Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3239
Peer-review started: July 8, 2014
First decision: September 15, 2014
Revised: October 23, 2014
Accepted: December 16, 2014
Article in press: December 16, 2014
Published online: March 21, 2015
Processing time: 255 Days and 1.6 Hours
AIM: To investigate urotensin-II (UII) and its effects on tumor necrosis factor (TNF)-α and interleukin (IL)-1β in early acute liver failure (ALF).
METHODS: We investigated the time-dependent alteration in UII levels and its effects on TNF-α and IL-1β in liver and blood in the early stage of lipopolysaccharide/D-galactosamine-induced ALF.
RESULTS: After lipopolysaccharide/D-galactosamine challenge, UII rose very rapidly and reached a maximal level 0.5 h, and the level remained significantly elevated after 2 h (P < 0.05). Six hours after challenge, UII began to degrade, but remained higher than at 0 h (P < 0.05). Pretreatment with urantide, an inhibitor of the UII receptor, suppressed the degree of UII increase in liver and blood at 6 h after challenge (P < 0.05 vs paired controls). In addition, liver and blood TNF-α increased from 1 to 6 h, and reached a peak at 1 and 2 h, respectively; however, IL-1β did not rise until 6 h after challenge. Urantide pretreatment inhibited the degree of TNF-α and IL-1β increase following downregulation of UII post-challenge (all P < 0.05).
CONCLUSION: UII plays a role in the pathogenesis and priming of ALF by triggering an inflammatory cascade and driving the early release of cytokines in mice.
Core tip: In this study, we found that urotensin-II (UII) increased before tumor necrosis factor (TNF)-α and interleukin (IL)-1β following lipopolysaccharide/D-galactosamine challenge. Furthermore, pretreatment with urantide, an inhibitor of the UII receptor, blocked TNF-α and IL-1β increases following downregulation of UII in liver and blood at different time points after challenge. Therefore, UII may play a pivotal role in the pathogenesis and priming of acute liver failure by triggering the inflammatory cascade, and initiating and driving the early release of TNF-α and IL-1β in lipopolysaccharide/D-galactosamine-challenged mice.
- Citation: Liu LM, Zhao L, Liang DY, Yu FP, Ye CG, Tu WJ, Zhu T. Effects of urotensin-II on cytokines in early acute liver failure in mice. World J Gastroenterol 2015; 21(11): 3239-3244
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3239
Urotensin-II (UII), a somatostatin-like cyclic vasoactive polypeptide, is released from numerous tissues[1,2], and exerts a wide range of actions in the progression of many diseases, including cardiovascular, endocrine, immune system, and kidney diseases[3]. A recent study found that UII level in plasma and liver was upregulated in patients with acute liver failure (ALF)[4]. UII and its G-protein coupled receptor, GPR14, are mainly expressed in Kupffer and endothelial cells in liver tissues during ALF[4]. Kupffer and endothelial cells are important immune inflammatory cells in organisms; thus, there seems to be an interrelationship between the high level of UII polypeptide and immune-mediated hepatic inflammatory injury in ALF.
ALF is an inflammatory process caused by a variety of proinflammatory cytokines, including interleukin (IL)-1β and IL-6, and particularly tumor necrosis factor (TNF)-α[5,6]. The cascades of these cytokines induced by the early burst of TNF-α result in acute inflammation in liver tissues, and lead to ALF[7]. Our recent study showed that high UII-mediated ALF was associated with the upregulation of proinflammatory cytokines[8]. However, the impact of UII on these cytokines in patients with ALF remains unclear. Therefore, we investigated the time-dependent alteration in UII level and its effects on TNF-α and IL-1β levels in the early stage of ALF.
Lipopolysaccharide (LPS) (Escherichia coli strain O55: B5) and D-galactosamine (D-GalN) were obtained from Sigma-Aldrich (St. Louis, MO, United States). Urantide was purchased from Peptides (Louisville, KY, United States). Male BALB/c mice (6 wk of age) weighing 20-22 g were obtained from the Animal Center of the First People’s Hospital Affiliated to Shanghai Jiaotong University, and maintained in specific pathogen-free air at a temperature of 22 ± 2 °C with a 12 h light/dark cycle and relative humidity of 50%. Animal care and treatment were humane and in compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Medical Scientific Research of the First People’s Hospital, Shanghai Jiaotong University (No: 2013KY041). All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Mice were injected intraperitoneally with 800 mg/kg D-GalN and 50 μg/kg LPS dissolved in 200 μL of pyrogen-free normal saline[9]. The mice were randomly divided into two groups: non-urantide, which received an intravenous injection of 100 μL normal saline, or urantide pretreatment, with 0.6 mg/kg urantide dissolved in 100 μL normal saline 30 min before the LPS/D-GalN injection, as previously described[8]. The mice were then anesthetized and killed at 0.0, 0.5, 1.0, 2.0 and 6.0 h after the LPS/D-GalN injection (n = 6 at each time point per group), and blood and liver were collected for testing.
Total RNA was extracted from liver tissues with TRIzol reagent (Invitrogen of Thermo Fisher Scientific, Waltham, MA, United States) following the manufacturer’s instructions. Two micrograms of total RNA were used for the synthesis of first-strand cDNA with an M-MLV reverse transcription (RT) kit (Fermentas of Thermo Fisher Scientific). The polymerase chain reaction (PCR) primers were designed by Primer Premier 6.0 software (PremierBiosoft, Palo Alto, CA, United States) from the reported sequences (GenBank accession number X66539 for TNF-α, NM031512 for IL-1β, NM011910 for UII, and NM031144 for β-actin) (Table 1). PCR was performed with the following thermal cycling conditions: denaturation at 94 °C for 5 min followed by 32 cycles of denaturation at 94 °C for 1 min, primer annealing at 58 °C (for UII) or 55 °C (for TNF-α and IL-1β) for 45 s, and primer extension at 72 °C for 45 s, with a final extension at 72 °C for 10 min.
| Gene | Primer sequences | Product size (bp) |
| UII | Sense: 5’-GAGCATTCCCTTCATCGTAG-3’ | 385 |
| Antisense: 5’-CATAGCGTTCACTGCTCATT-3’ | ||
| TNF-α | Sense: 5’-GGCGGTGCCTATGTCTACG-3’ | 354 |
| Antisense: 5’-GACAAGCCTGTAGCCCACC-3’ | ||
| IL-1β | Sense: 5’-CCAGTGAAATGATGGCTTATTACAG-3’ | 151 |
| Antisense: 5’-GTAGTGGTGGTCGTAGATTCGTA-3’ | ||
| β-actin | Sense: 5’-CCTGGCACCCAGCACAAT-3’ | 156 |
| Antisense: 5’-GGGCCGGACTCGTCATAC-3’ | ||
| Sense: 5’-ATATCGCTGCGCTGGTCGTC-3’ | 517 | |
| Antisense: 5’-AGGATGGCGTGAGGGAGAGC-3’ |
Serum cytokine levels, including TNF-α and IL-1β, were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, United States) according to the manufacturer’s protocol; and serum UII levels were determined using an enzyme immunoassay kit (Phoenix Biotech, Beijing, China), based on the principle of a “competitive” enzyme immunoassay[10], according to the manufacturer’s guidelines.
SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, United States) was used in the study. The results are expressed as means ± standard deviation. A P < 0.05 was considered statistically significant.
A rapid increase in UII level was observed in the very early stage of the LPS/D-GalN challenge in mice with or without urantide pretreatment. As shown in Figure 1, LPS/D-GalN induced a significant increase in UII, which reached a peak from 0.5 to 2.0 h and remained elevated in liver and blood at 6 h (both P < 0.05). However, in urantide-pretreated mice, UII levels were statistically lower from 0.5 to 6.0 h after challenge compared with the paired control (all P < 0.05).
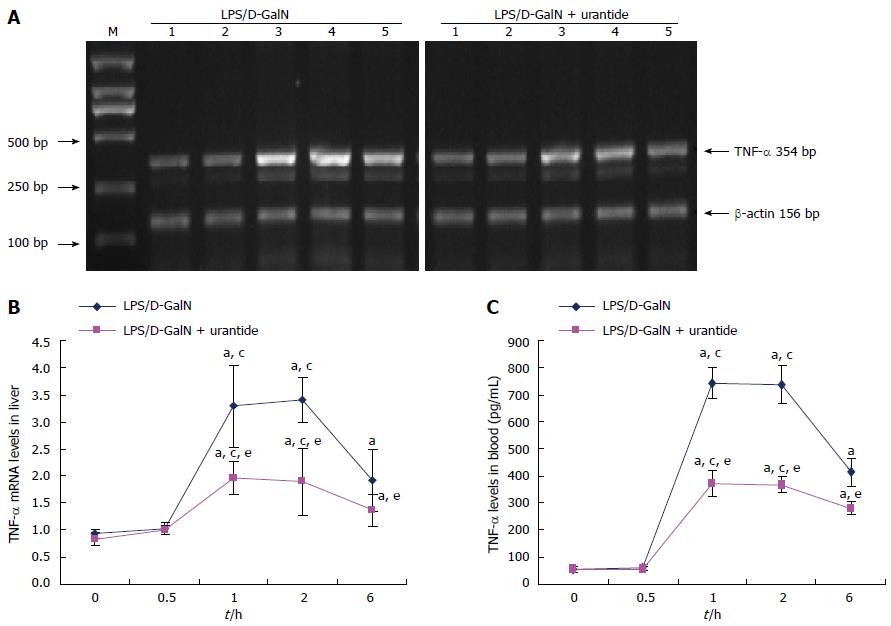
TNF-α levels were measured in the early stage of the LPS/D-GalN challenge in liver and blood in mice with or without urantide pretreatment. As shown in Figure 2, TNF-α increased and peaked at 1 and 2 h, and remained elevated at 6 h after drug administration in liver and blood (both P < 0.05). TNF-α levels in liver and blood were not significantly different between 0 and 0.5 h. However, TNF-α levels in liver and blood in urantide-pretreated mice were significantly lower than in paired control mice from 1 to 6 h after challenge (all P < 0.05).
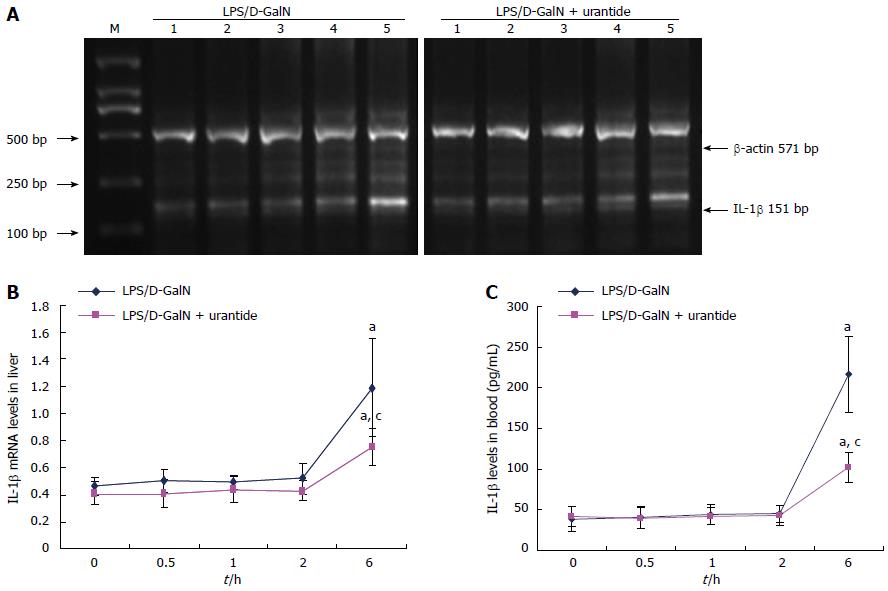
The time-dependent alteration in IL-1β following urantide pretreatment was also determined in the early stage of the LPS/D-GalN challenge. As shown in Figure 3, IL-1β did not increase in liver and blood until 6 h after the LPS/D-GalN challenge (P < 0.05). IL-1β levels were not significantly different at 0.0, 0.5, 1.0, and 2 h. However, urantide pretreatment lowered IL-1β levels in liver and blood compared with paired control mice at 6 h after challenge (P < 0.05).
LPS induces lethal ALF in mice sensitized by D-GalN[11]. For more than 20 years, LPS/D-GalN-induced hepatitis in mice has been regarded as a well-established model for gaining insight into the mechanism of ALF[12,13]. Our previous reports showed that the simultaneous administration of LPS and D-GalN led to high mortality due to a severe hepatic inflammatory response followed by massive cell apoptosis and necrosis in mice[5,8,14]. Elevated levels of UII were also observed in the liver and blood in this animal model, and blockade of the signal with urantide markedly suppressed liver apoptosis and acute inflammation[8].
UII is a cyclic polypeptide that exerts a wide range of actions in health and disease[15,16], and has an important effect on inflammation-related diseases, such as hypertension[17], coronary atherosclerosis[18], chronic glomerulonephritis[19], and hepatic cirrhosis[20]. Patients with ALF also exhibit enhanced expression of UII in the liver[4]. Watanabe et al[21] and Ban et al[22] showed that UII is associated with endothelial dysfunction-related diseases and immune-driven inflammatory diseases. To validate the role of UII signals in the pathogenesis of ALF, we serially tested the levels of UII in liver and blood to investigate the sequence of events preceding acute liver damage, which was not fully apparent until 6 h after co-administration of LPS/D-GalN[5]. An early event prior to obvious injury may reveal the pathophysiologic mechanisms of ALF. We found that UII is significantly induced in liver and plasma 6 h after LPS/D-GalN challenge, increasing and reaching a maximal level very rapidly. At 6 h after the challenge, UII levels began to degrade, but remained high. In addition, we also observed that urantide pre-treatment suppresses the degree of this increase, suggesting an autocrine loop in the in vivo production of UII. With positive feedback, early enhancement of UII expression may be induced, finally leading to hepatic inflammatory injury after the LPS/D-GalN challenge. Therefore, UII has cytokine-like activity.
To gain further mechanistic insights, we determined whether UII has an effect on proinflammatory cytokines, including TNF-α and IL-1β, the pacing factors in the inflammatory response to hepatic injury. TNF-α in liver and blood increases following UII upregulation, but not until 1 h after the LPS/D-GalN challenge. This is subsequently followed by elevations of liver and blood IL-1β levels, the secretion of which did not rise until 6 h after the LPS/D-GalN challenge. This time-dependent alteration suggests a causal relationship between UII and both TNF-α and IL-1β in early ALF.
To confirm this deduction, the potency of the UII receptor antagonist, urantide, was evaluated by the expression of TNF-α and IL-1β. We found that urantide pretreatment suppresses the increase in UII, and reduces the degree of increase of TNF-α and IL-1β at different time points in the early stage of the LPS/D-GalN challenge. These results extend our previous finding on the inhibitory effect of urantide on the production of proinflammatory cytokines in mice with ALF[8]. From these results, we suggest that the sharp and rapid upregulation of UII induces early expression and secretion of TNF-α and IL-1β.
TNF-α is known to play a central role in the pathogenesis of LPS/D-GalN-induced liver failure[23] by inducing the release of a variety of proinflammatory cytokines, including IL-1β[7]. Previous reports showed that UII induced the expression of IL-6[24], and was upregulated by IFN-γ[25]. Therefore, UII is involved in the vicious cycle of inflammatory cytokine release in immune-related tissue injury.
In conclusion, UII can cause acute liver injury by triggering the inflammatory response, and by initiating and driving the early release of proinflammatory cytokines in LPS/D-GalN-challenged mice.
Urotensin-II (UII) plays a role in inflammation-related diseases and is upregulated in acute liver failure (ALF), an inflammatory process caused by proinflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-1β. However, the impact of UII on these cytokines remains elusive.
This study examines the mechanisms of immune-mediated inflammatory injury in acute liver failure, and the role of the urotensin system in tissue damage and inflammation.
This study demonstrated that UII may cause acute liver injury by triggering the inflammatory response, and by initiating and driving the early release of proinflammatory cytokines in lipopolysaccharide/D-galactosamine-challenged mice.
The urotensin system may be a new research hotspot in mechanistic studies, and may provide a new drug target for the future treatment of ALF.
ALF is a life-threatening clinical syndrome with a sudden loss of hepatic function in patients with no preexisting history of liver disease. The pathologic feature of ALF is the death of a large number of parenchymal hepatocytes resulting from cell apoptosis and necrosis. Massive cell loss leads to functional impairment of the liver, and ultimately, multi-organ failure and death. Mortality is high in patients with ALF (approximately 90%). UII, initially isolated from the teleost urophysis, is a somatostatin-like cyclic neuropeptide widely distributed in many tissues in many classes of vertebrates, including humans, and exerts biologic actions in both physiologic and pathologic conditions.
The paper investigated the role of UII in modifying the levels of TNF-α and IL-1β in a well-established model of ALF. The major finding is the demonstration of the role of UII in initiating the proinflammatory cascade in ALF.
P- Reviewer: Ma YJ S- Editor: Decsi T, Hashimoto N, Vezali E L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Tostivint H, Lihrmann I, Vaudry H. New insight into the molecular evolution of the somatostatin family. Mol Cell Endocrinol. 2008;286:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Chen YH, Yandle TG, Richards AM, Palmer SC. Urotensin II immunoreactivity in the human circulation: evidence for widespread tissue release. Clin Chem. 2009;55:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, Fournier A, Tonon MC, Pelletier G, Conlon JM, Leprince J. Urotensin II, from fish to human. Ann N Y Acad Sci. 2010;1200:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Leifeld L, Clemens C, Heller J, Trebicka J, Sauerbruch T, Spengler U. Expression of urotensin II and its receptor in human liver cirrhosis and fulminant hepatic failure. Dig Dis Sci. 2010;55:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Liu LM, Zhang JX, Luo J, Guo HX, Deng H, Chen JY, Sun SL. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized rats. Dig Dis Sci. 2008;53:1316-1324. [PubMed] |
| 6. | Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202-R1209. [PubMed] |
| 7. | Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, Collins RD, Hawiger J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434-48442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Liang DY, Liu LM, Ye CG, Zhao L, Yu FP, Gao DY, Wang YY, Yang ZW, Wang YY. Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-κB pathway in ALF mice. PLoS One. 2014;8:e64895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Gong X, Luo FL, Zhang L, Li HZ, Wu MJ, Li XH, Wang B, Hu N, Wang CD, Yang JQ. Tetrandrine attenuates lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. Int Immunopharmacol. 2010;10:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 278] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Yin X, Gong X, Jiang R, Zhang L, Wang B, Xu G, Wang C, Wan J. Synthetic RGDS peptide attenuated lipopolysaccharide/D-galactosamine-induced fulminant hepatic failure in mice. J Gastroenterol Hepatol. 2014;29:1308-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Kemelo MK, Wojnarová L, Kutinová Canová N, Farghali H. D-galactosamine/lipopolysaccharide-induced hepatotoxicity downregulates sirtuin 1 in rat liver: role of sirtuin 1 modulation in hepatoprotection. Physiol Res. 2014;63:615-623. [PubMed] |
| 13. | Chojkier M, Fierer J. D-Galactosamine hepatotoxicity is associated with endotoxin sensitivity and mediated by lymphoreticular cells in mice. Gastroenterology. 1985;88:115-121. [PubMed] |
| 14. | Liu LM, Zhang JX, Wang XP, Guo HX, Deng H, Luo J. Pim-3 protects against hepatic failure in D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest. 2010;40:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1156-R1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Kiss RS, You Z, Genest J, Behm DJ, Giaid A. Urotensin II differentially regulates macrophage and hepatic cholesterol homeostasis. Peptides. 2011;32:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Cheung BM, Leung R, Man YB, Wong LY. Plasma concentration of urotensin II is raised in hypertension. J Hypertens. 2004;22:1341-1344. [PubMed] |
| 18. | Hassan GS, Douglas SA, Ohlstein EH, Giaid A. Expression of urotensin-II in human coronary atherosclerosis. Peptides. 2005;26:2464-2472. [PubMed] |
| 19. | Balat A, Karakök M, Yilmaz K, Kibar Y. Urotensin-II immunoreactivity in children with chronic glomerulonephritis. Ren Fail. 2007;29:573-578. [PubMed] |
| 20. | Liu D, Chen J, Wang J, Zhang Z, Ma X, Jia J, Wang Y. Increased expression of urotensin II and GPR14 in patients with cirrhosis and portal hypertension. Int J Mol Med. 2010;25:845-851. [PubMed] |
| 21. | Watanabe T, Arita S, Shiraishi Y, Suguro T, Sakai T, Hongo S, Miyazaki A. Human urotensin II promotes hypertension and atherosclerotic cardiovascular diseases. Curr Med Chem. 2009;16:550-563. [PubMed] |
| 22. | Ban Y, Watanabe T, Suguro T, Matsuyama TA, Iso Y, Sakai T, Sato R, Idei T, Nakano Y, Ota H. Increased plasma urotensin-II and carotid atherosclerosis are associated with vascular dementia. J Atheroscler Thromb. 2009;16:179-187. [PubMed] |
| 23. | Fukuda T, Mogami A, Tanaka H, Yoshikawa T, Hisadome M, Komatsu H. Y-40138, a multiple cytokine production modulator, protects against D-galactosamine and lipopolysaccharide-induced hepatitis. Life Sci. 2006;79:822-827. [PubMed] |
| 24. | Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, Steplewski K, Aiyar N, Douglas SA. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:238-250. [PubMed] |
| 25. | Birker-Robaczewska M, Boukhadra C, Studer R, Mueller C, Binkert C, Nayler O. The expression of urotensin II receptor (U2R) is up-regulated by interferon-gamma. J Recept Signal Transduct Res. 2003;23:289-305. [PubMed] |