Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3537
Peer-review started: September 1, 2014
First decision: October 14, 2014
Revised: November 6, 2014
Accepted: December 20, 2014
Article in press: December 22, 2014
Published online: March 28, 2015
Processing time: 210 Days and 18 Hours
AIM: To investigate the effect of Qingyi decoction on the expression of secreted phospholipase A2 (sPLA2) in intestinal barrier injury.
METHODS: Fifty healthy Sprague-Dawley rats were randomly divided into control, severe acute pancreatitis (SAP), Qingyi decoction-treated (QYT), dexamethasone-treated (DEX), and verapamil-treated (VER) groups. The SAP model was induced by retrograde infusion of 1.5% sodium deoxycholate into the biliopancreatic duct of the rats. All rats were sacrificed 24 h post-SAP induction. Arterial blood, intestine, and pancreas from each rat were harvested for investigations. The levels of serum amylase (AMY) and diamine oxidase (DAO) were determined using biochemical methods, and serum tumor necrosis factor (TNF)-α level was measured by an enzyme linked immunosorbent assay. Pathologic changes in the harvested tissues were investigated by microscopic examination of hematoxylin and eosin-stained tissue sections. The expressions of sPLA2 at mRNA and protein levels were detected by reverse transcriptase PCR and Western blot, respectively. A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay was used to investigate apoptosis of epithelial cells in the intestinal tissues.
RESULTS: Compared to the control group, the expression of sPLA2 at both the mRNA and protein levels increased significantly in the SAP group (0.36 ± 0.13 vs 0.90 ± 0.38, and 0.16 ± 0.05 vs 0.64 ± 0.05, respectively; Ps < 0.01). The levels of AMY, TNF-α and DAO in serum were also significantly increased (917 ± 62 U/L vs 6870 ± 810 U/L, 59.7 ± 14.3 ng/L vs 180.5 ± 20.1 ng/L, and 10.37 ± 2.44 U/L vs 37.89 ± 5.86 U/L, respectively; Ps < 0.01). The apoptosis index of intestinal epithelial cells also differed significantly between the SAP and control rats (0.05 ± 0.02 vs 0.26 ± 0.06; P < 0.01). The serum levels of DAO and TNF-α, and the intestinal apoptosis index significantly correlated with sPLA2 expression in the intestine (r = 0.895, 0.893 and 0.926, respectively; Ps < 0.05). The levels of sPLA2, AMY, TNF-α, and DAO in the QYT, VER, and DEX groups were all decreased compared with the SAP group, but not the control group. Qingyi decoction intervention, however, gave the most therapeutic effect against intestinal barrier damage, although the onset of its therapeutic effect was slower.
CONCLUSION: Qingyi decoction ameliorates acute pancreatitis-induced intestinal barrier injury by inhibiting the overexpression of intestinal sPLA2. This mechanism may be similar to that of verapamil.
Core tip: Secreted phospholipase A2 (sPLA2) is a damage factor that stimulates excessive inflammatory responses, which can lead to the degradation and hydrolysis of biologic membranes, thus promoting epithelial injury. We demonstrate that sPLA2 is overexpressed at the mRNA and protein levels in a rat model of severe acute pancreatitis-induced intestinal barrier injury. However, a traditional Chinese medicine, Qingyi decoction, effectively antagonized this overexpression of sPLA2 to alleviate the severity of the disease. This observation was comparable to the inhibitory effect of verapamil on sPLA2 expression.
- Citation: Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol 2015; 21(12): 3537-3546
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3537.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3537
Severe acute pancreatitis (SAP) is a common surgical acute abdominal disease and can lead to the early death of patients because of associated systemic inflammatory response syndrome and multiple organ dysfunction syndrome[1]. One severe complication is often intestinal barrier damage. This permits great quantities of gut bacteria and endotoxin to enter the blood and lymphatic circulations, and eventually whole internal organs[2]. Also, the body’s mononuclear macrophage system is activated, which leads to release of large quantities of tumor necrosis factor (TNF), interferons, interleukins, and other inflammatory factors to trigger cascade of inflammatory events that further cause tissue damage[3,4].
Secreted phospholipase A2 (sPLA2), the main phospholipase A subtype, is a damage factor whose function depends on intracellular calcium ion concentration. Under the stimulus of excessive sPLA2 expression, large amounts of inflammatory factors cause the degradation of cell surface active substances and hydrolyze biologic membranes to aggravate lecithin damage in organs and tissues[5,6]. The efficacy of the Chinese medicine Qingyi decoction (QYT) has been demonstrated through clinical practice and animal experiments for years; QYT is an effective prescription for the treatment of acute pancreatitis[6]. It is generally well tolerated by patients, and induces purgation, promotes blood circulation, eliminates blood stasis, and reduces inflammation. It can also directly neutralize endotoxins and protect the intestinal barrier. In our previous study on the intervening role of QYT in patients following acute pancreatitis, it was shown that QYT administration reduced lung injury by decreasing the transcription of sPLA2, thereby protecting pulmonary function[6].
Dexamethasone (DEX) is a glucocorticoid with several beneficial functions including anti-inflammatory activity, microcirculation promotion, and oxygen free radical scavenging. Verapamil (VER) can effectively reduce tissue damage, especially intestinal damage, by reducing intracellular calcium ion concentration which plays a decisive role in sPLA2 activation[7]. VER is a commonly used calcium blocker. Considering its inhibitory effect on sPLA2, VER may be used to protect against intestinal tissue and pancreatic injuries during SAP.
The present study aimed to examine the role of sPLA2 in a rat model of SAP-induced intestinal barrier injury and the intervening roles of QYT and VER.
Fifty clean-grade healthy male Sprague-Dawley rats (180-220 g, age: 8 wk) were purchased from the specific-pathogen-free Animal Center of Dalian Medical University (Dalian, China). The animals were randomly divided into five groups (n = 10 per group): controls, untreated SAP, and SAP treated with QYT (Chinese Medicine Preparations Division, First Affiliated Hospital of Dalian Medical University, Dalian, China, Supplementary 1), DEX (Ling Rui Pharmaceutical, Zhengzhou, China), or VER (Harvest Pharmaceutical, Shanghai, China). This study was carried out in strict accordance with the recommendations in the European Union Animal Management Practices (1986). The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Dalian Medical University (Dalian, China).
The SAP intestinal barrier damage model was established using bile pancreatic duct retrograde injection of 1.5% deoxycholic acid sodium salt (Baier Di Biotechnology, Beijing, China). The rats were subject to preoperative fasting of 12 h with free access to drinking water, and administered 10% chloral hydrate anesthesia (ip, 3 mL/kg) prior to operation. Under sterile conditions, the needle of a 1 mL syringe was inserted into the major duodenal papilla of the rat and 1.5% deoxycholic acid sodium salt (1 mL/kg dose, speed of 0.1 mL/min) was injected through the bile pancreatic duct into the pancreas. The control group only had their pancreas marginally rotated to avoid any incidence of mild acute pancreatitis that could arise following the injection of the solvent (water) used to dissolve the salt. The very short time (24 h) required for the manifestation of chemically induced SAP would not permit complete resolution of such mild acute pancreatitis in the control rats, which would in turn compromise the principal clinical differences between the control and the SAP groups. The DEX group (10 mg/kg body weight/dose, 5 mg/mL concentration) and VER group (1.25 mg/kg body weight/dose, 2.5 mg/mL concentration) were given their respective drugs intravenously immediately, 6 and 12 h post-operation. The QYT group, however, was orally treated with QYT (10 mL/kg body weight/dose) 0.5 h before the induction of SAP (to permit enough time for the absorption of the traditional drug into the blood), and then 6 and 12 h post-operation. At 24 h post-operation, animals were anesthetized and abdominal aortic blood was taken for serum collection and storage at -80 °C until use. The pancreas and intestinal tissues were harvested, and part of each was either immediately stored at -80 °C or fixed in neutral phosphate formaldehyde.
Pathologic observations were made under an optical microscope (Leica DMIRB; Leica, Solms, Germany). Pancreas and intestinal tissues previously fixed in neutral phosphate formaldehyde were paraffin embedded and sectioned (2 μm serial sectioning) for pathologic morphology observation after routine hematoxylin and eosin staining.
Serum amylase content was determined using a fully automatic biochemical analyzer (Abbott Laboratories, ML, United States) from 50 μL of rat serum that was diluted six times with MilliQ (Millipore Corp, Billerica, MA, United States) water before acquisition.
Serum TNF-α level was determined using an enzyme-linked immunosorbent assay kit (Lengton Company, Shanghai, China) according to the manufacturer’s instructions.
For diamine oxidase (DAO) detection, 80 μL of serum was added to 800 μL of detection reagent (Tris-HCl, reduced coenzyme, glutamate dehydrogenase, 1.4 d diamine mixture), mixed, and incubated for 20 s, and absorbance was read at 340 nm wavelength for the value of A1. The mixture was placed in a water bath (37 °C) for 10 min, and then the absorbance at 340 nm was read again for the A2 value. The DAO activity (U/L) = {[A1 × A2]/[A2 × cuvette diameter (cm) × 6.3 × NADH mmol extinction coefficient] × [reaction liquid volume (μL)/sample volume (μL)]} × 1000.
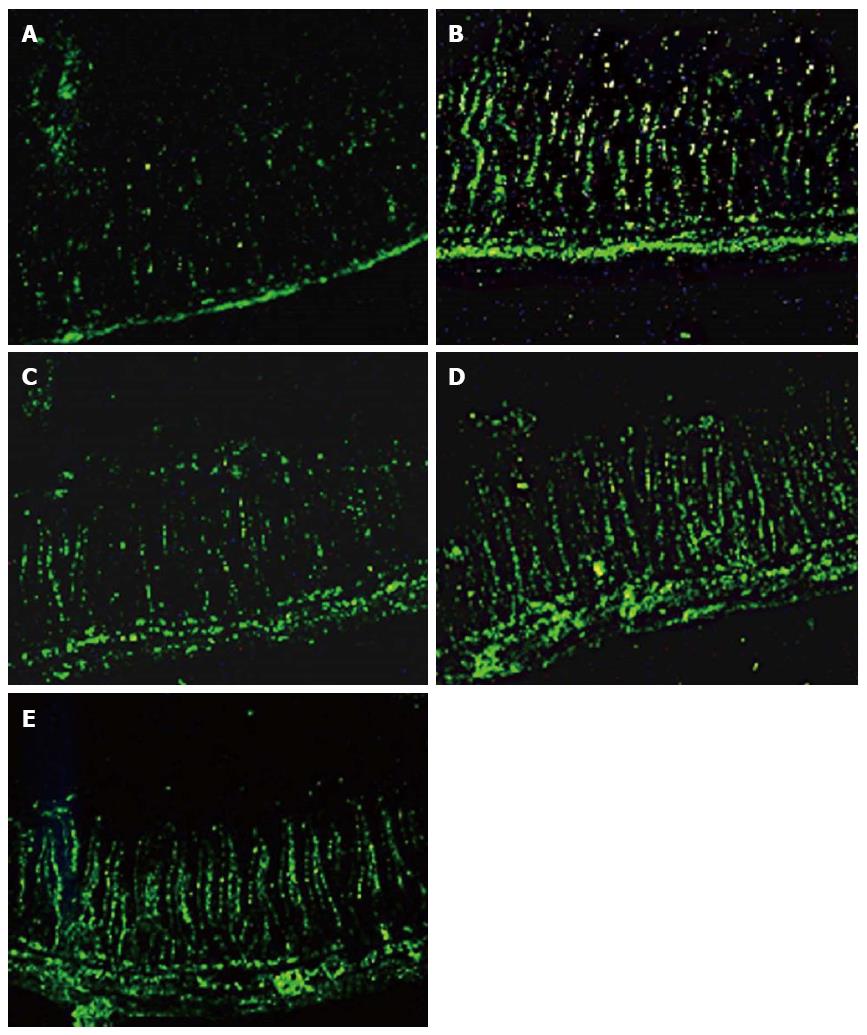
A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay kit (Keygen, Nanjing, China) was used to detect apoptosis of epithelial cells in paraffin-embedded intestinal tissues according to the manufacturer’s instructions. Under high magnification (200 ×), five randomly selected areas were observed. The apoptosis index (AI) was calculated as the percentage of fluorescein isothiocyanate-positive cells out of 500 intestinal epithelial cells.
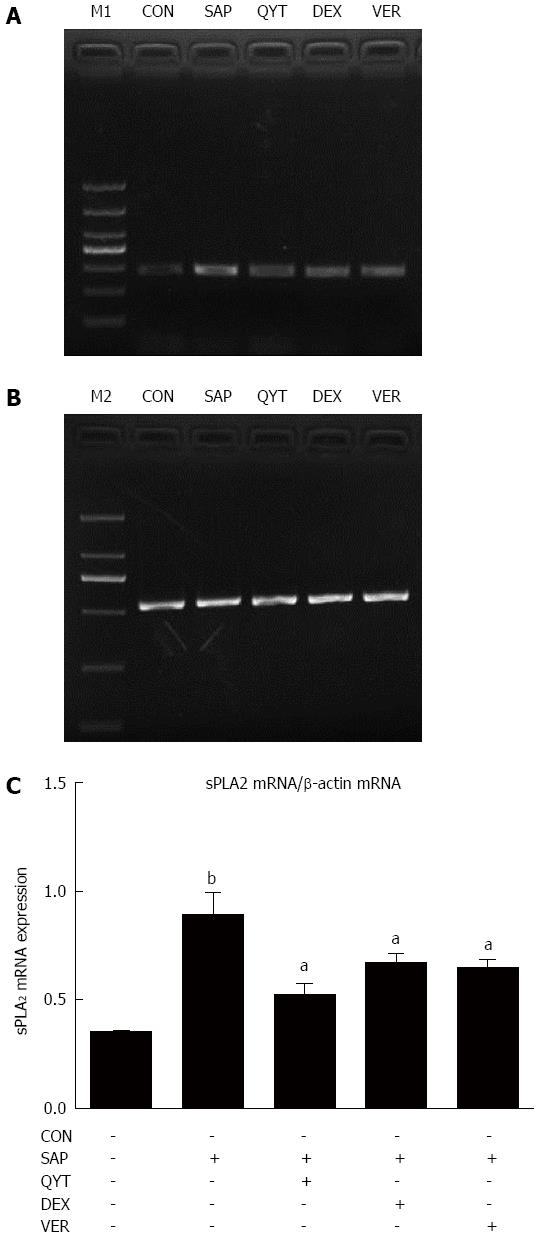
Total RNA was extracted from intestinal tissue using RNAisoPlus (Takara Bio Inc., Otsu, Shiga, Japan) according to the manufacturer’s instructions. Briefly, 1 mL of RNAisoPlus was added to 50 mg of tissue and homogenized on ice. Then 200 μL of chloroform was added and the mixture centrifuged before the supernatant was collected. Isopropyl alcohol was used to precipitate the RNA from the chloroform. Precipitated RNA was dissolved in RNase-free water and its quality measured using an ultraviolet spectrophotometer. The observed values of A260/A280 ranged between 1.8 and 2.0. The routine PCR condition included 30 cycles at an annealing temperature of 60 °C for 30 s. Primers (Takara) were as follows: sPLA2, 5′-GTGGCAGGATCCCCCAAGG-3′ (upstream), 5′-GCAACTGGGCGTGTTCCCTCTGCA-3′ (downstream), product length, 283 bp; and β-actin, 5′-GGAGTCCTGTGGCATCCACG-3′ (upstream), 5′-CTAGAAGCATTTGCGGTGGA-3′ (downstream), product length, 531 bp. An ultraviolet imaging system (Protein Simple; Alphalmade HP, Santa Clara, CA, United States) was used to read the PCR products after agarose gel electrophoresis. Intestinal tissue sPLA2 mRNA expression level was estimated as the ratio of intestinal sPLA2 mRNA gray value to its corresponding internal control (β-actin) grey value.
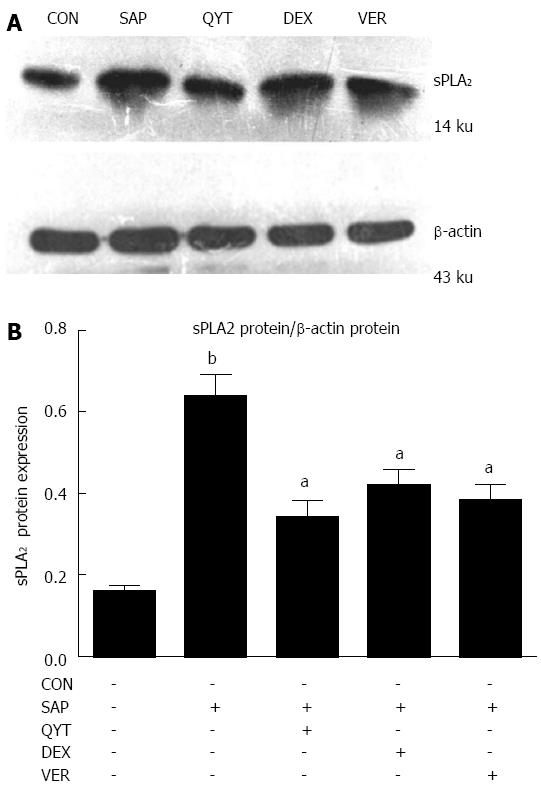
Total protein was extracted using 1 mL of RIPA lysis buffer supplemented with 1 μL protease inhibitor solution, 5 μL PMSF and 10 μL phosphatase inhibitor (all purchased from Keygen, Nanjing, China) for every 100 mg of intestinal tissue; protein (100 μg) from each sample was separated on SDS-PAGE and transferred onto a nitrocellulose membrane. Sections of the membrane were cut according to the estimated molecular weight of proteins of interest and blocked in 5% non-fat milk at 37 °C for 1 h, then incubated in blocking buffer with diluted primary antibody (sPLA2, 1:400 or β-actin, 1:1000; Santa Cruz Biotechnologies, Dallas, TX, United States) at 4 °C overnight with gentle rocking. After incubation, the membrane was rinsed three times and incubated in HRP-conjugated secondary antibody (1:10000) at 37 °C with gentle rocking for 1 h, then rinsed three times and bands visualized using enhanced chemiluminescence followed by film exposure. Band intensities were analyzed using ImageJ software, version 1.35d (National Institutes of Health, Bethesda, MD, United States).
Statistical analysis was conducted using SPSS software (version 16.0; SPSS, Inc., Chicago, IL, United States). All data are reported as mean ± SD, and analysis of variance was used for comparisons among the groups. A P < 0.05 was considered statistically significant. The Pearson product-moment correlation was used for correlation analysis.
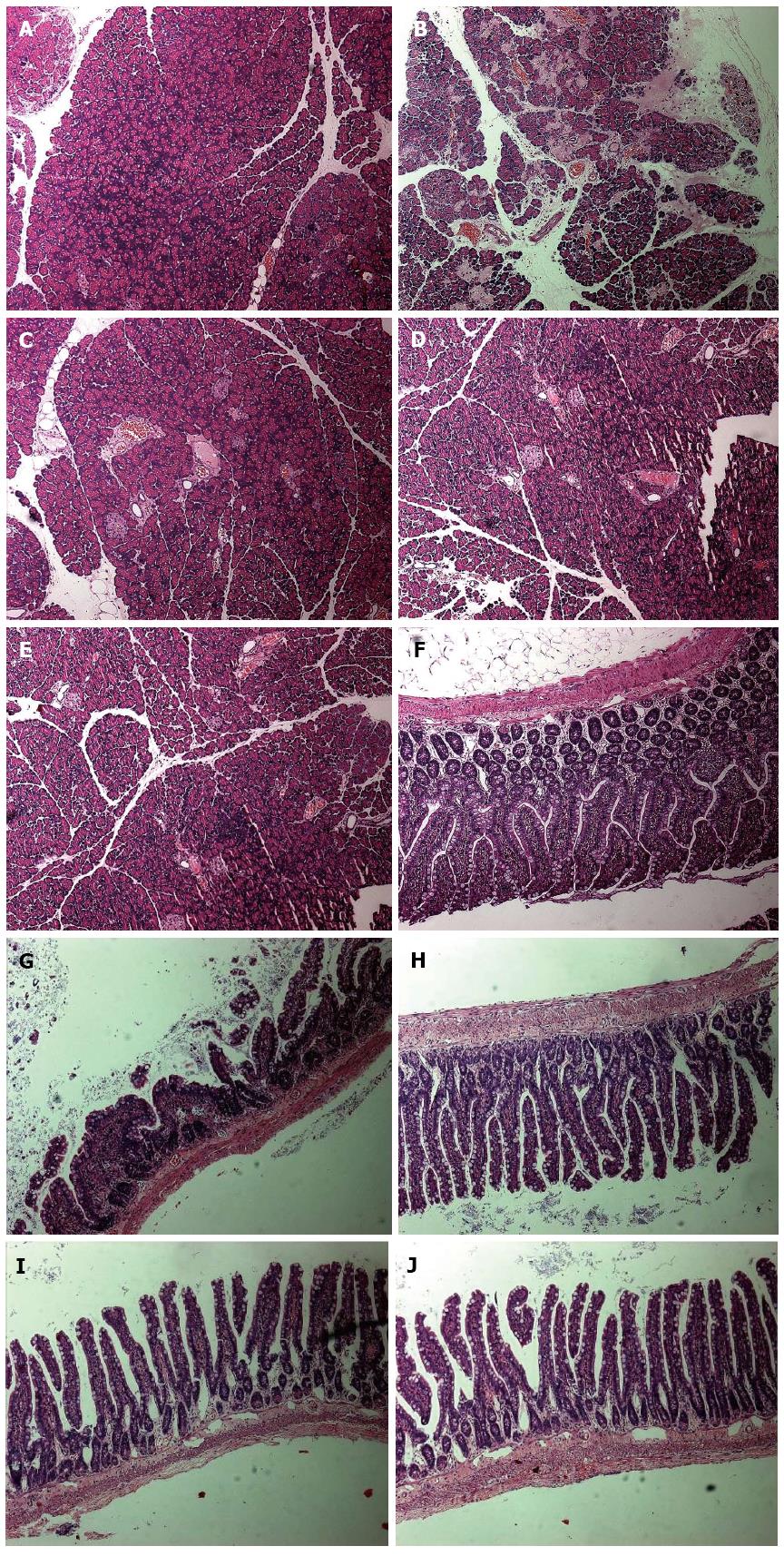
Substantial pathologic changes were observed in the pancreatic and intestinal tissues from each group of rats except the control group, where mucosa lobular structures did not exhibit edema or bleeding. Other features observed in the disease groups included blurred SAP pancreas lobular structures, large numbers of inflammatory cell infiltration, extensive hemorrhaging, and intestinal epithelial cell necrosis of > 50%. However, the pathologic damage in QYT, DEX, and VER groups was greatly reduced compared to the SAP group. The QYT and VER groups exhibited intestinal mucosal epithelial cell swelling, generally of normal morphology, whereas the DEX group, in addition to cell swelling deformation, showed varying degrees of inflammatory cell infiltration. Their pancreatic lobule structures were clear with only a small amount of edema, hemorrhage, and inflammatory cell invasion. Necrotic areas were the smallest compared to the DEX and SAP groups (Figure 1).
Compared with the control group, serum AMY levels in the SAP rats significantly increased (P < 0.01) (Table 1). However, serum AMY levels in the treated (QYT, DEX or VER) groups were significantly lower compared with the SAP group (Ps < 0.05). Furthermore, serum TNF-α levels were significantly elevated in the SAP group compared to controls (P < 0.01). The serum TNF-α levels significantly decreased in the SAP rats following QYT, DEX, or VER intervention (Ps < 0.05). Serum DAO levels in the SAP group were significantly higher than controls (P < 0.01). Compared with the SAP group, levels of serum DAO in the QYT, DEX and VER groups decreased significantly (Ps < 0.05).
| Group (n = 12) | AMY (U/L) | TNF-α(ng/L) | DAO (U/L) | AI |
| Control | 917 ± 62 | 59.7 ± 14.3 | 10.37 ± 2.44 | 0.05 ± 0.02 |
| SAP | 6870 ± 810b | 180.5 ± 20.1b | 37.89 ± 5.86b | 0.26 ± 0.06b |
| QYT | 4048 ± 511a | 122.4 ± 15.2a | 22.43 ± 2.13a | 0.13 ± 0.04a |
| DEX | 3363 ± 200a | 137.0 ± 23.4a | 24.27 ± 3.36a | 0.16 ± 0.03a |
| VER | 3852 ± 234 a | 125.2 ± 16.5a | 26.96 ± 5.56a | 0.19 ± 0.03a |
The intestinal epithelial cell AI in the SAP group was significantly higher compared with controls (P < 0.01). However, compared with the SAP group, the AIs of the QYT, DEX, or VER groups were significantly lower (Ps < 0.05). The AI of the QYT group was the most reduced among the treated cohort (Table 1) (Figure 2).
Compared with the control group, intestinal tissue sPLA2 mRNA expression in the SAP group was significantly higher (P < 0.01) (Figure 3). The sPLA2 mRNA expression level in the QYT, DEX, and VER groups, however, were significantly lower compared with the SAP group (Ps < 0.05).
The expression of sPLA2 protein in the intestinal tissue of the SAP group was significantly higher compared with the control group (P < 0.01) (Figure 4). Upon intervention with QYT, DEX, or VER in SAP rats, the protein level decreased significantly (Ps < 0.05). The inhibition of the sPLA2 protein expression was superior in the QYT treatment group. Nonetheless, the intestinal expression of sPLA2 protein in the QYT, DEX, and VER groups was significantly higher compared with controls (Ps < 0.05). The protein expression level of sPLA2 positively correlated with serum TNF-α and DAO levels in the SAP rats (Ps < 0.05) (Table 2).
| Statistic | DAO | TNF-α | AI |
| r | 0.895 | 0.893 | 0.926 |
| P-value | < 0.05 | < 0.05 | < 0.05 |
sPLA2 is widely present in mammalian tissues and cells, and functions to rebuild phospholipids, transmit signals in cell physiologic processes, and plays an important role in some diseases, such as SAP[7]. Overexpression of sPLA2 promotes a large release of arachidonic acid, prostaglandin, platelet-activating factors, and other bioactive substances[8-10]. Overexpression of sPLA2 is mainly stimulated by a large number of inflammatory mediators, and it is an important factor in intestinal ischemia-reperfusion injury[11,12]. sPLA2 can degrade phospholipid components of cell membranes, and thus directly damage the intestinal mucosa. It can also indirectly cause ischemia-reperfusion injury and deregulate the inflammatory cytokine network, and thus attack the intestinal barrier in the development and progression of SAP[13-15]. The overall consequences include decreased intestinal peristalsis and intestinal epithelial cell apoptosis and necrosis[16-18]. These activities may promote the multiplication of intestinal bacteria and their translocation into the blood, which can cause gut origin sepsis and endotoxemia[19-21]. Destruction of the intestinal mucosa mechanical barrier also results in the release of a large amount of diamine oxidase enzymes and active substances[22-24]. Wilmore et al[25] first proposed that intestinal barrier injury may lead to systemic inflammatory response syndrome and multiple organ dysfunction syndrome[26-30]. Therefore, the study of the role of sPLA2 in the pathogenesis of intestinal barrier damage in SAP is of great significance.
We found that SAP successfully developed in the rats after 24 h of disease induction, characterized by pancreatic and intestinal tissue injury, and pathologic changes. The serum levels of AMY, TNF-α, and DAO significantly increased in the SAP group compared with either the controls or any of the treated groups. Intestinal epithelial cell apoptosis index and sPLA2 expression at both the mRNA and protein levels were also significantly increased in the SAP group. Additionally, the expression level of sPLA2 positively and significantly correlated with serum TNF-α and DAO levels. The extent of damage to the intestinal barrier positively correlated with intestinal sPLA expression level, thus suggesting the involvement of sPLA2 in the process of intestinal barrier damage in SAP.
QYT, DEX and VER demonstrated the potential to reduce damage to the pancreatic and intestinal tissues. These drugs also decreased serum AMY, TNF-α, and DAO levels, the intestinal epithelial cell AI, and the expression of sPLA2 mRNA and protein. However, the interventional effect of QYT and VER were better. Thus, administration of QYT may help reduce symptoms of intestinal paralysis[31]. QYT is also suggested to promote the inhibition of intestinal phospholipase overexpression, the release of inflammatory mediators and toxic substances, and reduce the proliferation of intestinal bacteria and the effect of their endotoxin, which when in the blood, triggers systemic inflammation[9,26]. However the precise mechanism by which QYT protects intestinal damage is yet to be comprehensively elucidated.
DEX is commonly used clinically as an anti-inflammatory agent as it inhibits inflammation and inflammation promoters, decreases vascular permeability, and antagonizes phospholipase A2-induced release of platelet-activating factor to ease inflammation and reduce tissue injury[32-34]. This study used DEX as a reference drug to compare the therapeutic effects of QYT and VER on intestinal barrier damage during SAP progression in rats. Both experimental drugs effectively reduced the expression of sPLA2 at the transcriptional and translational levels as compared to the reference drug, and offered superior therapeutic effect against the extent of intestinal barrier damage. Preliminary results (data not shown) indicated that sPLA2 expression was significantly higher in intestinal mucosa than in the lung tissue of SAP rats. Thus, suggesting that the role of sPLA2 in intestinal barrier injury deserves attention.
Six hours post-operation and drug administration, the vitality of the rats in a descending order was in the DEX, QYT, and VER groups. However, 24 h post-operation, vitality was superior and disease progression was also gentler in the QYT and VER groups compared with the DEX group. The onset of QYT therapeutic effect, although slower, persisted longer compared with DEX. QYT has been widely used in clinical settings, particularly for the treatment of SAP and lung injury[35,36]. Acute pancreatitis is currently diagnosed based on clinical staging, disease evolution, and other characteristics. Its treatment may be greatly enhanced if Western orthodox medicine is combined with traditional medicinal preparations, such as QYT, for a synergistic therapeutic effect, and also to ameliorate some of the commonly associated complications, including intestinal barrier injury.
Severe acute pancreatitis (SAP) can lead to the early death of patients because of associated systemic inflammatory response syndrome and multiple organ dysfunction syndromes. However, its pathogenesis has not been fully elucidated, which has impaired the development and availability of specific clinical treatments to date.
It is currently recognized that intestinal barrier injury is the initiating factor for SAP-associated multiple organ failure.
The therapeutic function of the traditional Chinese medicine, Qingyi decoction, was comparable to the Western orthodox drug verapamil in a rat model of SAP. Qingyi decoction, although slower in onset of action, effectively inhibited the overexpression of secreted phospholipase A2 (sPLA2), which is known to play an essential pathologic role in the development of intestinal barrier injury, a common complication in SAP.
The intestinal transcription and protein expression levels of sPLA2 positively correlated with the serum levels of proinflammatory factors tumor necrosis factor-α and diamine oxidase, and therefore, may be of diagnostic and/or prognostic significance in SAP disease.
SAP remains a serious clinical problem with significant morbidity and mortality. Studies suggest that loss of the gut barrier function is instrumental in the local and systemic infectious complications associated with a severe course of the disease. Improvement of intestinal barrier function may be a useful strategy to alleviate the severity and possibility of infectious complication in SAP. This study explored the involvement of sPLA2 in intestinal barrier injury in SAP, and the intervening role of Qingyi decoction and verapamil in comparison with dexamethasone as a reference treatment. Qingyi decoction is a traditional Chinese prescription in treatment of SAP. This study is interesting and important to the field.
P- Reviewer: Pezzilli R, Urganci N, Xu CF, Yago Maria D S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Shen J, Wan R, Shen Z, Gao J, Wang X, Qian L, Lu H, Han W, Wang X. Chemokine receptor CXCR3 is involved in the acute pancreatitis-associated lung injury. Biomed Pharmacother. 2012;66:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Liu G, Zhang J, Chen H, Wang C, Qiu Y, Liu Y, Wan J, Guo H. Effects and mechanisms of alveolar type II epithelial cell apoptosis in severe pancreatitis-induced acute lung injury. Exp Ther Med. 2014;7:565-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Li Q, Wang C, Tang C, He Q, Li N, Li J. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques*. Crit Care Med. 2013;41:1938-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Wu JH, Xu GG, Guo YH. Changes of phospholipase A2 in the patients with acute pancreatitis and therapeutic effect of verapamil. Zhongguo Weizhongbing Jijiu Yixue. 2007;27:103-105. [DOI] [Full Text] |
| 6. | Zhang XM, Chen HL, Wang ZH. Expression of secretory type II phospholipase A2 in acute lung injury following acute pancreatitis and interventional effect of Qingyi decoction on it. Zhongguo Weizhongbing Jijiu Yixue. 2010;22:518-521. [DOI] [Full Text] |
| 7. | Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem J. 2005;387:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Bingham CO, Austen KF. Phospholipase A2 enzymes in eicosanoid generation. Proc Assoc Am Physicians. 1999;111:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Furue S, Hori Y, Kuwabara K, Ikeuchi J, Onoyama H, Yamamoto M, Tanaka K. Increased activity of group II phospholipase A2 in plasma in rat sodium deoxycholate induced acute pancreatitis. Gut. 1997;41:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Sham HP, Yu EY, Gulen MF, Bhinder G, Stahl M, Chan JM, Brewster L, Morampudi V, Gibson DL, Hughes MR. SIGIRR, a negative regulator of TLR/IL-1R signalling promotes Microbiota dependent resistance to colonization by enteric bacterial pathogens. PLoS Pathog. 2013;9:e1003539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | McHowat J, Liu S. Interleukin-1beta stimulates phospholipase A2 activity in adult rat ventricular myocytes. Am J Physiol. 1997;272:C450-C456. [PubMed] |
| 12. | Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 793] [Cited by in RCA: 880] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 13. | Guo ZZ, Wang P, Yi ZH, Huang ZY, Tang CW. The crosstalk between gut inflammation and gastrointestinal disorders during acute pancreatitis. Curr Pharm Des. 2014;20:1051-1062. [PubMed] |
| 14. | Siggers J, Ostergaard MV, Siggers RH, Skovgaard K, Mølbak L, Thymann T, Schmidt M, Møller HK, Purup S, Fink LN. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol. 2013;304:G864-G875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Rodrigues RS, Oliveira RA, Li Y, Zaja-Milatovic S, Costa LB, Braga Neto MB, Kolling GL, Lima AA, Guerrant RL, Warren CA. Intestinal epithelial restitution after TcdB challenge and recovery from Clostridium difficile infection in mice with alanyl-glutamine treatment. J Infect Dis. 2013;207:1505-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wang ZT, Yao YM, Xiao GX, Sheng ZY. Risk factors of development of gut-derived bacterial translocation in thermally injured rats. World J Gastroenterol. 2004;10:1619-1624. [PubMed] |
| 17. | Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26:1586-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Müller M, Colcuc S, Drescher DG, Eckardt AJ, von Pein H, Taube C, Schumacher J, Gockel HR, Schimanski CC, Lang H. Murine genetic deficiency of neuronal nitric oxide synthase (nNOS(-/-) ) and interstitial cells of Cajal (W/W(v) ): Implications for achalasia? J Gastroenterol Hepatol. 2014;29:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5259] [Cited by in RCA: 5202] [Article Influence: 208.1] [Reference Citation Analysis (0)] |
| 20. | Testini M, Gurrado A, Portincasa P, Scacco S, Marzullo A, Piccinni G, Lissidini G, Greco L, De Salvia MA, Bonfrate L. Bovine pericardium patch wrapping intestinal anastomosis improves healing process and prevents leakage in a pig model. PLoS One. 2014;9:e86627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Zhang HQ, Zhou CH, Wu YQ. Effect of emodin on small intestinal peristalsis of mice and relevant mechanism. World J Gastroenterol. 2005;11:3147-3150. [PubMed] |
| 22. | Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 24. | Luiten EJ, Hop WC, Endtz HP, Bruining HA. Prognostic importance of gram-negative intestinal colonization preceding pancreatic infection in severe acute pancreatitis. Results of a controlled clinical trial of selective decontamination. Intensive Care Med. 1998;24:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Wilmore DW, Smith RJ, O’Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917-923. [PubMed] |
| 26. | Sun JJ, Chu ZJ, Liu WF, Qi SF, Yang YH, Ge PL, Zhang XH, Li WS, Yang C, Zhang YM. Perirenal space blocking restores gastrointestinal function in patients with severe acute pancreatitis. World J Gastroenterol. 2013;19:8752-8757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ruyssers NE, De Winter BY, De Man JG, Ruyssers ND, Van Gils AJ, Loukas A, Pearson MS, Weinstock JV, Pelckmans PA, Moreels TG. Schistosoma mansoni proteins attenuate gastrointestinal motility disturbances during experimental colitis in mice. World J Gastroenterol. 2010;16:703-712. [PubMed] |
| 28. | Li HY, Yan X, Xue QL, Zhou YN, Gao Y, Wang R, Liu YM, Ran JT. Effects of nociceptin/orphanin FQ on rats with cathartic colon. World J Gastroenterol. 2007;13:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Crowther GS, Chilton CH, Todhunter SL, Nicholson S, Freeman J, Baines SD, Wilcox MH. Comparison of planktonic and biofilm-associated communities of Clostridium difficile and indigenous gut microbiota in a triple-stage chemostat gut model. J Antimicrob Chemother. 2014;69:2137-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Yue C, Wang W, Tian WL, Huang Q, Zhao RS, Zhao YZ, Li QR, Li JS. Lipopolysaccharide-induced failure of the gut barrier is site-specific and inhibitable by growth hormone. Inflamm Res. 2013;62:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Qiu Y, Li YY, Li SG, Song BG, Zhao GF. Effect of Qingyitang on activity of intracellular Ca2+-Mg2+-ATPase in rats with acute pancreatitis. World J Gastroenterol. 2004;10:100-104. [PubMed] |
| 32. | Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Potter DM, Connelly AK, Dibridge SA, Whiteside TL, Okada H. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32:2050-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Yılmaz T, Gedikli Ö, Yildirim M. Evaluation of spatial memory and locomotor activity during hypercortisolism induced by the administration of dexamethasone in adult male rats. Brain Res. 2015;1595:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Morley SC. The actin-bundling protein L-plastin supports T-cell motility and activation. Immunol Rev. 2013;256:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Wang G, Chen HL, Ren F, Li J, Li YQ. [Expression of Cav-1, AQP1 and AQP5 in lung of acute pancreatitis-associated lung injury rats and the therapeutic role of Qingyitang]. Zhonghua Yi Xue Zazhi. 2010;90:2564-2569. [PubMed] |
| 36. | Chen YF, Sha JP, Wu ZM. Synergetic effect of yihuo qingyi decoction (see text) and recombinant staphylokinase in treatment of severe acute pancreatitis of rats. J Tradit Chin Med. 2011;31:103-106. [PubMed] |