Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3429
Peer-review started: September 19, 2014
First decision: October 29, 2014
Revised: November 17, 2014
Accepted: December 22, 2014
Article in press: December 22, 2014
Published online: March 21, 2015
Processing time: 185 Days and 2.5 Hours
A 61-year-old male from Northeast China presented with a 2-mo history of abdominal distension, pruritus and jaundice. Laboratory testing revealed an elevated serum IgG4 level. A computed tomography scan showed a typical feature of autoimmune pancreatitis (AIP) and cholecystocholangitis. Early gastric cancer was incidentally discovered when endoscopic untrasound-guided fine needle aspiration (EUS-FNA) of the pancreas was carried out. The patient underwent radical subtotal gastrectomy for gastric cancer combined with cholecystectomy. Helicobacter pylori (H. pylori) and IgG4-positive plasmacytes were detected in gastric cancer tissue, pancreatic EUS-FNA sample and resected gallbladder specimen by immunohistochemistry. The patient was diagnosed with H. pylori-positive IgG4-related AIP and sclerosing cholecystocholangitis as well as H. pylori-positive gastric cancer. He responded well to steroid therapy and remains healthy with no signs of recurrence at one year follow-up. We speculate that H. pylori might act as a trigger via direct or indirect action in the initiation of onset of gastric cancer and multiorgan IgG4-related disease.
Core tip: We report a rare case of a 61-year-old male patient who suffered from Helicobacter pylori (H. pylori)-positive IgG4-related autoimmune pancreatitis and sclerosing cholecystocholangitis as well as H. pylori-positive gastric cancer. The patient responded well to corticosteroid therapy after he underwent radical subtotal gastrectomy for gastric cancer combined with cholecystectomy. This report supports the role of H. pylori in the initiation of onset of gastric cancer and multiorgan IgG4-related disease.
-
Citation: Li M, Zhou Q, Yang K, Brigstock DR, Zhang L, Xiu M, Sun L, Gao RP. Rare case of
Helicobacter pylori -positive multiorgan IgG4-related disease and gastric cancer. World J Gastroenterol 2015; 21(11): 3429-3434 - URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3429
IgG4-related disease (IgG4-RD) is a recently recognized disorder that is characterized by the enlargement of involved organs, elevated levels of serum IgG4, and abundant infiltration of IgG4-positive plasmacytes in the affected organs[1]. Autoimmune pancreatitis (AIP) has been divided into types 1 and 2. Type 1 AIP is the pancreatic manifestation of IgG4-RD[2]. IgG4-associated cholangitis and chronic sclerosing sialadenitis are other common manifestations of IgG4-RD[3]. Most cases of AIP in Japan and Korea are of the type 1 form, whereas type 2 is quite rare[2]. The prevalence and clinical features of AIP and other forms of IgG4-RD in China have yet to be fully clarified.
Recent investigations from Japan indicate that the standardized incidence ratio for malignances in IgG4-RD patients is higher than that in the general population and that the affected cancerous tissues can be infiltrated by IgG4 positive plasmacytes to various degrees[4,5]. On the contrary, the latest report from the United States indicates that cancer risk before and after diagnosis of AIP is similar to that in control subjects[6]. Malignancies in patients with IgG4-RD have included lung cancer, colon cancer, prostate cancer and lymphoma[4,6-9]. The question of whether synchronous carcinoma and IgG4-RD have a true association or are the result of a nonspecific peri-cancerous IgG4 reaction remains to be clarified.
Infection with Helicobacter pylori (H. pylori) has been shown to play a major role in gastric carcinogenesis[10,11]. The interplay of infectious agents with other etiological factors such as the genetic susceptibility of the host or the external environment is becoming increasingly recognized as an important component in the occurrence of gastric cancer. Over the last decade, H. pylori was thought to contribute to the development of AIP through induction of autoimmunity and apoptosis[12,13]. However, the relationship between H. pylori infection and multiorgan IgG4-RD has yet to be clarified.
In this report, we describe a rare case of concurrent H. pylori-positive gastric cancer and multiorgan IgG-RD from northeast China.
A 61-year-old male from northeast China presented with a two-month history of abdominal distension, pruritus, jaundice and a 25-pound weight loss. The patient denied any history of alcohol, tobacco, or illicit drug use. On physical examination, the patient had yellow staining of the skin and sclera. He had mild epigastric tenderness to deep palpation.
Routine blood tests showed a high percentage (11%) of eosinophils in the white blood cell count, and the erythrocyte sedimentation rate (ESR) was markedly raised (89 mm/h). Serum biochemical data on admission were as follows: total bilirubin 179 μmol/L, total bile acids 184.1 μmol/L, alkaline phosphatase 487 U/L, alanine aminotransferase (ALT) 319 U/L, aspartate aminotransferase (AST) 128 U/L, amylase 183 U/L, and lipase 127 U/L. Serum immunological testing displayed high levels of IgG4 (17.5 g/L) and IgG (18.1 g/L).
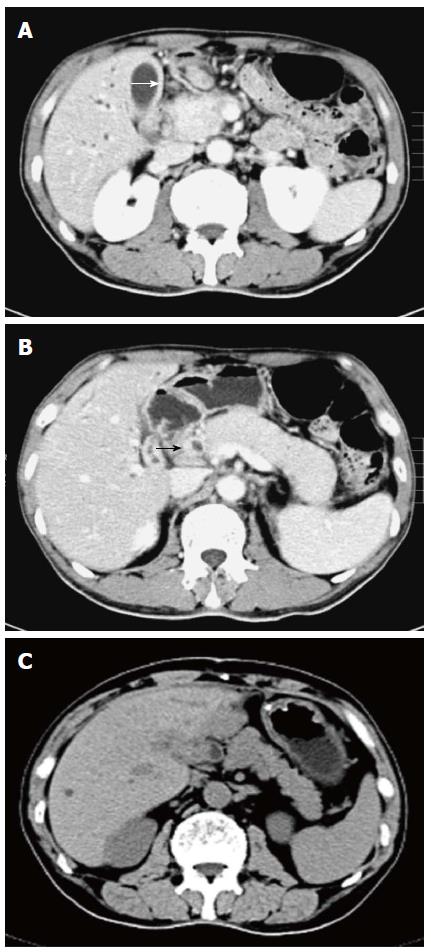
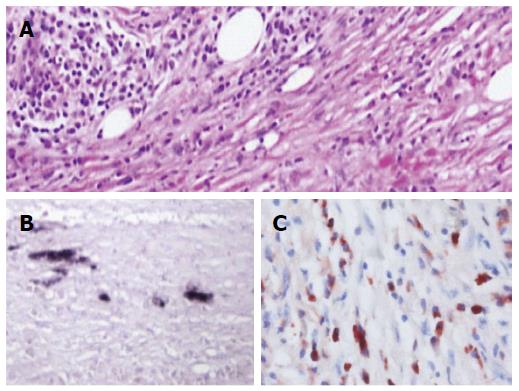
A computed tomography scan of the abdomen revealed diffuse gallbladder wall thickening and intrahepatic bile duct dilatation (Figure 1A), a patchy thickening of the distal common bile duct and diffuse enlargement of the pancreas with loss of lobulation, consistent with AIP (Figure 1B). Endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNA) of the pancreas revealed dense lymphoplasmacyte infiltration and storiform-type fibrosis (Figure 2A). Immunohistochemical staining showed several H. pylori-positive cells (Figure 2B) and numerous IgG4-positive plasma cells (Figure 2C) in the needle specimen sections of the patient, which met the diagnostic criteria for IgG4-related AIP with H. pylori infection.
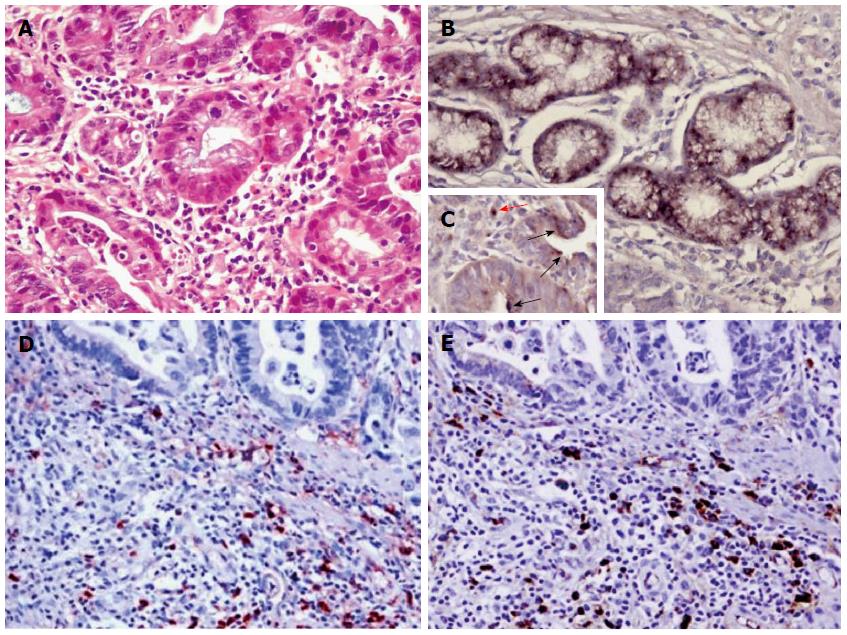
Endoscopic biopsy specimens from the pylorus showed an early moderately differentiated gastric adenocarcinoma limited to the mucosal and submucosal layers with abundant infiltration of lymphoplasmacytes and eosinophil cells in the tumor stroma by HE staining (Figure 3A), as well as the presence of H. pylori in the epithelial cells, cancer cells or mesenchymal cells by immunohistochemistry (Figure 3B, C). In contrast, only sparse and patchy IgG4-positive or IgG-positive plasma cells were seen in the tumor stroma by immunohistochemical staining (Figure 3D, E). Neither dense fibrosis nor phlebitis was observed in the gastric specimen of the patient (Figure 3A).
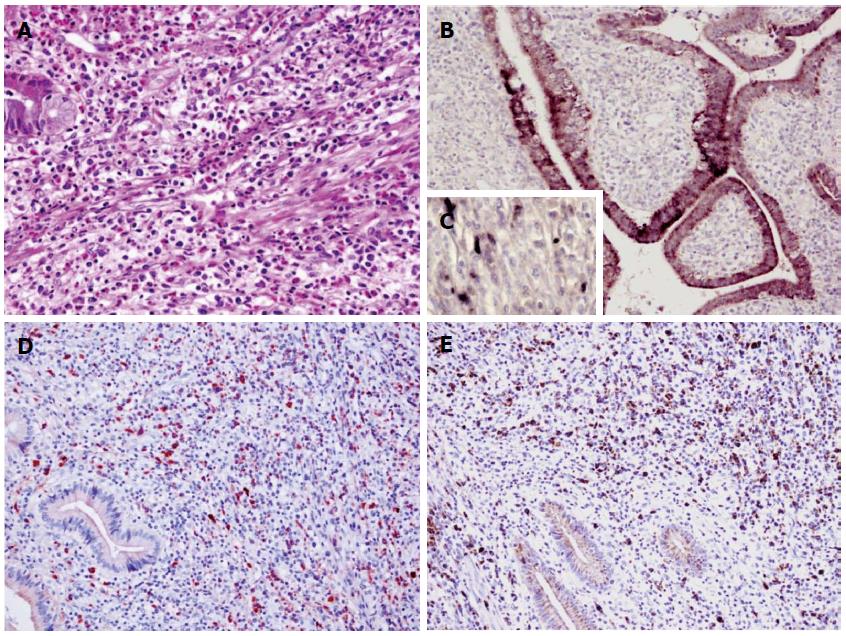
The patient underwent radical subtotal gastrectomy for gastric cancer combined with cholecystectomy and T-tube drainage on the 14th day after admission. HE staining of the resected gallbladder specimen revealed numerous lymphoplasmacyte and eosinophil cell infiltration as well as fibrosis (Figure 4A). Immunohistochemical staining showed presence of H. pylori in the cholecyst epithelial cells or mesenchymal cells (Figure 4B, C). Numerous IgG4-positive plasmacytes were evident in the cholecystectomy specimen, with a ratio of IgG4/IgG-positive plasmacytes of more than 40%, which met the diagnostic criteria for IgG4-related sclerosing cholecystitis (Figure 4D, E).
On the 3rd day after surgery, the patient was diagnosed with H. pylori-positive multiorgan IgG4-RD. He received a first-line therapy (a proton pump inhibitor, clarithromycin and metronidazole) for eradication of H. pylori and 40 mg/d of prednisone for seven days without any side effects, and was then discharged with the same steroid dose alone for the following 3 wk. After 4 wk of daily oral prednisone therapy, the patient exhibited no signs of either abdominal distension or body itching. Abnormal liver function test results returned to normal levels. Elevated serum levels of amylase and lipase returned to normal value ranges. 14C-urea breath test was negative for H. pylori. The enlarged pancreas returned to its normal size and lobulated form (Figure 1C). The patient received a long-term maintenance dose of 10 mg/d of prednisone after steroid tapering. At 12-mo follow-up, his illness had not recurred, but the serum level of IgG4 was high (15.6 g/L).
IgG4-RD is a recently recognized systemic condition characterized by elevated serum IgG4 levels and steroid responsiveness. IgG4-RD shows organ enlargement or nodular lesions consisting of abundant infiltration of lymphocytes and IgG4-positive plasma cells (ratio of IgG4/IgG-positive plasma cells > 40%) and fibrosis in various organs[5]. A histological diagnosis of IgG4-RD requires the presence of at least two of three characteristic histological features including: (1) dense lymphoplasmacytic infiltration; (2) fibrosis arranged at least focally in a storiform pattern; and (3) obliterative phlebitis[5]. The patient in this study fully met the diagnostic criteria for IgG4-related sclerosing cholecystitis and type 1 AIP[5]. Even so, biopsy specimens from the bile duct are difficult to obtain using standard procedures, except cholangiectomy. Diagnostic criteria developed in Japan to establish IgG4-related sclerosing cholangitis include diffuse or segmental narrowing of the introhepatic and/or extrahepatic bile duct associated with the thickening of the bile duct wall and coexistence of AIP/or high serum levels of IgG4[14,15]. Thus, the patient in this study also met the diagnostic criteria for IgG4-related sclerosing cholangitis[1,14,15].
Comprehensive clinical diagnostic criteria for IgG4-related gastric disease have so far not been constituted[5,16]. Recently, Koizumi et al[16] suggested that there appeared to be two kinds of IgG4-related gastric disease. One was a gastric lesion showing marked thinking of the wall of the stomach, consisting of dense fibrosis with abundant infiltration of IgG4-positive plasma cells; the other was an IgG4-related pseudotumor in the gastric region, showing polypoid or mass-like lesions. In this study, only sparse and patchy IgG4-positive or IgG-positive plasma cells were identified in the cancer stroma and the gastric submucosa adjacent to the carcinoma (data not shown), and either storiform-type fibrosis or obliterative phlebitis was absent. Thus, our data support a peri-cancerous IgG4 reaction rather than IgG4-related gastric disease.
Since the gallbladder, common bile duct, pancreas and stomach were involved synchronously in this case, we believed there might be a common factor contributing to the pathogenesis seen in the lesions of these adjacent organs. Over the last decade, molecular mimicry of host structures by constituents of H. pylori is thought to be connected with the development of autoimmune sequelae in hepatobiliary- or pancreatic-tissue destruction through antibody cross-reactivity[13,17]. The binding motif of the HLA molecule DRB1*0405 was found to be located in the homologous segments between carbonic anhydrase II (CAII) of the human pancreas and alpha-carbonic anhydrase of H. pylori, and serum anti-CAIIAb levels in AIH were significantly higher than those in chronic pancreatitis and pancreatic adenocarcinoma[18,19]. Additionally, in the case of H. pylori infection, T cell-mediated apoptotic signals and granulocyte recruitment as well as activation of the oxidative burst were also thought to contribute to the pathogenesis of AIP[12]. In this study, the patient presented with H. pylori infection in the stomach, gallbladder, and pancreas and with abundant infiltration of IgG4-positive plasma cells in the gastric submucosa, gallbladder stroma, and pancreatic tissues. Thus, we speculate that H. pylori might act as a trigger via direct or indirect action (immune cross-response) in the initiation of onset of multiorgan IgG4-RD and that IgG4 reaction might increase the H. pylori-associated risk of developing gastric cancer. However, further identification and characterization of such cases is required to elucidate the mechanism of this association.
High serum IgG4 levels have been proposed as a marker of AIP and also showed good accuracy in distinguishing between AIP and pancreatic cancer and other autoimmune diseases[20]. Additionally, AIP patients with elevated serum IgG4 had higher incidence of jaundice at onset, more frequent diffuse pancreatic enlargement at imaging, and more frequent extrapancreatic lesions compared to those with normal serum IgG4 levels[21]. Over the last decade, the usefulness of serum IgG4 as a marker of the efficacy of steroid treatment has been paid great attention[20]. Recently, response to steroids in AIP patients was recognized regardless of serum IgG4 level, however, a long-term maintenance therapy was required more frequently amongst patients with elevated IgG4 compared to those with normal IgG4[21].
In summary, we report a rare case of H. pylori-positive multiorgan IgG4-RD and gastric cancer. This report supports the role of H. pylori in the initiation of onset of gastric cancer and multiorgan IgG4-RD. Since the cancer was discovered unexpectedly and resected successfully, a careful examination of IgG4-AIP is required to rule out the presence of gastric malignant tumor before steroid therapy.
A 61-year-old male from northeast China presented with a 2-mo history of abdominal distension, pruritus and jaundice.
The patient was diagnosed with Helicobacter pylori (H. pylori)-positive IgG4-related autoimmune pancreatitis (AIP) and sclerosing cholecystocholangitis as well as H. pylori-positive gastric cancer.
The differential diagnosis included IgG4-related gastric disease and primary sclerosing cholangitis.
The patient was diagnosed with H. pylori-positive multiorgan IgG4-related disease (IgG4-RD) as well as H. pylori-positive gastric cancer on the basis of the presence of H. pylori and IgG4-positive plasmacytes in gastric cancer tissue, pancreatic endoscopic untrasound-guided fine needle (EUS-FN) sample and resected gallbladder specimen, and a high serum IgG4 level.
A computed tomography scan revealed diffuse gallbladder wall thickening and intrahepatic bile duct dilation, a patchy thickening of the distal common bile duct and diffuse enlargement of the pancreas with loss of lobulation.
Immunohistochemistry revealed H. pylori and IgG4-positive plasmacyte infiltration in resected gastric cancer tissue and gallbladder specimen as well as pancreatic EUS-FN sample.
The patient received 30 mg/d of prednisone for 4 wk, and a long-term maintenance dose of 10 mg/d of prednisone.
The relationship between H. pylori infection and multiorgan IgG4-RD has not yet been clarified.
AIP is a form of pancreatitis with a presumed autoimmune etiology and is currently recognized as two subtypes. Type 1 AIP is a pancreatic lesion of IgG4-related disease, while type 2 AIP is related to granulocytic epithelial lesion.
This report supports the role of H. pylori in the initiation of onset of gastric cancer and multiorgan IgG4-RD. Since the gastric cancer was discovered unexpectedly and resected successfully, a careful examination of IgG4-AIP is required to rule out the presence of gastric malignant tumor before steroid therapy.
This is a well-written manuscript about a rare case with H. pylori-positive IgG4-related diseases. However, the role of H. pylori in the pathogenesis of IgG4-related disease should be more discussed. Please explain why IgG4 levels are still high after treatment and the correlation between the IgG4 levels and the clinical presentation/imaging findings.
P- Reviewer: Buzas GM, Hsu JT S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Masaki Y, Kurose N, Umehara H. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st century. J Clin Exp Hematop. 2011;51:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Notohara K, Shimosegawa T. Two clinicopathologic subtypes of autoimmune pancreatitis: LPSP and IDCP. Gastroenterology. 2010;139:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140-148; quiz e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | James B. Electrical stimulation of the brain: JRSM November 1983, p 905. J R Soc Med. 1984;77:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1774] [Article Influence: 136.5] [Reference Citation Analysis (0)] |
| 6. | Hart PA, Law RJ, Dierkhising RA, Smyrk TC, Takahashi N, Chari ST. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas. 2014;43:417-421. [PubMed] |
| 7. | Hart PA, Smyrk TC, Chari ST. IgG4-related prostatitis: a rare cause of steroid-responsive obstructive urinary symptoms. Int J Urol. 2013;20:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Fujimoto M, Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Koyanagi I, Aini W, Tsuruyama T, Date H, Haga H. Stromal plasma cells expressing immunoglobulin G4 subclass in non-small cell lung cancer. Hum Pathol. 2013;44:1569-1576. [PubMed] |
| 9. | Inoue T, Hayama M, Kobayashi S, Oyaizu T, Nakazato Y, Honma K, Chida M. Lung Cancer Complicated with IgG4-related Disease of the Lung. Ann Thorac Cardiovasc Surg. 2014;20 Suppl:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Figueiredo C, Garcia-Gonzalez MA, Machado JC. Molecular pathogenesis of gastric cancer. Helicobacter. 2013;18 Suppl 1:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract. 2013;2013:393015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Kountouras J, Zavos C, Chatzopoulos D. Autoimmune pancreatitis, Helicobacter pylori infection, and apoptosis: a proposed relationship. Pancreas. 2005;30:192-193. [PubMed] |
| 13. | Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Okazaki K, Uchida K, Ikeura T, Takaoka M. Current concept and diagnosis of IgG4-related disease in the hepato-bilio-pancreatic system. J Gastroenterol. 2013;48:303-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Okazaki K, Uchida K, Koyabu M, Miyoshi H, Ikeura T, Takaoka M. IgG4 cholangiopathy - current concept, diagnosis, and pathogenesis. J Hepatol. 2014;61:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Koizumi S, Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, Endo Y, Kuwata G, Koizumi K, Shimosegawa T. Immunoglobulin G4-related gastrointestinal diseases, are they immunoglobulin G4-related diseases? World J Gastroenterol. 2013;19:5769-5774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Zen Y, Nakanuma Y. Pathogenesis of IgG4-related disease. Curr Opin Rheumatol. 2011;23:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741-744. [PubMed] |
| 19. | Talar-Wojnarowska R, Gąsiorowska A, Olakowski M, Dranka-Bojarowska D, Lampe P, Śmigielski J, Kujawiak M, Grzegorczyk J, Małecka-Panas E. Utility of serum IgG, IgG4 and carbonic anhydrase II antibodies in distinguishing autoimmune pancreatitis from pancreatic cancer and chronic pancreatitis. Adv Med Sci. 2014;59:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Morselli-Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol. 2009;24:15-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Matsubayashi H, Sawai H, Kimura H, Yamaguchi Y, Tanaka M, Kakushima N, Takizawa K, Kadooka M, Takao T, Hebbar S. Characteristics of autoimmune pancreatitis based on serum IgG4 level. Dig Liver Dis. 2011;43:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |