosa-associated lymphoid tissue-type lymphomas, and 7 undiagnosed. The deep and large biopsy technique provided a definitive and conclusive diagnosis in 29 (80.6%) of the 36 patients. The 12 gastric linitis plastica and 6 lymphoma patients received chemotherapy and avoided surgery. Minor oozing of blood in 2 mucosal resection wounds was managed by argon plasma coagulation and in 5 cases after deep biopsies by epinephrine (0.001%). Neither severe hemorrhage nor perforation occurred in any patient.
CONCLUSION: The deep and large biopsy technique is superior to ordinary endoscopic biopsy for achieving an accurate diagnosis of gastric infiltrating tumors. This procedure guided by EUS is an effective and safe diagnostic method for gastric infiltrating tumors in which endoscopic biopsy results were negative for malignancy.
Core tip: The diagnosis of gastric infiltrating tumors is challenging, which is often delayed due to negative endoscopic and histological tests. We for the first time investigated the deep and large biopsy technique for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies. This biopsy technique combined bite-on-bite technique with or without endoscopic mucosal resection. Endoscopic ultrasound was used to select the thickest site for biopsy. The biopsy provided a definitive and conclusive diagnosis in 29 (80.6%) of the 36 patients. Neither severe hemorrhage nor perforation occurred. It is an effective and safe diagnostic method for gastric infiltrating tumors with negative endoscopy biopsies.
- Citation: Zhou XX, Pan HH, Usman A, Ji F, Jin X, Zhong WX, Chen HT. Endoscopic ultrasound-guided deep and large biopsy for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies. World J Gastroenterol 2015; 21(12): 3607-3613
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3607
The most common gastric infiltrating tumors are gastric linitis plastica (GLP) and gastric lymphoma. GLP is a diffuse, infiltrating carcinoma characterized by thickening and rigidity of the stomach wall. Generally, GLP infiltrates the submucosal layer without destroying the structure of the stomach wall, and thus specific findings in the mucosal layer are insufficient for making a diagnosis[1,2]. Few patients are curable because at diagnosis the tumor is frequently advanced, with invasion of neighboring organs or distant metastasis[2]. On the other hand, primary gastric lymphoma comprises only 5% of gastric malignant tumors[3]. Most gastric lymphomas originate in the submucosa, and diagnosis via gastroscopy and forceps biopsy is often difficult[4]. The distinction between gastric lymphoma and GLP is also important for the treatment.
Endoscopic ultrasound (EUS) is a reliable nonsurgical technique for diagnosis and staging of gastrointestinal malignancies. The EUS examination has become an integral part of the pre-therapeutic evaluation in patients suspected of submucosal tumors of the upper gastrointestinal tract[5,6]. EUS can be used to ascertain the echogenicity, location, size, and depth of lesions and perigastric lymph nodes that are the diagnostic criteria for GLP or gastric lymphoma[4,7]. On EUS images, GLP is more likely to feature a pattern of vertical spread, while horizontal spread is more typical of gastric lymphoma[4,8]. Although some lesions have distinctive EUS characteristics, using these diagnostic criteria alone to distinguish lymphoma from GLP is inadequate. Consequently, tissue sampling is necessary to establish a conclusive diagnosis.
Specimens obtained from a standard endoscopic biopsy rarely provide a confirmative diagnosis because lesions in the submucosa are difficult to reach with forceps. To clarify the diagnosis, repeated biopsies or deep biopsy is required. It has been reported that the bite-on-bite technique is effective and safe for subepithelial lesions[9], but the number of cases was limited and lesions did not appear to be hypervascular or under a thick overlying epithelium.
Endoscopic mucosal resection (EMR), which recently has been widely applied for the treatment of early stomach cancer, may be useful in the diagnosis of GLP and gastric lymphoma[10]. EMR can obtain a larger tissue specimen and therefore may increase the rate of positive diagnostic findings compared with conventional biopsy. However, the procedure is associated with an increased risk of complications, including perforation and bleeding[11]. Performing EMR under the guidance of EUS may reduce operational risk and complications. However, no systematic study of EMR combined with bite-on-bite technique for diagnosis of gastric infiltrating tumors has been reported.
In the present study, we retrospectively investigated the safety and efficacy of EMR and bite-on-bite technique under the guidance of EUS for diagnosis of gastric infiltrating tumors that had been determined nonmalignant through endoscopic biopsy.
From January 2009 to March 2014 in our department, 36 patients (19 men, average age 53.5 years, age range: 31-77 years) suspected of gastric infiltrating tumors underwent deep and large biopsies guided by EUS. All patients had undergone ordinary biopsies 2 to 4 times and pathology showed negative results. During routine endoscopic examinations, among the 36 patients, 6 were asymptomatic, while 11, 6, 6, 4 and 3 patients presented with abdominal pain, gastrointestinal tract hemorrhage, abdominal circumference, obstruction, and mass, respectively. They provided informed consent for deep and large biopsies. Therapy, pathology, and image data were extracted. The institutional review board at Zhejiang University approved this study.
All patients received deep and large biopsies under the guidance of EUS. A 12-MHz probe (GF-UM 2R, Olympus, Tokyo, Japan) and two-channel endoscope (GIF-2T240, Olympus, Tokyo, Japan) were used for ultrasonographic study. The lesion was scanned after filling the stomach with water. By EUS, the location, echogenicity, and infiltrated depth of tumors were characterized, and the maximum thickness of the gastric wall, perigastric lymph nodes, and ascites were noted.
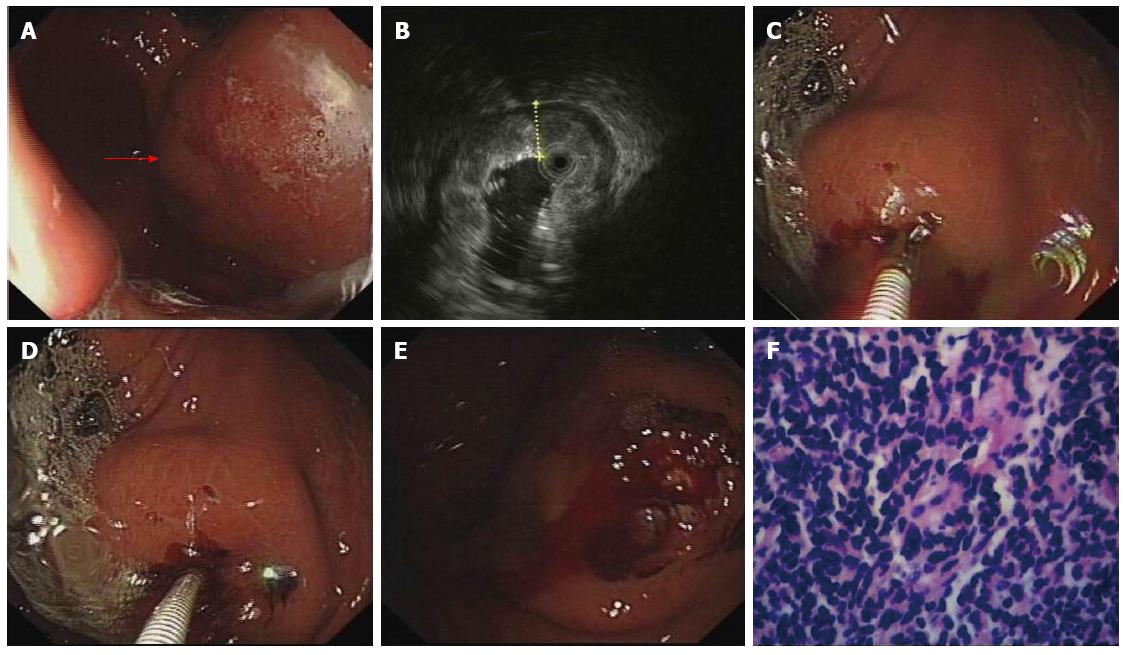
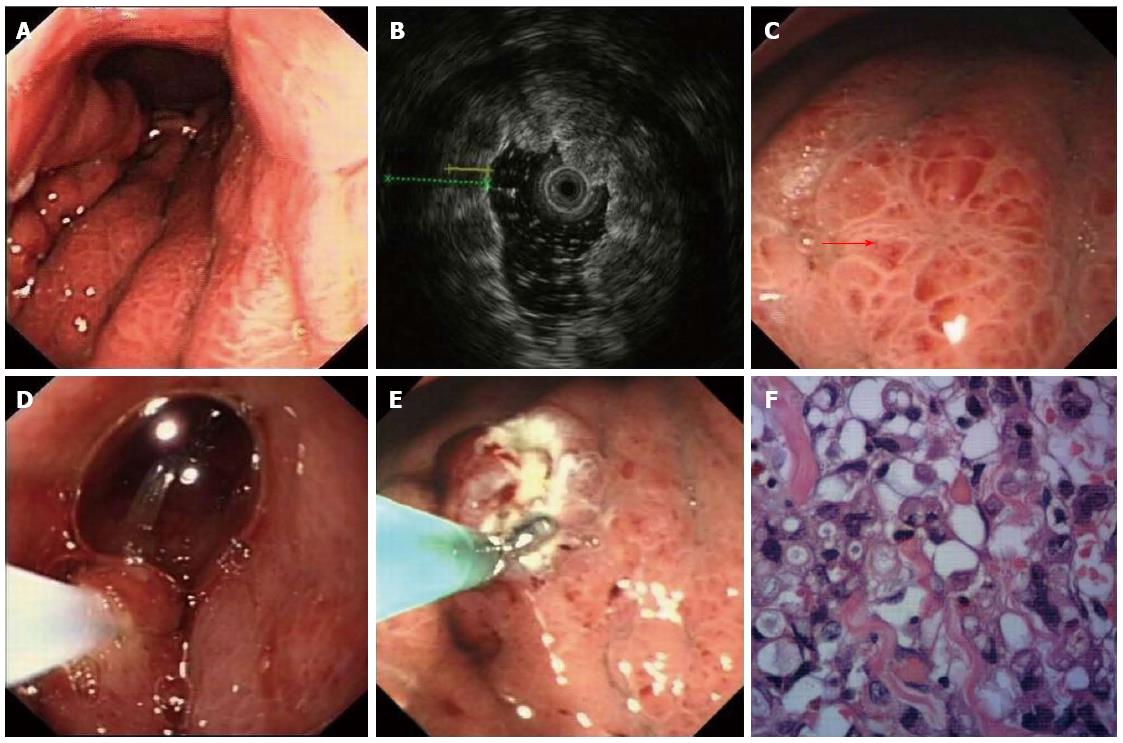
EUS was used to select the thickest site for biopsy. If the lesion protruded into the cavity, EMR was performed for removal of the overlying mucosa and then bite-on-bite technique was conducted in the resected area to obtain submucosal tissue. If the lesion appeared to be flat or was difficult to lift by injection, the bite-on-bite technique was directly used. EMR was performed with a conventional electrosurgical snare (FD-IU, Olympus, Tokyo, Japan) and an electrosurgical unit (VIO 200D, ERBE, Tubingen, Germany). The lesion was lifted by submucosal injection of indigo carmine (0.002%) and epinephrine (0.001%), and the mucosa was then resected. The bite-on-bite technique was performed as previously reported[11] using a biopsy forceps with needle (Radial jaw 3 standard capacity, Boston Scientific). Each bite was directly taken from top of the previous bite in an attempt to burrow into the lesion. Two to eight bites per lesion were performed for every patient. All specimens were sent for pathologic study, some of which were assayed by immunohistochemistry. Procedural risks and complications such as perforation and hemorrhage were recorded.
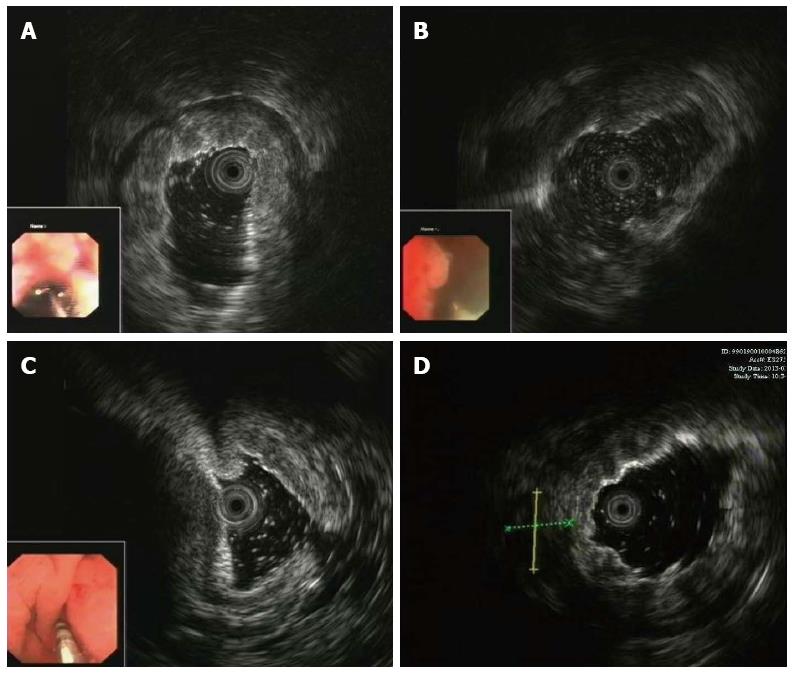
Thirty-six patients were examined using EUS, and gastric infiltrating tumors were diagnosed. The lesions were diffusely located in 13 cases, and in 10, 5, 5, and 3 cases located in the body and antrum, fundus and cardia, body only, and antrum only, respectively. EUS showed that the lesion site had been replaced by a hypoechoic or medium-echoic thickened gastric wall. In 24 of the 36 lesions, the muscularis propria was invaded and the first three sonographic layers were blurred or even indistinguishable and absent (Figure 1A), while in the remaining 12 lesions the five sonographic layers were invaded (Figure 1B). The maximum full thickness of the stomach wall ranged from 10 mm to 29 mm, with an average of 16.3 mm. Perigastric lymph nodes were seen in 3 patients and perigastric ascites in 6 patients (Figure 1C and 1D). In 2 patients, both perigastric lymph nodes and perigastric ascites were found.
The deep and large biopsy procedure was performed under the guidance of EUS to determine appropriate biopsy sites (Figures 2 and 3). The choice of EMR or bite-on-bite technique was based on the endoscopic results. Twenty-eight of the 36 patients underwent combined EMR and bite-on-bite technique (Figure 3), while the remaining 8 patients were given bite-on-bite technique alone (Figure 2). Minor oozing of blood in 2 mucosal resection wounds was managed by argon plasma coagulation (APC) and in 5 cases after deep biopsies by epinephrine (0.001%). None of the patients suffered from severe hemorrhage or perforation.
Postoperative histological results showed that 23 of the 36 lesions were poorly differentiated adenocarcinomas (including 9 signet-ring cell carcinomas), 2 diffuse large B cell lymphomas, 4 mucosa-associated lymphoid tissue-type lymphomas, and 7 of unknown type. The diagnostic yield of the bite-on-bite technique was 6 (75%) of 8, whereas that of EMR with bite-on-bite technique was 23 (82.2%) of 28. The deep and large biopsy technique provided a definitive diagnosis in 29 (80.6%) of the 36 patients (Table 1). Based on the systemic assessment, 3 GLP patients underwent surgery. Twelve unresected GLP and 6 lymphoma patients received chemotherapy, and 5 GLP patients received both surgery and chemotherapy. Three GLP patients refused treatment. Five patients with negative results underwent surgery and the pathologic results showed poorly differentiated adenocarcinomas. Two patients without a definitive diagnosis were confirmed as adenocarcinomas by endoscopy biopsies at the 3- and 6-mo follow-up, respectively.
| n | GLP | Gastric lymphoma | No diagnosis | |
| Bite-on-bite technique | 8 | 5 (62.5) | 1 (12.5) | 2 (25.0) |
| EMR combined with bite-on-bite technique | 28 | 18 (64.3) | 5 (17.9) | 5 (17.9) |
| Total | 36 | 23 (63.9) | 6 (16.7) | 7 (19.4) |
Making a diagnosis of gastric infiltrating tumors is challenging, and is often delayed due to false negative endoscopic and histological tests[1,2,12]. These tests can be false negative because lesions in the submucosa are beyond the reach of conventional-sized forceps. In cases of GLP, the false-negative rate with endoscopic mucosal forceps biopsy can be as high as 55.9%[13]. In our department, from January 2009 to March 2014 there were at least 36 patients with gastric infiltrating tumors determined by EUS, with negative malignant endoscopy biopsies. It has been shown that, for diagnosis of GLP, the accuracy of computed tomography (74.6%, 44/59) was significantly higher than that of gastroscopy (44.1%, 26/59; P < 0.001), yet this is still not effective enough for making a clear diagnosis[13].
EUS may be a viable pre-surgical diagnostic method, increasing diagnostic accuracy and safely predicting gastric infiltrating tumors on the basis of endosonographic characteristics[4,14]. According to a prospective multicenter study by Rösch et al[15], EUS had a sensitivity of 64% and a specificity of 80% in differentiating between malignant and benign submucosal tumors. However, the differential diagnosis between GLP and gastric lymphoma is not an easy task. In the present study, EUS showed that in all patients the lesion site had been replaced by a hypoechoic or medium-echoic thickened gastric wall. The invaded sonographic layered structures were blurred or even indistinguishable. Although some of these lesions had distinctive classifiable EUS features, endosonographic criteria alone were inadequate and could not confirm a clear diagnosis.
For tissue acquisition of gastric submucosal lesions, a variety of deep and large techniques have been developed, such as jumbo biopsy, EUS-guided fine needle aspiration (EUS-FNA), endoscopic submucosal resection, endoscopic submucosal dissection and the bite-on-bite technique[11,16-20]. It was reported that EUS-FNA provided a definitive diagnosis for sub-epithelial lesions in 14 (45.1%) of 31 patients, while the rate of a clearly definitive diagnosis using the jumbo biopsy forceps was 76 (58.9%) of 129 patients[17]. According to a retrospective study by Cantor et al[11], for the evaluation of sub-epithelial tumors the diagnostic yield was 17% (4/23) using the jumbo forceps and 87% (20/23) for endoscopic resection. However, these studies were performed with many limitations. EUS-FNA is not reliable for obtaining valid tissue and may be inadequate or inaccurate for diagnosis[16,18,20]. The use of jumbo forceps or EMR may increase the surface area of the tissue sample, but does not significantly increase its depth[11], and there are procedural risks and complications such as perforation and hemorrhage[17]. The bite-on-bite technique for deep biopsy of the stomach wall yields valid submucosal tissues, which may increase the accuracy rate for clear and positive diagnoses. Nevertheless, gastric infiltrating tumors usually have a thickened epithelium which may limit the use of bite-on-bite technique. In this study, we assessed the diagnostic yield of combined EMR and bite-on-bite technique for gastric infiltrating tumors that had received negative results for malignancy via endoscopy biopsies. Based on the endoscopic results, 28 of 36 patients were treated by combined EMR and bite-on-bite technique, and the other 8 patients only underwent bite-on-bite technique. The deep and large biopsy technique provided a definitive and confirmative diagnosis in 29 (80.6%) of the 36 patients.
Before planning an appropriate therapy, definitive pathology tests and results are essential for diagnosis of gastric infiltrating tumors. In the present study, based on the systemic assessment patients given a definite diagnosis underwent individualized treatment. The 12 unresected GLP and 6 lymphoma patients received chemotherapy and avoided surgery. Thus, deep and large biopsy technique helps to improve decision making in the management of gastric infiltrating tumors.
Previous studies showed that deep and large biopsy techniques for submucosal lesions have been associated with a relative risk of complications, mainly hemorrhage and perforation[11,19-22]. To reduce the complication rate, Cantor et al[11] proposed that endoscopic resection should be performed in obviously symptomatic patients (e.g., with gastrointestinal bleeding or abdominal pain or obstruction). In asymptomatic patients, it should be limited to lesions that are either malignant or suspected malignant. EUS-guided biopsy has the potential to reduce the complication rate. In our study, EUS was used to select the correct excision site. Deep and large biopsies were performed successfully in all the 36 patients. Minor oozing of blood occurred in 7 patients, which was easily managed with APC or epinephrine (0.001%) during the procedure. Neither severe hemorrhage nor perforation occurred in any patient.
In conclusion, the deep and large biopsy is superior to ordinary biopsy in its ability to achieve an accurate and positive diagnosis of gastric infiltrating tumors. The procedure guided by EUS is an effective and safe diagnostic method for gastric infiltrating tumors with negative endoscopy biopsies, and is also suitable for the diagnosis of other sub-epithelial lesions of the gastrointestinal tract. In addition, diagnostic results can provide key information for decision making in the management of gastric infiltrating tumors.
The diagnosis of gastric infiltrating tumors is challenging, which is often delayed due to false negative endoscopic and histological tests. These tests can be false negative because lesions in the submucosa are beyond the reach of conventional-sized forceps.
For tissue acquisition of gastric submucosal lesions, a variety of deep and large techniques have been developed. However, the procedures are associated with an increased risk of complications, including perforation and bleeding. In this study, we for the first time investigated the diagnostic yield and safety of a deep and large biopsy technique under the guidance of endoscopic ultrasound (EUS) for diagnosis of gastric infiltrating tumors with negative malignant endoscopy biopsies.
The deep and large biopsy was superior to ordinary biopsy in its ability to achieve an accurate and positive diagnosis of gastric infiltrating tumors. Patients received deep and large biopsies under the guidance of EUS without severe complications. The diagnostic results help patients to make decisions in their next therapies.
EUS-guided deep and large biopsy technique is an effective and safe diagnostic method for gastric infiltrating tumors with negative endoscopy biopsies, and is also suitable for the diagnosis of other sub-epithelial lesions of the gastrointestinal tract. Diagnostic results can provide key information for decision making in the management of gastric infiltrating tumors.
The deep and large biopsy technique combined bite-on-bite technique with or without endoscopic mucosal resection (EMR) technique to obtain submucosal tissue from lesions. The bite-on-bite technique was used with each bite taken from the top of the previous bite in an attempt to burrow into the lesion. Two to eight bites per lesion were performed for every patient.
The authors present important research findings and the paper is timely as findings demonstrate improvement in diagnostic approach. The article describes an elegant solution by the combination of EUS and EMR as well as “inkwell” biopsy.
P- Reviewer: Merrett ND, Pani SP, Yamagata M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Park MS, Ha HK, Choi BS, Kim KW, Myung SJ, Kim AY, Kim TK, Kim PN, Lee NJ, Lee JK. Scirrhous gastric carcinoma: endoscopy versus upper gastrointestinal radiography. Radiology. 2004;231:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Nakamura R, Saikawa Y, Wada N, Yoshida M, Kubota T, Kumai K, Kitajima M. Retrospective analysis of prognosis for scirrhous-type gastric cancer: one institution’s experience. Int J Clin Oncol. 2007;12:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | al Mofleh IA. Endoscopic features of primary upper gastrointestinal lymphoma. J Clin Gastroenterol. 1994;19:69-73; discussion 73-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Vetro C, Chiarenza A, Romano A, Amico I, Calafiore V, Di Raimondo C, Coppolino F, Di Raimondo F. Prognostic assessment and treatment of primary gastric lymphomas: how endoscopic ultrasonography can help in tailoring patient management. Clin Lymphoma Myeloma Leuk. 2014;14:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Zhou XX, Ji F, Xu L, Li L, Chen YP, Lu JJ, Wang CW, Huang W. EUS for choosing best endoscopic treatment of mesenchymal tumors of upper gastrointestinal tract. World J Gastroenterol. 2011;17:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Mortensen MB, Edwin B, Hünerbein M, Liedman B, Nielsen HO, Hovendal C. Impact of endoscopic ultrasonography (EUS) on surgical decision-making in upper gastrointestinal tract cancer: an international multicenter study. Surg Endosc. 2007;21:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Okanobu H, Hata J, Haruma K, Hara M, Nakamura K, Tanaka S, Chayama K. Giant gastric folds: differential diagnosis at US. Radiology. 2003;226:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Chen TK, Wu CH, Lee CL, Lai YC, Yang SS. Endoscopic ultrasonography in the differential diagnosis of giant gastric folds. J Formos Med Assoc. 1999;98:261-264. [PubMed] |
| 9. | Ji JS, Lee BI, Choi KY, Kim BW, Choi H, Huh M, Chung WC, Chae HS, Chung IS. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Heo J, Jeon SW. The clinical significance and management of noncurative endoscopic resection in early gastric cancer. Clin Endosc. 2013;46:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Song W, Chen CY, Xu JB, Ye JN, Wang L, Chen CQ, Zhang XH, Cai SR, Zhan WH, He YL. Pathological diagnosis is maybe non-essential for special gastric cancer: case reports and review. World J Gastroenterol. 2013;19:3904-3910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kim JI, Kim YH, Lee KH, Kim SY, Lee YJ, Park YS, Kim N, Lee DH, Kim HH, Park do J. Type-specific diagnosis and evaluation of longitudinal tumor extent of borrmann type IV gastric cancer: CT versus gastroscopy. Korean J Radiol. 2013;14:597-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Bohle W, Scheidig A, Zoller WG. Endosonographic tumor staging for treatment decision in resectable gastric cancer. J Gastrointestin Liver Dis. 2011;20:135-139. [PubMed] |
| 15. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 16. | Komanduri S, Keefer L, Jakate S. Diagnostic yield of a novel jumbo biopsy “unroofing” technique for tissue acquisition of gastric submucosal masses. Endoscopy. 2011;43:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 17. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Tae HJ, Lee HL, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS. Deep biopsy via endoscopic submucosal dissection in upper gastrointestinal subepithelial tumors: a prospective study. Endoscopy. 2014;46:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Manner H, Rabenstein T, May A, Pech O, Gossner L, Werk D, Manner N, Günter E, Pohl J, Vieth M. Long-term results of endoscopic resection in early gastric cancer: the Western experience. Am J Gastroenterol. 2009;104:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |