Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3402
Peer-review started: October 6, 2014
First decision: October 29, 2014
Revised: November 12, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: March 21, 2015
Processing time: 166 Days and 0 Hours
Although endoscopic ultrasound (EUS)-guided drainage has become the standard procedure for pancreatic pseudocysts in recent years and is generally regarded as a safe and effective method, there have been few reports of EUS-guided drainage of postoperative intra-abdominal abscesses. Here we report our experience with 4 cases of postoperative intra-abdominal abscesses for which EUS-guided drainage was performed between May 2011 and May 2014. Distal pancreatectomy had been performed in 3 cases, whereas low anterior resection for rectal cancer was performed in the remaining case. All patients underwent transgastric naso-cystic drainage, which resulted in clinical improvement without complications, even when performed within 4 wk after surgery. On average, the naso-cystic drain was removed 10 d after placement, with no abscess recurrence. Based on these findings, we believe that EUS-guided drainage of postoperative intra-abdominal abscesses is a safe and effective method, although further large-scale investigations are required to confirm our findings.
Core tip: There have been few reports of endoscopic ultrasound (EUS)-guided drainage of postoperative intra-abdominal abscesses, although EUS-guided drainage has become the standard procedure for pancreatic pseudocysts in recent years. Here we report our experience with 4 cases. Transgastric naso-cystic drainage was performed for all patients and resulted in clinical improvement without complications, even when performed within 4 wk after surgery. On average, the naso-cystic drain was removed 10 d after placement, with no abscess recurrence. We believe that EUS-guided drainage of postoperative intra-abdominal abscesses is a safe and effective method, although further large-scale investigations are required to confirm our findings.
- Citation: Mandai K, Uno K, Yasuda K. Endoscopic ultrasound-guided drainage of postoperative intra-abdominal abscesses. World J Gastroenterol 2015; 21(11): 3402-3408
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3402
Traditionally, postoperative intra-abdominal abscesses have been managed by percutaneous or surgical drainage. Image-guided percutaneous drainage is reported to be a minimally invasive and effective method[1].
Although endoscopic ultrasound (EUS)-guided drainage for pancreatic pseudocysts is regarded as a safe and effective standard method[2-7], there have been few reports of EUS-guided drainage of postoperative intra-abdominal abscesses[8,9].
Herein, we examined 4 cases of EUS-guided drainage of postoperative intra-abdominal abscesses performed at our institution to evaluate the safety and efficacy of this procedure.
Between May 2011 and May 2014, we experienced 4 cases of postoperative intra-abdominal abscesses for which EUS-guided drainage was performed. The indication of EUS-guided drainage was limited to cases in which adhesion of the abscess and digestive wall was highly suspected on computed tomography (CT).
All procedures were performed using a convex-type echoendoscope (GE-UC2000P or GF-UCT260; Olympus Medical Systems, Tokyo, Japan). A 19-gauge needle (EchoTip; Cook Medical, Winston-Salem, NC, United States or Expect; Boston Scientific Japan, Tokyo, Japan, or SonoTip; Medi-Globe, Rosenheim, Germany) was used to puncture the abscess. The fluid content was aspirated, and a sample of the aspirate was sent for Gram staining and culture. Subsequently, a small dose of contrast agent was injected, and a 0.025-inch guidewire (VisiGlide; Olympus Medical Systems) was introduced through the needle and coiled into the abscess under fluoroscopic guidance. In cases of only naso-cystic drainage, the fistula was dilated using a 7-Fr dilation catheter (Soehendra Biliary Dilation Catheter; Cook Medical), and a 6-Fr pigtail nasal biliary catheter (nasal biliary drainage set; Cook Medical) was subsequently placed. In cases of both naso-cystic and internal drainage, the fistula was dilated using a 7-Fr dilation catheter (Soehendra Biliary Dilation Catheter; Cook Medical) and a balloon catheter (Maxforce TTS biliary balloon dilator, 6 mm; Boston Scientific Japan). Next, two 0.025-inch guidewires (VisiGlide; Olympus Medical Systems) were placed in the abscess using a double lumen catheter (Uneven Double Lumen Catheter; PIOLAX, Kanagawa, Japan), and a 6-Fr pigtail nasal biliary catheter (nasal biliary drainage set; Cook Medical) and 7-Fr double pigtail biliary stent (Zimmon Biliary Stent; Cook Medical) were subsequently placed. The naso-cystic drainage catheter was irrigated once daily with 10-20 mL of sterile saline in all patients. Characteristics of the 4 cases are summarized in Table 1.
| Case | Age (yr)/sex | Primary disease | Surgical procedure | Abscess size (mm) | Primary treatment for abscess | EUS-D route | Time to EUS-D (d) | EUS-D modality | Drainage length (d) | Complications/abscess recurrence |
| 1 | 60/F | Rectal cancer | Low anterior rectal resection | 61 × 55 | Surgical drainage | TG | 69 p.o. | 6-Fr NB | 11 | None |
| 2 | 46/F | MCN | Distal pancreatectomy | 54 × 33 | None | TG | 26 p.o. | 6-Fr NB | 11 | None |
| 3 | 74/M | PDAC | Distal pancreatectomy | 62 × 35 | None | TG | 10 p.o. | 7-Fr stent | 20 | None |
| 66 × 42 | None | TG | 21 p.o. | 6-Fr NB | 8 | None | ||||
| 4 | 69/M | IPMN | Distal pancreatectomy | 70 × 45 | Percutaneous drainage | TG | 18 p.o. | 7-Fr stent, 6-Fr NB | 7-Fr stent: no removal 6-Fr NB: 10 per-cutaneous: 24 | None |
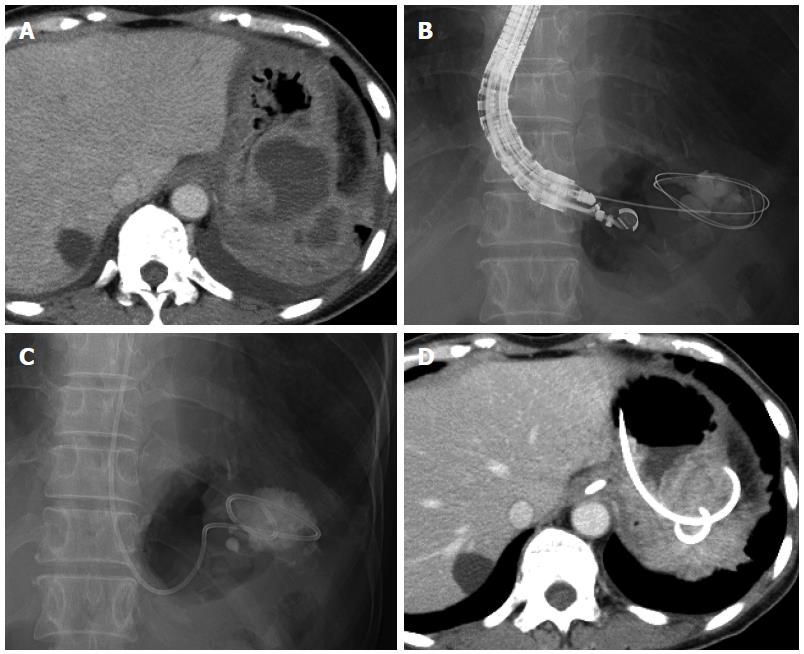
A 60-year-old woman underwent laparoscopic low anterior resection for rectal cancer. She developed a high fever after surgery, and CT revealed fluid collection around the anastomotic intestine and in the subdiaphragmatic area. We diagnosed this fluid collection as an abscess, and surgical drainage was consequently performed on postoperative day 17. Although the drain was removed and the patient was discharged on postoperative day 44, she was readmitted for high fever on postoperative day 64. CT revealed an encapsulated fluid collection (maximum axis, 61 mm) in the left subdiaphragmatic area close to the fornix of the stomach (Figure 1A). We again diagnosed this fluid collection as an abscess; and, as antibiotic administration did not improve the patient’s condition, EUS-guided transgastric drainage was performed, and a 6-Fr pigtail nasal biliary catheter was placed on postoperative day 69 (Figure 1B, C). Gram staining and culture of a sample of the aspirate confirmed extended-spectrum beta lactamase (ESBL)-producing Escherichia coli, Enterococcus, and anaerobic bacteria. Antibiotics were administered until postoperative day 74, and CT on postoperative day 75 showed that the abscess had decreased in size (Figure 1D). The nasal biliary catheter was removed on postoperative day 80. The patient was discharged on postoperative day 99. At the latest follow-up (3 years after discharge), no abscess recurrence was noted.
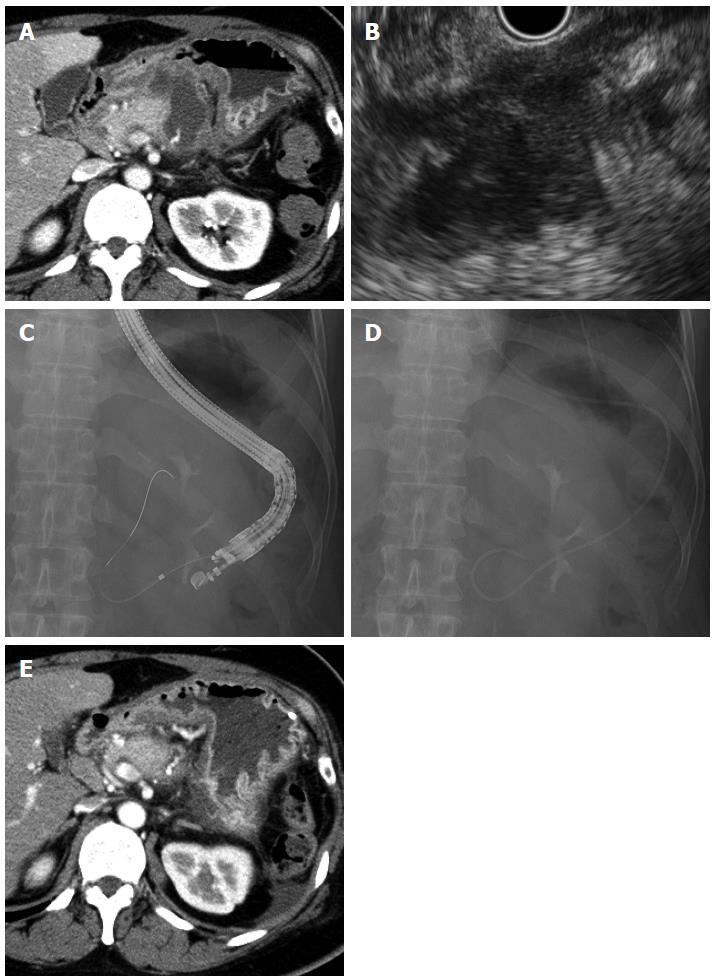
A 46-year-old woman underwent distal pancreatectomy for a mucinous cystic neoplasm of the pancreas. She developed high fever on postoperative day 24, and CT revealed irregular fluid collection (maximum axis, 54 mm) around the resection stump of the pancreas close to the gastric wall (Figure 2A). The fluid collection was diagnosed as an abscess; and, accordingly, EUS-guided transgastric drainage was performed and a 6-Fr pigtail nasal biliary catheter was placed on postoperative day 26 (Figure 2B-D). Gram staining and culture of a sample of the aspirate confirmed methicillin-resistant Staphylococcus aureus (MRSA). CT on postoperative day 37 showed that the abscess had decreased in size (Figure 2E), and the nasal biliary catheter was removed on the same day. Antibiotics were administered until postoperative day 38. The patient was discharged on postoperative day 40. At the latest follow-up (2 years after discharge), no abscess recurrence was observed.
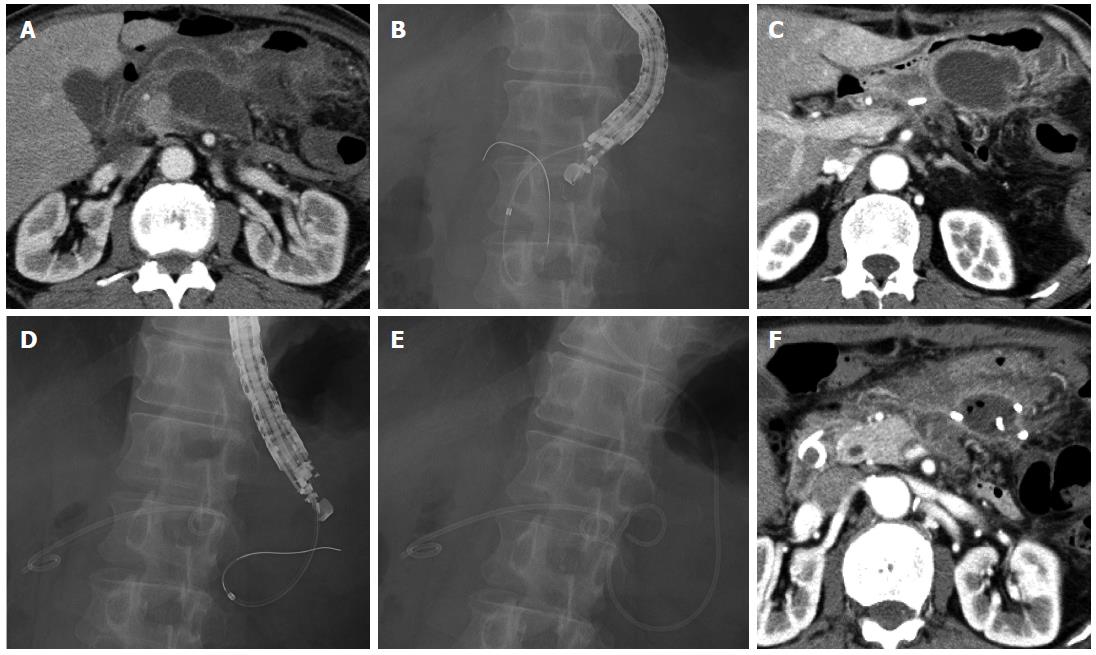
A 74-year-old man underwent distal pancreatectomy for pancreatic ductal adenocarcinoma. He developed high fever on postoperative day 9, and CT revealed fluid collection (maximum axis, 62 mm) around the resection stump of the pancreas close to the gastric wall (Figure 3A). We diagnosed this fluid collection as an abscess, and EUS-guided transgastric drainage was performed and a 7-Fr double pigtail stent was placed on postoperative day 10 (Figure 3B). Gram staining and culture of a sample of the aspirate confirmed methicillin-susceptible Staphylococcus aureus (MSSA), and antibiotics were administered until postoperative day 15. However, the patient developed high fever after drainage, and CT on postoperative day 17 revealed another fluid collection (maximum axis, 66 mm) at the left side of the previously drained abscess (Figure 3C). EUS-guided transgastric drainage was performed again, and a 6-Fr pigtail nasal biliary catheter was placed on postoperative day 21 (Figure 3D, E). Gram staining and culture of a sample of the aspirate again confirmed MSSA, and antibiotics were administered until postoperative day 24. CT on postoperative day 28 showed that the abscess had decreased in size (Figure 3F), and the nasal biliary catheter and the 7-Fr double pigtail stent were removed on postoperative days 29 and 30, respectively. The patient was discharged on postoperative day 32. At the latest follow-up (1 year after discharge), no abscess recurrence was observed.
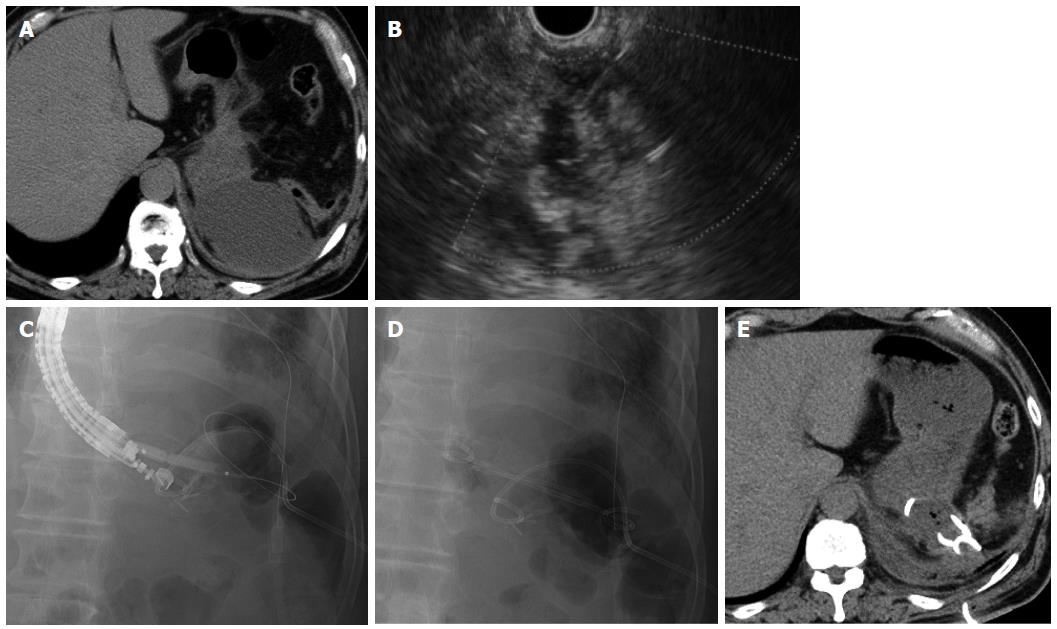
A 69-year-old man underwent distal pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas. He developed high fever on postoperative day 9, and CT revealed a fluid collection (maximum axis, 70 mm) at the dorsal side of the fornix close to the gastric wall (Figure 4A). We diagnosed this fluid collection as an abscess, and conventional ultrasound-guided percutaneous drainage was performed on the same day. However, the high fever persisted after drainage, and EUS-guided transgastric drainage was consequently performed together with placement of a 7-Fr double pigtail stent and a 6-Fr pigtail nasal biliary catheter on postoperative day 18 (Figure 4B-D). Gram staining and culture of a sample of the aspirate revealed Enterococcus, and antibiotics were administered until postoperative day 21. CT on postoperative day 28 showed that the abscess had decreased in size (Figure 4E), and the nasal biliary catheter and the percutaneous drain were removed on postoperative days 28 and 33, respectively. The 7-Fr double pigtail stent was not removed, and the patient was discharged on postoperative day 36. At the latest follow-up (6 mo after discharge), no abscess recurrence was noted.
Image-guided percutaneous drainage has been performed for postoperative intra-abdominal abscesses as a minimally invasive method. Azeem et al[10] reported that endoscopic drainage with or without EUS was as effective as percutaneous drainage in patients with pancreatic fluid collection after distal pancreatectomy, and that primary endoscopic drainage may be associated with a shorter hospital stay. Although their report suggested the importance of endoscopic drainage of pancreatic fluid collection after pancreatic resection, to our knowledge, there have been no reports comparing the outcomes of EUS-guided drainage and image-guided percutaneous drainage for the treatment of postoperative intra-abdominal abscesses.
In this study, EUS-guided transgastric drainage for postoperative intra-abdominal abscesses was performed in all 4 patients and resulted in clinical improvement in all cases. Furthermore, naso-cystic drainage was performed within 4 wk of surgery in 3 of the 4 patients, with no complications in any patient, suggesting that early EUS-guided drainage after surgery is safe. Similarly, Tilara et al[8] also reported that early drainage (within 30 d after surgery) of postoperative pancreatic fluid collections was not associated with an increased risk of complications.
In all 4 of our cases, naso-cystic drainage was performed. The naso-cystic drain was removed on day 11 after placement in cases 1 and 2, day 8 in Case 3, and day 10 in Case 4 (mean, day 10 after naso-cystic drainage), and there was no recurrence of the abscess after naso-cystic drain removal in any case. Taken together, these outcomes suggest the efficacy of naso-cystic drainage, but further investigations are required to confirm these results and to determine whether naso-cystic drainage alone is effective, as internal drainage and percutaneous drainage were also performed before naso-cystic drainage in Cases 3 and 4, respectively. Furthermore, the necessity of internal drainage stent removal and the appropriate time of stent removal should be investigated in cases of both naso-cystic and internal drainage. Several recent studies of EUS-guided drainage of pancreatic pseudocysts recommend both internal and naso-cystic drain placement by first using the double guidewire technique, followed by naso-cystic drain removal after improvement of infection[3,6]; however, there is no consensus regarding the optimal drainage method and time for drain removal.
Thus, based on our present cases, we believe that EUS-guided drainage of postoperative intra-abdominal abscesses is a safe and effective method; however, further large-scale investigations are required to confirm our findings.
The 4 patients (2 male, 2 female) developed high fever after abdominal surgery (pancreatectomy or low anterior resection for rectal cancer).
In addition to high fever, all patients had general fatigue.
Postoperative intra-abdominal fluid collection was performed to diagnose infection.
Gram staining and culture of samples of collected fluid confirmed extended-spectrum beta lactamase-producing Escherichia coli, Enterococcus, and anaerobic bacteria in the first patient, methicillin-resistant Staphylococcus aureus in the second patient, methicillin-susceptible Staphylococcus aureus in the third patient, and Enterococcus in the fourth patient.
Computed tomography revealed fluid collection close to the gastric wall in all 4 patients.
Pathological examination of the fluid collection was not performed in any of the patients.
All 4 patients underwent endoscopic ultrasound (EUS)-guided transgastric naso-cystic drainage, and in 2 of the 4 patients, internal drainage was also performed.
Earlier reports state that EUS-guided drainage of postoperative peripancreatic fluid collections is safe and effective, and we believe that this case report adds our findings that early EUS-guided transgastric drainage within 4 wk of surgery is also safe and effective.
Naso-cystic drainage describes a method of external drainage via the nose.
This case report presents data showing that early EUS-guided drainage within 4 wk of surgery for postoperative intra-abdominal abscesses is safe and effective.
This is a well-written case report of a novel application of therapeutic EUS. Although 3 of 4 cases death with post-pancreatic surgery fluid collections, the 4th case is the first to report drainage of an abscess by EUS after colorectal surgery.
P- Reviewer: Chisthi MM, Teshima CW, Yoshida H S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Kassi F, Dohan A, Soyer P, Vicaut E, Boudiaf M, Valleur P, Pocard M. Predictive factors for failure of percutaneous drainage of postoperative abscess after abdominal surgery. Am J Surg. 2014;207:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Itoi T, Itokawa F, Tsuchiya T, Kawai T, Moriyasu F. EUS-guided pancreatic pseudocyst drainage: simultaneous placement of stents and nasocystic catheter using double-guidewire technique. Dig Endosc. 2009;21 Suppl 1:S53-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, Groth S, Omar S, Soehendra N. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Sadik R, Kalaitzakis E, Thune A, Hansen J, Jönson C. EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. World J Gastroenterol. 2011;17:499-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 6. | Kato S, Katanuma A, Maguchi H, Takahashi K, Osanai M, Yane K, Kim T, Kaneko M, Takaki R, Matsumoto K. Efficacy, Safety, and Long-Term Follow-Up Results of EUS-Guided Transmural Drainage for Pancreatic Pseudocyst. Diagn Ther Endosc. 2013;2013:924291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Lin H, Zhan XB, Sun SY, Yang XJ, Jin ZD, Zou DW, Li ZS. Stent selection for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a multicenter study in china. Gastroenterol Res Pract. 2014;2014:193562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Tilara A, Gerdes H, Allen P, Jarnagin W, Kingham P, Fong Y, DeMatteo R, D’Angelica M, Schattner M. Endoscopic ultrasound-guided transmural drainage of postoperative pancreatic collections. J Am Coll Surg. 2014;218:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Varadarajulu S, Wilcox CM, Christein JD. EUS-guided therapy for management of peripancreatic fluid collections after distal pancreatectomy in 20 consecutive patients. Gastrointest Endosc. 2011;74:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Azeem N, Baron TH, Topazian MD, Zhong N, Fleming CJ, Kendrick ML. Outcomes of endoscopic and percutaneous drainage of pancreatic fluid collections arising after pancreatic tail resection. J Am Coll Surg. 2012;215:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |