Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3325
Peer-review started: September 7, 2014
First decision: October 14, 2014
Revised: November 3, 2014
Accepted: December 8, 2014
Article in press: December 8, 2014
Published online: March 21, 2015
Processing time: 193 Days and 20.8 Hours
AIM: To compare the number of regulatory T-cells (Tregs) measured by flow cytometry with those obtained using a real-time quantitative PCR (qPCR) method in patients suffering from inflammatory bowel disease (IBD).
METHODS: Tregs percentages obtained by both flow cytometry and qPCR methods in 35 adult IBD patients, 18 out of them with Crohn´s disease (CD) and 17 with ulcerative colitis (UC) were compared to each other as well as to scores on two IBD activity questionnaires using the Harvey Bradshaw Index (HBI) for CD patients and the Simple Colitis Clinical Activity Index (SCCAI) for UC patients. The Treg percentages by flow cytometry were defined as CD4+CD25highCD127lowFOXP3+ cells in peripheral blood mononuclear cells, whereas the Treg percentages by qPCR method were determined as FOXP3 promoter demethylation in genomic DNA.
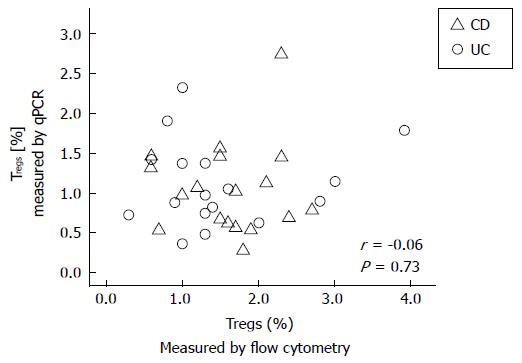
RESULTS: We found an average of 1.56% ± 0.78% Tregs by using flow cytometry, compared to 1.07% ± 0.53% Tregs by using qPCR in adult IBD patients. There were no significant correlations between either the percentages of Tregs measured by flow cytometry or qPCR and the HBI or SCCAI questionnaire scores in CD or UC patients, respectively. In addition, there was no correlation between Treg percentages measured by qPCR and those measured by flow cytometry (r = -0.06, P = 0.73; Spearman Rho). These data suggest that, either Treg-related immune function or the clinical scores in these IBD patients did not accurately reflect actual disease activity. Until the cause(s) for these differences are more clearly defined, the results suggest caution in interpreting studies of Tregs in various inflammatory disorders.
CONCLUSION: The two methods did not produce equivalent measures of the percentage of total Tregs in the IBD patients studied which is consistent with the conclusion that Tregs subtypes are not equally detected by these two assays.
Core tip: In our study neither regulatory T-cells (Tregs) percentages measured by flow cytometry defined as CD4+CD25highCD127lowFOXP3+ cells in peripheral blood mononuclear cells or by real-time PCR measured as forkhead box P3 promoter demethylation in genomic DNA correlated with self-reported inflammatory bowel disease activity. This suggests that either Treg-related immune function or the clinical scores did not accurately reflect actual disease activity. We conclude that natural and induced Tregs are not equally detected by the assays applied.
- Citation: Brandhorst G, Petrova DT, Weigand S, Eberle C, von Ahsen N, Schmitz J, Schultze FC, Raddatz D, Karaus M, Oellerich M, Walson PD. Lack of correlation between Treg quantification assays in inflammatory bowel disease patients. World J Gastroenterol 2015; 21(11): 3325-3329
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3325.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3325
The immune system has been postulated to be involved in the pathogenesis of inflammatory bowel disease (IBD). Either IBD associated immune dysfunction leads to excessive responses to normal intestinal microflora or changes in the intestinal microflora or epithelial barrier function somehow lead to exaggerated or abnormal reactions by the mucosal immune system[1]. Regardless of whether immune changes are the cause of or the result of IBD, the percentage of regulatory T-cells (Tregs) has been used as a marker of immune function in IBD patients[2] and some authors have suggested that Treg imbalances correlate with IBD activity[3].
Expression of the forkhead box P3 (FOXP3) gene has been claimed to be a specific marker of Tregs[4] and a number of both research and commercial FOXP3 based methods have been used to assess Treg percentages in whole blood. However, contrary to what has been reported to be true in murine models[5] and in human cord blood[6] a number of Treg subtypes have been identified in human whole blood[7,8]. The two main subpopulations in humans seem to be thymus-derived natural Tregs (nTregs) and peripherally generated induced Tregs (iTregs). While many different biomarkers have been proposed to differentiate between nTregs and iTregs[9] these assays vary in their ability to correctly identify these two subpopulations[10]. To our knowledge Treg percentages measured by different methods in the same IBD patients have not been adequately assessed.
The purpose of the studies reported here was to compare the Treg percentages measured by flow cytometry to those obtained with a methylation sensitive, real-time PCR method specific for detection of the Treg-specific demethylated region (TSDR) in the same adult IBD patients and to compare the results of both methods to self-reported IBD activity assessed by approved questionnaires.
This work was part of a larger study approved by the institutional review board and designed to evaluate whether a number of laboratory measures of immune function could be used as surrogate markers of disease activity in adults with either Crohn´s disease (CD) or ulcerative colitis (UC). Written informed consent was obtained from all patients before enrolment[11]. Treg percentages obtained by both flow cytometry and quantitative PCR (qPCR) in 35 adult IBD patients (18 with CD and 17 with UC) were compared to each other as well as to results of two IBD self-assessing activity questionnaires according to the Harvey Bradshaw Index (HBI) for CD patients and the Simple Colitis Clinical Activity Index (SCCAI) for UC patients. Patients were not preselected by their disease activity.
Flow cytometry analysis was performed as described previously[12]. The Treg percentages were defined as CD4+CD25highCD127lowFOXP3+ cells. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using the Lymphoprep®-protocol (Axis-Shield; Oslo, Norway) and stored at -80 °C in 10% (v/v) dimethylsulfoxide and 90% (v/v) bovine serum albumin until analysis. In order to control the pre-analytical steps, it was verified that the freezing and thawing of PBMCs did not affect the flow cytometry results in comparison to fresh samples. PBMCs were incubated with 20 μL anti-human CD4-FITC (Catalogue number # 555346, all conjugates used by Becton Dickinson Pharmingen, Heidelberg, Germany), 5 μL anti-human CD25-PE-Cy7 (# 560920) and 20 μL anti-human CD127-Alexa Fluor 647 (# 558558). After cell fixation and membrane permeabilization according to the manufacturer´s protocol, 20 μL FOXP3-PE (clone 259D/C7) and corresponding mouse isotype control antibodies were added. The quantification of regulatory T cells was done using an 8-color flow cytometer (FACS Canto II, Becton Dickinson; Germany). CD4+CD25highCD127low T-cells were gated out of the lymphocyte region (forward/sideward scatter). From these, the intersection between CD4+CD25high and CD25highCD127low gates was formed followed by the identification of Treg cells according to their level of FOXP3 expression. Data were analyzed with BD FACS Diva software (Becton Dickinson) to identify the percentage of CD4+CD25highCD127lowFOXP3+ cells.
As a second method of assessing the Treg percentage the FOXP3 promoter demethylation signature was determined using methylation-sensitive real-time PCR as previously described[6,13,14] and adapted in our laboratory[15]. Briefly, genomic DNA was isolated by NucleoSpin® columns (Macherey-Nagel; Düren, Germany) and treated with bisulfite for conversion of unmethylated cytosine into uracil (EZ DNA Methylation GoldTM, Zymo Research, Irvine, California). After quantification in triplicates of methylated and unmethylated FOXP3-specific PCR products by use of real-time PCR and methylation-specific primers on a LightCycler® 480 (Roche Diagnostics; Mannheim, Germany), the FOXP3 demethylation status was calculated as a ratio. Due to X-chromosomal inactivation of the FOXP3 gene the results for female patients were corrected by a factor of two.
We found an average of 1.56% Tregs (SD: 0.78%) by using flow cytometry, compared to 1.07% Tregs (SD: 0.53%) by using qPCR in these adult IBD patients (Table 1). There were no statistically significant correlations between either the flow cytometry or qPCR measured percentages of Tregs and the HBI or SCCAI questionnaire scores in CD or UC patients, respectively. There were no significant differences between these correlations for either male vs female patients, the presence or absence of remission, or the drug therapies currently used (Table 1). In addition, there was no significant correlation between Treg percentages measured by qPCR and those measured with the flow cytometry (r = -0.06, P = 0.73; Spearman Rho); (Figure 1).
| Subjects | n | Age (yr) | Flow cytometry (%) | qPCR (%) | r | P value |
| Average ± SD | Average ± SD | Average ± SD | ||||
| Patients with Crohn´s disease | 18 | 38 ± 10 | 1.62 ± 0.62 | 1.05 ± 0.56 | -0.10 | 0.68 |
| Female | 10 | 39 ± 11 | 1.44 ± 0.66 | 1.00 ± 0.70 | -0.18 | 0.61 |
| Male | 8 | 37 ± 9 | 1.83 ± 0.53 | 1.12 ± 0.35 | -0.30 | 0.47 |
| Patients with ulcerative colitis | 17 | 43 ± 17 | 1.50 ± 0.93 | 1.10 ± 0.52 | -0.05 | 0.85 |
| Female | 8 | 45 ± 14 | 1.39 ± 0.63 | 0.99 ± 0.45 | -0.06 | 0.89 |
| Male | 9 | 41 ± 20 | 1.60 ± 1.17 | 1.19 ± 0.58 | -0.03 | 0.93 |
| Current drug therapy | ||||||
| Infliximab | 4 | 46 ± 11 | 1.65 ± 0.97 | 0.98 ± 0.34 | 0.20 | 0.80 |
| Azathiaprine | 14 | 41 ± 18 | 1.57 ± 0.81 | 1.07 ± 0.60 | -0.21 | 0.48 |
| Systemic corticosteroids | 9 | 39 ± 12 | 1.73 ± 0.98 | 0.91 ± 0.40 | -0.06 | 0.88 |
These data suggest that, at least in this small cohort of IBD patients, either Treg-related immune function or the clinical scores did not accurately reflect actual disease activity. It is possible that either a different scoring system or measurement of tissue e.g., from intestine biopsies rather than circulating Treg percentages would have been more predictive[3,16-18]. Interestingly, in a small study that included septic patients a weak correlation between flow cytometry and demethylation PCR methods could be demonstrated[19]. However, the lack of correlation between the two measures of Treg percentages is consistent with studies that suggest that FOXP3 activity is not confined to CD4+CD25highCD127low cells and can be expressed in non-Treg cells[18,20]. This is also consistent with reports that the flow cytometry methods that have been previously used to identify Tregs are incapable of separating induced from natural Tregs[17]. More recently Neuropilin 1 (Nrp1) expression has been proposed as a method capable of distinguishing between nTregs and iTregs[21]. Measuring demethylation status at the FOXP3 locus using TSDR may also aid in the differentiation of Treg subtypes due to the different degree of methylation in nTregs and iTregs[10]. Thus TSDR demethylation assays would be expected to identify primarily nTregs which would explain why the two assays found different percentages of Tregs in these IBD patients. Differences can also be caused by analytical interference from drug therapy. For example basiliximab has been shown to interfere with the detection of CD25 in flow cytometry assays[22]. However, for tumour necrosis factor alpha antibodies like infliximab are unlikely to interfere due to their different mode of action.
In the present preliminary study a cohort selection effect might be a limiting factor. In addition to self-assessment scores the disease activity could be assessed using other methods including endoscopy and/or by more objective, cheaper and conventional biochemical markers for inflammation such as fecal calprotectin, C-reactive protein in serum, platelets, leukocytosis, IL-6, etc. Determination of Treg populations in peripheral blood would be an expensive routine measure of disease activity. Finally, Treg proportions in the blood may not really represent Treg proportions in the lamina propria. The determination of Treg subtypes in the inflamed mucosa might have more pathogenic relevance.
Until the cause(s) for these differences are more clearly defined, the results suggest caution in interpreting studies of Tregs in various inflammatory disorders.
In conclusion, in this study neither Treg percentages in whole blood measured by flow cytometry or qPCR correlated with self-reported disease activity. This suggests that either Treg-related immune function or the clinical scores did not accurately reflect actual disease activity. Additionally, the flow cytometry and qPCR methods did not produce equivalent measures of the percentage of Tregs in these 35 adult IBD patients which is consistent with the conclusion that Tregs subtypes are not equally detected by these two assays.
The technical help of R. Andag, J. Engelmayer, S. Götze, and C. Scholz (all from the Department of Clinical Chemistry, Goettingen, Germany) is greatly appreciated. Furthermore, it was part of the Inaugural Dissertation for the doctoral degree for Sebastian Weigand.
The immune system has been postulated to be involved in the pathogenesis of inflammatory bowel disease (IBD).
Regulatory T-cell (Treg) percentages measured by different methods in the same IBD patients have not been adequately assessed.
Either Treg-related immune function or the clinical scores in these IBD patients did not accurately reflect actual disease activity.
The lack of correlation between these two assays for quantification of Tregs suggests caution in interpreting studies of Tregs in various inflammatory disorders.
Expression of the forkhead box P3 (FOXP3) gene has been claimed to be a specific marker of Tregs.
The authors clearly show that there is poor correlation between two different metods for measuring Tregs in perfiferal blood. Thus, studies on Tregs in various inflammatory disorders should be read with great caution.
P- Reviewer: Sorrentino D, Tommasini A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] |
| 2. | Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Maul J, Zeitz M. Ulcerative colitis: immune function, tissue fibrosis and current therapeutic considerations. Langenbecks Arch Surg. 2012;397:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Dummer CD, Carpio VN, Gonçalves LF, Manfro RC, Veronese FV. FOXP3+ regulatory T cells: from suppression of rejection to induction of renal allograft tolerance. Transpl Immunol. 2012;26:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68-73. [PubMed] |
| 6. | Liu J, Lluis A, Illi S, Layland L, Olek S, von Mutius E, Schaub B. T regulatory cells in cord blood--FOXP3 demethylation as reliable quantitative marker. PLoS One. 2010;5:e13267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245-1253. [PubMed] |
| 8. | Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13-20. [PubMed] |
| 9. | Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol. 2013;4:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 10. | Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6:116-123. [PubMed] |
| 11. | Brandhorst G, Weigand S, Eberle C, Raddatz D, Karaus M, Oellerich M, Walson PD. CD4+ immune response as a potential biomarker of patient reported inflammatory bowel disease (IBD) activity. Clin Chim Acta. 2013;421:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, Faustin V, Riggert J, Oellerich M, Hasenfuss G. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring). 2013;21:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701-1711. [PubMed] |
| 14. | Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K, Pratschke J. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Schultze FC, Andag R, Alwahsh SM, Toncheva D, Maslyankov S, Yaramov N, von Ahsen N, Brandhorst G, Walson PD, Oellerich M. FoxP3 demethylation is increased in human colorectal cancer and rat cholangiocarcinoma tissue. Clin Biochem. 2014;47:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hölttä V, Sipponen T, Westerholm-Ormio M, Salo HM, Kolho KL, Färkkilä M, Savilahti E, Vaarala O, Klemetti P. In Crohn’s Disease, Anti-TNF-α Treatment Changes the Balance between Mucosal IL-17, FOXP3, and CD4 Cells. ISRN Gastroenterol. 2012;2012:505432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Shalev I, Selzner N, Shyu W, Grant D, Levy G. Role of regulatory T cells in the promotion of transplant tolerance. Liver Transpl. 2012;18:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol. 2012;40:186-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Tatura R, Zeschnigk M, Adamzik M, Probst-Kepper M, Buer J, Kehrmann J. Quantification of regulatory T cells in septic patients by real-time PCR-based methylation assay and flow cytometry. PLoS One. 2012;7:e49962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Litjens NH, Boer K, Betjes MG. Identification of circulating human antigen-reactive CD4+ FOXP3+ natural regulatory T cells. J Immunol. 2012;188:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623-630. [PubMed] |
| 22. | Abadja F, Alamartine E, Berthoux F, Mariat C, Genin C, Lambert C. Quantification of circulating regulatory T cells by flow cytometry in kidney transplant patients after basiliximab induction therapy. Transplantation. 2010;89:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |