Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3256
Peer-review started: July 20, 2014
First decision: August 15, 2014
Revised: September 2, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: March 21, 2015
Processing time: 244 Days and 1.6 Hours
AIM: To evaluate the prognostic value of high-mobility group box 1 (HMGB1) expression in intrahepatic cholangiocarcinoma (IHCC) and the possible underlying mechanism.
METHODS: Tissue microarray was constructed from 65 IHCC patients. Immunohistochemistry was performed to validate expression of HMGB1 and Vascular endothelial growth factor C (VEGF-C). Real-time PCR and Western blot analyses were used to study transcript and protein levels. The interaction between HMGB1 and VEGF-C was evaluated by siRNA, real-time PCR, and enzyme-linked immuno assays. The correlation between HMGB1 expression and other clinicopathologic parameters was analyzed by χ2 test, and the univariate as well as multivariate analyses were accomplished by Kaplan-Meier method and Cox-regression model, respectively.
RESULTS: Overall, overexpression of HMGB1 was found in 38/65 (58.8%) IHCCs, whereas VEGF-C overexpression was present in 30/65 (46.2%) cases. Overexpression of HMGB1 was significantly correlated with lymphatic microvessel density (P = 0.031, r = 0.268) and VEGF-C expression (P = 0.041, r = 0.254). With univariate analysis, both HMGB1 (P = 0.001) and VEGF-C (P = 0.004) were identified to be significantly associated with overall survival rate. Multivariate analysis indicated that HMGB1 could be served as an unfavorable independent prognostic factor in IHCCs (P = 0.005). siRNA knockdown of HMGB1 inhibited transforming growth factor-β-induced epithelial-mesenchymal transition (EMT) by elevating E-Cadherin expression and reducing expression of N-Cadherin, Vimentin and Snail in RBE cells. Further in vitro study revealed that HMGB1 silencing significantly decreased the level of VEGF-C, whereas the recombinant HMGB1 increased the VEGF-C level in RBE cells (both P < 0.05), which suggested that HMGB1 could promote lymphatic microvessel density, and subsequently lymphatic invasion, via promoting VEGF-C expression.
CONCLUSION: Our results define an important role of HMGB1 in the progression of cholangiocarcinoma, and HMGB1 may serve as a prognostic marker for IHCC patients.
Core tip: Cholangiocarcinoma is a lethal malignancy of the biliary tract, for which novel biomarkers are urgently needed for its management and treatment. This study shows that high-mobility group box 1 (HMGB1) is an independent prognostic factor in intrahepatic cholangiocarcinoma that positively correlates with lymphatic microvessel density and vascular endothelial growth factor C expression. Furthermore, HMGB1 enhances the secretion of vascular endothelial growth factor C and promotes epithelial-mesenchymal transition of RBE cells. Together, these results define an important role of HMGB1 in the progression of cholangiocarcinoma, which may serve as a prognostic marker for intrahepatic cholangiocarcinoma patients.
- Citation: Xu YF, Ge FJ, Han B, Yang XQ, Su H, Zhao AC, Zhao MH, Yang YB, Yang J. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol 2015; 21(11): 3256-3265
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3256.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3256
Cholangiocarcinoma (CCA) is the second most common cancer after hepatocellular carcinoma, and accounts for approximately 7%-10% of all primary hepatic cancers[1]. CCA is characterized by poor responsiveness to chemotherapy and radiotherapy in the majority of cases[2]. So far, surgical resection is the only potentially curative option. The morbidity and mortality of CCA, especially intrahepatic cholangiocarcinoma (IHCC), have been increasing worldwide in recent years[3]. IHCC is characterized by silent clinical signatures, early regional invasiveness, distant metastasis, and a poor prognosis[4]. Therefore, new insights into the biologic process of IHCCs and identification of novel biomarkers are urgently needed for cancer management and treatment.
High-mobility group box 1 (HMGB1) is a proinflammatory cytokine and chromatin-binding molecule[5], and is involved in a variety of biologic processes, including transcription, DNA repair, differentiation, and extracellular signal transduction[6]. Emerging data have suggested that HMGB1 could promote tumor progression via promoting proliferation and invasiveness of cancer cells[7]. Clinically, overexpression of HMGB1 has been reported in multiple malignancies including melanoma[8], gastric cancer[9], colorectal cancer[10], prostate cancer[11], and nasopharyngeal carcinoma[12]. However, to the best our knowledge, there has been no study so far to investigate the role of HMGB in IHCC.
Vascular endothelial growth factor C (VEGF-C) is a key mediator of lymphangiogenesis, acting via its receptors VEGF-R2 and VEGF-R3. Multiple studies have suggested that increased levels of VEGF-C correlate with lymphangiogenesis and distant metastasis[13]. Interestingly, Moriwaka et al[14] demonstrated a link between HMGB1 expression and lymph vessel density as well as VEGF-C expression in colon cancer. Additionally, HMGB1 has been suggested to promote lymphangiogenesis and invasive capacity of tumor cells through a VEGF-C-related pathway in oral squamous cell carcinoma[15].
Epithelial-mesenchymal transition (EMT), an early embryonic development program in which cells convert from the epithelial to the mesenchymal state, has been shown to play a critical role during cancer progression and metastasis[16]. During this process, the epithelial cancer cells lose epithelial characteristics and acquire mesenchymal properties resulting in reduction of adhesions and improvement of motility, thus promoting invasion and metastasis[17]. HMGB1 has been reported as a key regulator in the EMT process in mesothelial cells[18]. However, the link between HMGB1 and the EMT process remains unclear in the context of IHCC progression.
In this study, for the first time, we evaluated the expression and prognostic significance of HMGB1 in IHCCs. The roles of HMGB1 in EMT processes of IHCC, as well as the relationship between HMGB1 expression and VEGF-C were also investigated.
Our study consisted of specimens from 65 IHCC patients (32 male; 33 female) who underwent surgical resections between 2005 and 2011 at the Qilu Hospital of Shandong University (Jinan, China). Detailed clinicopathologic profiles were obtained from medical records. The specimens were reviewed by two pathologists (Han B and Yang XQ). Tumor staging and histologic classification were assessed according to the 7th edition of American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) classification. The mean age of the patients was 56.9 ± 10.9 years (range, 28-83 years). Follow-up data were available for 57 patients, ranging from 3 to 96 mo (mean: 27.6 ± 27.3 mo). This study was approved by the Institutional Review Board at the School of Medicine of Shandong University and written consent was obtained from all patients.
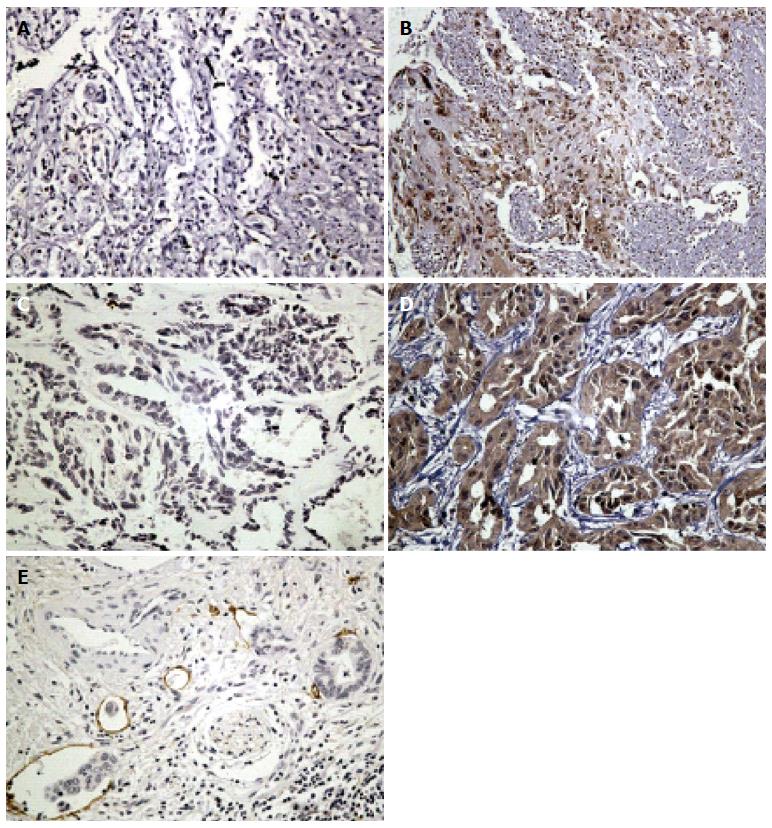
The tissue microarray was constructed as previously described[19]. Two cores (1.0 mm in diameter) were taken from the representative areas of each tumor block and re-embedded into the recipient block. IHC was performed as previously described[20]. Briefly, 4 μm sections were deparaffinized and rehydrated. Sections were submerged into antigenic retrieval buffer (pH 6.0 citric acid) for heat-mediated retrieval by microwave for 15 min. The slides were incubated with primary antibodies for HMGB1 (GTX101277, 1:500; GeneTex Inc., Irvine, CA, United States) and VEGF-C (ab9546, 1:500; Abcam, Cambridge, United Kingdom) overnight at 4 °C, then visualized using 3, 3-diaminobenzidine tetrahydrochloride as the chromogen. Slides incubated without primary antibody were considered as the negative control. The slides were then evaluated by two independent observers (Han B and Yang XQ) who were blind to the clinicopathologic data. The expression of HMGB1 and VEGF-C were evaluated with a semiquantitative scoring system based on intensity and distribution of positive-stained cells[21-23]. Briefly, the staining intensity (range, 0-3) and the percentage of positive cells (0, 0%-10%; 1, 11%-25%; 2, 26%-50%; 3, 51%-75%; 4, 76%-100%) were multiplied. Overexpression and non-overexpression were designated by a score of ≥ 8 or < 8, respectively.
The quantitative vessel counts were performed according to the method described by Weidner et al[24]. Lymphatic microvessel density (LMVD) of the tumor was determined by using the D2-40 antibody (ab77854, 1:500; Abcam); the methodology and validation criteria were in compliance with the international consensus on evaluation of angiogenesis quantification in solid human tumors[25]. Regions with the highest LMVD were initially selected with low magnification (× 100) scanning, and then five “hotspot” fields in the corresponding area were selected and observed at a magnification of × 200. Any brown-stained, separated endothelial cell cluster was considered a single, countable lymphatic microvessel. The average amounts of lymphatic microvessels in the three fields were recognized as the value of LMVD[26]. The average score of LMVD of all samples was selected as the cut-off. The cut-off of LMVD was 12.7 and separated LMVD into high and low group[27].
The IHCC cell line RBE and perihilar cholangiocarcinoma cell line QBC939 were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China), The IHCC cell line HUCCT-1 was obtained from RIKEN Bioresource Center (Japan). All lines were cultured in RPMI-1640 medium supplemented with 10% FBS (Gibco of Thermo Fisher Scientific, Waltham, MA, United States). Human recombinant HMGB1 (rHMBG1) was purchased from Sino Biological Inc., Beijing, China (Cat No. 10326-H08H).
Small interfering RNA (siRNA) was used to knockdown HMGB1 expression. Three specific siRNAs were designed and synthesized by Songon (Shanghai, China). The most effective single siHMGB1 (sense, 5’-CCUGUCCAUUGGUGAUGUUTT-3’; anti-sense, 5’-AACAUCACCAAUGGACAGGTT-3’) was used for further experiments. A scrambled siRNA (scHMGB1) sequence was used as a control: sense, 5’-UUCUCCCAACGUGUCACG-3’; anti-sense, 5’-ACGUGACACGUUCGGAGAATT-3’.
Total RNA was extracted from the RBE cells and cDNA was synthesized by reverse transcription. A SYBR Green Realtime PCR Master Mix (Toyobo Co., Osaka, Japan) and ABI Prism 7700 Sequence Detection System (Applied Biosystems of Thermo Fisher Scientific) were used in this experiment. The primers of HMGB1 were as follows: sense, 5’-TTTAGATCTATGGCAAAGGAGATCCTAAGAAG-3’; anti-sense, 5’-TTTGAATTCTTATTCATCATCATCATCTTCTTCTTCATCT-3’. The relative HMGB1 expression was normalized to GAPDH (sense 5’-GAGTCAACGGATTTGGTCGT-3’; anti-sense, 5’-TTGATTTTGGAGGGATCTC-3’). PCR assays were performed in triplicate, and fold induction was calculated using the 2-ΔCT method[4].
Cells were lysed and protein was extracted as previously described[20]. After SDS-PAGE, proteins were transferred to nitrocellulose membranes (BioTrace NT Nitocellulose; Pall Corp., Port Washington, NY, United States). The membrane was incubated with primary antibodies overnight at 4 °C: Anti-HMGB1 (1:500), and antibodies against E-cadherin, N-cadherin, vimentin, slug, snail, claudin-1, ZO-1 and β-catenin (1:1000, #9782; Cell Signaling Technology Danvers, MA, United States). The secondary anti-rabbit antibody (Beyotime Company, China) was used at a dilution of 1:10000 and the blot was developed with RapidStep ECL Reagent (Millipore Corp., Billerica, MA, United States). For the EMT assay, RBE cells were stimulated with 10 ng/mL transforming growth factor (TGF)-β for 72 h, and the cells were lysed and protein was extracted for further analysis.
RBE cells cultured in 12-well plate at a density of 2.5 × 104 cells per well were treated with scHMGB1, siHMGB1, or RPMI-1640 supplemented with 2 μg/mL rHMBG1. Cells treated with Lipofectamine 2000 alone served as a mock transfection group. The supernatants from each group were collected and centrifuged at 1000 rpm for 3 min after 48-h transfection/incubation. The levels of VEGF-C were detected using the human VEGF-C ELISA kit (Boster Systems Inc., Pleasanton, CA, United States) according to the manufacturer’s instructions. Absorbance at a wavelength of 450 nm in every well was measured in spectrophotometer.
All the statistical analyses were performed by SPSS 17.0 software (SPSS, Chicago, IL, United States). The associations between HMGB1 expression and clinicopathologic parameters were assessed by a χ2 test. Spearman’s Rank correlation coefficient was used to identify the correlation between HMGB1 and LMVD. Cumulative overall survival rates were calculated by the Kaplan-Meier method and survival curves were compared by a log-rank test. For multivariate analysis, factors from univariate analysis were selected with a P = 0.20 cutoff[28]. Forward stepwise multivariate analysis was used to identify independent prognostic factors. P < 0.05 was considered as significant.
HMGB1 expression was mainly identified in the nucleus with slight penetration to cytoplasm or extracellular milieu, and the penetration tendency was manifest especially in area adjacent to necrosis. Overall, HMGB1 was overexpressed in 38/65 (58.8%) patients with IHCCs. VEGF-C expression was identified in the cytoplasm and overexpression was found in 32/65 (49.2%) IHCC cases. Representative IHC images of HMGB1 and VEGF-C are shown in Figure 1.
The relationship between HMGB1 overexpression and various clinicopathologic parameters are demonstrated in Table 1. Overall, overexpression of HMGB1 was significantly associated with lymph node metastasis (P = 0.011). No significant correlation was identified between HMGB1 overexpression and age, gender, tumor size, histologic classification, or microvascular or perineural invasion. Similarly, VEGF-C overexpression correlated with histologic classification (P = 0.016) and lymph node metastasis (P = 0.007).
| Parameters | HMGB1 | P value | VEGF-C | P value | ||
| Low | High | Low | High | |||
| Age (yr) | ||||||
| < 60 | 18 (42.9) | 24 (57.1) | 25 (59.5) | 17 (40.5) | ||
| ≥ 60 | 9 (39.1) | 14 (60.9) | 0.771 | 10 (43.5) | 13 (56.5) | 0.056 |
| Gender | ||||||
| Male | 15 (46.9) | 17 (63.6) | 15 (46.9) | 17 (53.1) | ||
| Female | 12 (36.4) | 21 (63.6) | 0.390 | 18 (54.5) | 15 (45.5) | 0.536 |
| Tumor size (cm) | ||||||
| < 5 | 8 (36.4) | 14 (63.6) | 10 (45.5) | 12 (54.5) | ||
| ≥ 5 | 19 (44.2) | 24 (55.8) | 0.545 | 23 (53.5) | 20 (46.5) | 0.540 |
| Histologic classification | ||||||
| Well | 8 (47.1) | 9 (52.9) | 12 (70.6) | 5 (29.4) | ||
| Moderate | 10 (32.3) | 21 (67.7) | 10 (32.3) | 21 (67.7) | ||
| Poor | 9 (52.9) | 8 (47.1) | 0.329 | 12 (70.6) | 5 (29.4) | 0.016 |
| Tumor stage | ||||||
| I + II | 21 (41.2) | 30 (8.8) | 25 (49.0) | 26 (51.0) | ||
| III + IV | 6 (42.9) | 8 (57.1) | 0.910 | 8 (57.1) | 6 (42.9) | 0.590 |
| Node stage | ||||||
| Negative | 25 (50) | 25 (50) | 30 (60.0) | 20 (40.0) | ||
| Positive | 2 (13.3) | 13 (86.7) | 0.011 | 3 (20.0) | 12 (80.0) | 0.007 |
| UICC stage | ||||||
| I + II | 18 (48.6) | 19 (51.4) | 21 (56.8) | 16 (43.2) | ||
| III + IV | 9 (32.1) | 19 (67.9) | 0.181 | 12 (42.9) | 16 (57.1) | 0.267 |
| Microvascular invasion | ||||||
| Negative | 20 (39.2) | 31 (60.8) | 26 (51.0) | 25 (49.0) | ||
| Positive | 7 (50.0) | 7 (50.0) | 0.458 | 7 (50.0) | 7 (50.0) | 0.948 |
| Perineural invasion | ||||||
| Negative | 22 (38.6) | 35 (61.4) | 30 (52.6) | 27 (47.4) | ||
| Positive | 5 (62.5) | 3 (37.5) | 0.2601 | 3 (37.5) | 5 (62.5) | 0.475a |
| Satellite nodular | ||||||
| Negative | 24 (44.4) | 30 (55.6) | 27 (50) | 27 (50) | ||
| Positive | 3 (27.3) | 8 (72.7) | 0.292 | 6 (54.5) | 5 (45.5) | 0.783 |
| HBV infection | ||||||
| Negative | 24 (42.1) | 33 (57.9) | 30 (52.6) | 27 (47.4) | ||
| Positive | 3 (37.5) | 5 (62.5) | 1.0001 | 3 (37.5) | 5 (62.5) | 0.475a |
| Calculus | ||||||
| Negative | 23 (40.4) | 34 (59.6) | 29 (51.8) | 27 (48.2) | ||
| Positive | 4 (50.0) | 4 (50.0) | 0.7121 | 3 (37.5) | 5 (62.5) | 0.708a |
| LMVD | ||||||
| Low | 15 (57.7) | 11 (42.3) | 18 (69.2) | 8 (30.8) | ||
| High | 12 (30.8) | 27 (9.2) | 0.031 | 15 (38.5) | 24 (61.5) | 0.015 |
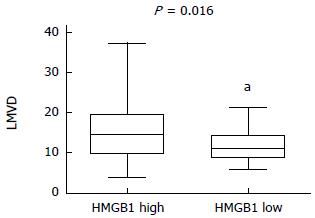
The LMVD values of the 65 specimens ranged from 4 to 37 (mean: 12.7 ± 5.9), and tumors below or equal to 12.7 were classified as the low LMVD group (n = 26) and tumors above 12.7 were classified as the high LMVD group (n = 39). HMGB1 overexpression in high and low LMVD groups was compared by a χ2 test, and the difference were statistically significantly (P = 0.016) (Figure 2).
In Spearman’s Rank correlation coefficient analysis, HMGB1 overexpression positively correlated with LMVD (P = 0.031, r = 0.268) and VEGF-C (P = 0.03, r = 0.268) (Table 2).
| HMGB1 | LVD | P value | r | VEGF-C | P value | r | ||
| Low | High | Negative | Positive | |||||
| Negative | 15 | 12 | 0.031 | 0.268 | 18 | 9 | 0.03 | 0.268 |
| Positive | 11 | 27 | 15 | 23 | ||||
Univariate analysis revealed that HMGB1 was an unfavorable prognostic factor (P = 0.001). Additionally, tumor size (P = 0.022), lymph node metastasis (P < 0.001), LMVD (P = 0.016), and VEGF-C (P = 0.004) were also significantly associated with overall survival. In a multivariate analysis, HMGB1 overexpression remained an independent prognostic factor (hazard ratio = 4.517, 95%CI: 1.458-13.992; P = 0.009) (Table 3). Notably, the IHCC patients who showed co-expression of HMGB1 and VEGF-C had the poorest survival compared with other subgroups when analyzed by the Kaplan-Meier method (Figure 3).
| Parameters | HR | 95%CI | P value |
| Node stage | |||
| Negative | 1.000 | ||
| Positive | 3.166 | 1.108-9.046 | 0.031 |
| Tumor size (cm) | |||
| < 5 | 1.000 | ||
| ≥ 5 | 4.212 | 1.429-12.420 | 0.009 |
| Microvascular invasion | |||
| Negative | 1.000 | ||
| Positive | 2.730 | 1.088-6.850 | 0.032 |
| HMGB1 | |||
| Negative | 1.000 | ||
| Positive | 4.517 | 1.458-13.992 | 0.009 |
| VEGF-C | |||
| Negative | 1.000 | ||
| Positive | 3.003 | 1.016-8.875 | 0.047 |
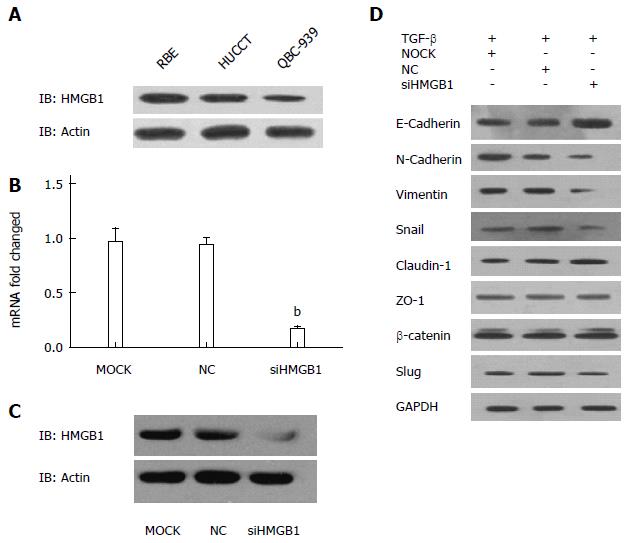
Western blotting was utilized to determine the expression levels of HMGB1 in a panel of CCA cell lines. As shown in Figure 4A, the protein expression level of HMGB1 was substantially higher in RBE cells compared with HUCCT-1 and QBC939 cells.
We firstly confirmed the decreased mRNA and protein expression levels of HMGB1 in siHMGB1-treated RBE cells when compared with those of control groups (Figure 4B, C). As shown in Figure 4D, knockdown of HMGB1 suppressed the TGF-β-induced EMT, as indicated by decreased protein expression of N-cadherin, vimentin, snail, and E-cadherin. By contrast, no significant change was identified in the protein expressions of claudin-1, ZO-1, β-catenin, or slug after si-HMGB1 treatment.
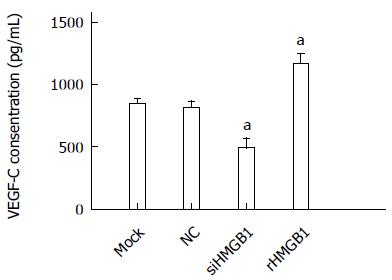
The level of VEGF-C in conditioned medium was significantly decreased in the siHMGB1 group compared with the control groups (P < 0.005). In the rHMGB1-treated group, the VEGF-C level was significantly increased compared with the control (P < 0.001) (Figure 5).
Although the pathogenesis of CCA is poorly understood, a number of risk factors have been identified, including primary sclerosing cholangitis, liver fluke, biliary calculus, and chronic infection of hepatitis C virus[29]. Chronic inflammation induced by these risk factors together with partial bile obstruction form a complex tumor-promoting microenvironment. Within the tumor microenvironment, cytokines, chemokines, and reactive oxygen species induce the recruitment of inflammation-mediating immune cells and lead the necrosis of biliary epithelial cells, resulting in active secretion and/or passive releasing of HMGB1 to the extracellular milieu. In turn, release of HMGB1 further stimulates inflammatory responses, hence, a feed-forward cycle of inflammation exists in the tumor microenvironment of CCA[30]. In the current study, HMGB1 expression was also identified in extracellular milieu, which suggests that HMGB1 can be actively secreted or passively released into extracellular space in the CCA.
Extracellular HMGB1 binding to its receptors (RAGE and Toll-like receptors) subsequently initiates a signaling cascade involving mitogen-activated protein kinase, nuclear factor-κB, phosphoinositide 3-kinase, and Cdc42 in the setting of cancer, may lead to tumor cell survival, expansion, and metastasis[31]. Elevated expression of HMGB1 has been reported in carcinomas of the stomach[32], liver[30], breast[33], and prostate[34]. Univariate and multivariate analyses revealed that HMGB1 overexpression is a poor prognosis factor in IHCC. Our results suggest that HMGB1 contributes to the development and progression of CCA.
Additionally, we show that overexpression of HMGB1 is associated with VEGF-C expression and high LMVD levels. Of note, VEGF-C is a prognostic factor and more importantly, the subset of IHCC patients with co-overexpression of HMGB1 and VEGF-C had the worst cancer-related survival. Previously, Chuangui et al[35] reported that the expression of HMGB1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. We thus speculate that HMGB1 playa a role in lymphangiogenesis through interaction with VEGF-C signaling pathways[36]. Further in vitro analysis showed that levels of VEGF-C in supernatants are significantly decreased with HMGB1 knockdown, and increased with rHMGB1 treatment. This indicates that HMGB1 expression in RBE cells promotes the secretion of VEGF-C. Alternatively, it is possible that HMGB1 binding to its receptors activates transduction signals such as Ras/mitogen-activated protein kinase and nuclear factor-κB, which stimulate the expression of VEGF-C, thereby, promoting lymphangiogenesis[37].
Metastasis is a central hallmark of malignancy during which cancer cells disseminate from the original site and transfer to distant organs[38]. EMT is considered as a critical step in this process[39]. The main molecular characteristic of EMT is the down-regulation of epithelial markers and the up-regulation of mesenchymal markers[40]. In the current study, for the first time, our data suggest that HMGB1 overexpression promotes EMT of RBE cells, and HMGB1 might endow CCA cells with the ability to metastasize via induction of EMT.
There are several limitations in our study. First, the IHCC cohort is relatively small. CCA is a rare malignancy, with only approximately 9760 new cases diagnosed annually in the United States[41], and IHCC only occupies about 20% of these cases[42]. Moreover, the majority of patients are diagnosed at an unresectable stage, which makes it difficult to obtain samples and perform a large randomized trial. Another limitation is the lack of in vivo data to further characterize the role of HMGB1 in IHCC. Although we have found that HMGB1 is an independent prognostic factor in IHCC, and HMGB1 expression is associated with lymph node metastasis, the underlying molecular mechanism of how HMGB1 contributes to faster CCA progression still remains unclear. More experiments are needed to elucidate the receptor and downstream signaling pathways initiated by HMGB1, as well as the entire signaling network of HMGB1. In addition, animal models are an essential tool to study the role of biomarkers in cancer progression. We hope that our results in vitro trigger further investigation on the role of HMGB1 in IHCC in vivo.
In summary, this is the first study to systematically characterize HMGB1 expression in an IHCC cohort. HMGB1 is shown to be an independent poor prognostic factor in IHCC, and our data suggest a link between HMGB1 and VEGF-C in IHCC. That is, overexpression of HMGB1 might promote lymphangiogenesis through a VEGF-C-related pathway. On the other hand, HMGB1 overexpression promotes progression of IHCC by promoting angiogenesis and EMT.
Cholangiocarcinoma (CCA) is a lethal malignancy of the biliary tract with very few treatment options. CCA is characterized by poor responsiveness to chemotherapy and radiotherapy in the majority of cases. So far, surgical resection is the only potentially curative option. The morbidity and mortality of CCA, especially intrahepatic cholangiocarcinoma (IHCC), have been increasing worldwide in recent years. IHCC is characterized by silent clinical signatures, early regional invasiveness, distant metastasis, and a poor prognosis.
High-mobility group box 1 (HMGB1) is involved in a variety of biologic processes, including transcription, DNA repair, differentiation, and extracellular signal transduction. Clinically, overexpression of HMGB1 has been reported in multiple malignancies, but there has been no study so far to investigate the role of HMGB in IHCC.
This study, for the first time, showed that HMGB1 is an independent prognostic factor in IHCC and positively correlates with lymphatic microvessel density and vascular endothelial growth factor C (VEGF-C) expression. In vitro analysis suggests that HMGB1 enhances the secretion of VEGF-C and promotes epithelial-mesenchymal transition of RBE cells.
This results defined an important role of HMGB1 in the progression of cholangiocarcinoma, and HMGB1 may serve as a prognostic marker for IHCC patients. These findings suggested that HMGB1 could be a potential molecular target in cholangiocarcinoma.
The authors evaluated HMBG1 as a possible novel prognostic marker for IHCC. Additionally, mechanistic studies were performed investigating a presumed functional link between HMBG1 and epithelial-mesenchymal transition and VEGF-C-dependent lymphangiogenesis. This study deals with an interesting topic; some of the findings herein have not been reported in this particular setting and may indeed give some new insights.
P- Reviewer: Schmelzle M, Schmeding M S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Kelley ST, Bloomston M, Serafini F, Carey LC, Karl RC, Zervos E, Goldin S, Rosemurgy P, Rosemurgy AS. Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy. Am Surg. 2004;70:743-748; discussion 748-749. [PubMed] |
| 3. | Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 652] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 4. | Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, Gaudio E, Alvaro D. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 5. | van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2013;32:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836-2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1949] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 8. | Poser I, Golob M, Buettner R, Bosserhoff AK. Upregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotype. Mol Cell Biol. 2003;23:2991-2998. [PubMed] |
| 9. | Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H. Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol. 2005;166:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Gnanasekar M, Kalyanasundaram R, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A. HMGB1: A Promising Therapeutic Target for Prostate Cancer. Prostate Cancer. 2013;2013:157103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Wu D, Ding Y, Wang S, Zhang Q, Liu L. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol. 2008;216:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762-4773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 637] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Moriwaka Y, Luo Y, Ohmori H, Fujii K, Tatsumoto N, Sasahira T, Kuniyasu H. HMGB1 attenuates anti-metastatic defense of the lymph nodes in colorectal cancer. Pathobiology. 2010;77:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Sasahira T, Kirita T, Oue N, Bhawal UK, Yamamoto K, Fujii K, Ohmori H, Luo Y, Yasui W, Bosserhoff AK. High mobility group box-1-inducible melanoma inhibitory activity is associated with nodal metastasis and lymphangiogenesis in oral squamous cell carcinoma. Cancer Sci. 2008;99:1806-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol. 2011;8:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2157] [Cited by in RCA: 2337] [Article Influence: 137.5] [Reference Citation Analysis (0)] |
| 18. | Qi F, Okimoto G, Jube S, Napolitano A, Pass HI, Laczko R, Demay RM, Khan G, Tiirikainen M, Rinaudo C. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am J Pathol. 2013;183:1654-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Köchli OR, Mross F, Dieterich H, Moch H, Mihatsch M. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 2001;159:2249-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 411] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 20. | Wang L, Zhang J, Yang X, Chang YW, Qi M, Zhou Z, Zhang J, Han B. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Moser B, Janik S, Schiefer AI, Müllauer L, Bekos C, Scharrer A, Mildner M, Rényi-Vámos F, Klepetko W, Ankersmit HJ. Expression of RAGE and HMGB1 in thymic epithelial tumors, thymic hyperplasia and regular thymic morphology. PLoS One. 2014;9:e94118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal tumors. BMC Cancer. 2013;13:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Yang X, Wang W, Wang C, Wang L, Yang M, Qi M, Su H, Sun X, Liu Z, Zhang J. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep. 2014;32:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 25. | Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564-1579. [PubMed] |
| 26. | Hall FT, Freeman JL, Asa SL, Jackson DG, Beasley NJ. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Möbius C, Demuth C, Aigner T, Wiedmann M, Wittekind C, Mössner J, Hauss J, Witzigmann H. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2007;33:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Fujita T. Analyzing risk factors for intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1862-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, Fan W, Li YQ. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008;40:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6742] [Article Influence: 449.5] [Reference Citation Analysis (0)] |
| 32. | Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Flohr AM, Rogalla P, Meiboom M, Borrmann L, Krohn M, Thode-Halle B, Bullerdiek J. Variation of HMGB1 expression in breast cancer. Anticancer Res. 2001;21:3881-3885. [PubMed] |
| 34. | Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathol Oncol Res. 2012;18:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28:1659-1666. [PubMed] |
| 37. | van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 38. | Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2783] [Cited by in RCA: 2981] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 39. | Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 40. | Janda E, Litos G, Grünert S, Downward J, Beug H. Oncogenic Ras/Her-2 mediate hyperproliferation of polarized epithelial cells in 3D cultures and rapid tumor growth via the PI3K pathway. Oncogene. 2002;21:5148-5159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |