Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3157
Peer-review started: November 15, 2014
First decision: December 11, 2014
Revised: December 19, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: March 21, 2015
Processing time: 126 Days and 22.1 Hours
Pancreatic ductal adenocarcinoma (PDAC) represents the fourth cause of death in cancer and has a 5-year survival of < 5%. Only about 15% of the patients present with a resectable PDAC with potential to undergo “curative” surgery. After surgery, local and systemic recurrence, is though very common. The median survival of resected patients with adjuvant chemotherapy after surgery is only 20-23 mo. This underscores the significant need to improve PDAC management strategies. Increased survival rate is dependent on new breakthroughs in our understanding of not at least tumor biology. The aim of this review is to update and comment on recent knowledge concerning PDAC biology and new diagnostics and treatment modalities. One fundamental approach to improve survival rates is by earlier and improved diagnosis of the disease. In recent years, novel blood-based biomarkers have emerged based on genetic, epigenetic and protein changes in PDAC with very promising results. For biomarkers to enter clinical practice they need to have been developed using adequate control groups and provide high sensitivity and specificity and by this identify patients at risk already in a pre-symptomatic stage. Another way to improve outcomes, is by employing neoadjuvant treatments thereby increasing the number of resectable cases. Novel systemic treatment regimes like FOLFIRINOX and nab-paclitaxel have demonstrated improvements in prolonging survival in advanced cases, but long-term survival is still scarce. The future improved understanding of PDAC biology will inevitably render new treatment options directed against both the cancer cells and the surrounding microenvironment.
Core tip: This review updates the current progress in the management of pancreatic cancer with focus on novel modes of diagnosis and treatment. New blood-based biomarkers for early detection based on genetic, epigenetic and protein changes in pancreatic cancer are discussed and new treatment strategies such as stromal depletion are highlighted. Pancreatic cancer is a systemic disease already at diagnosis and demands multimodal managements strategies in order to improve outcomes.
- Citation: Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: Surgery is not enough. World J Gastroenterol 2015; 21(11): 3157-3165
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3157
Pancreatic cancer is a devastating disease. The estimated number of deaths attributed to the disease is over 266000 worldwide every year[1]. It represents the fourth cause of death in cancer but may, by the time of 2030, have moved to the second place if no significant treatment advances are made[2]. The overall 5-year survival rate is < 5% and a majority of patients presents with inoperable and non-curable tumors. Pancreatic cancer refers to adenocarcinoma arising from the ductal epithelium in the exocrine part of the gland. Pancreatic ductal adenocarcinoma (PDAC) accounts for about 90% and is the form mainly addressed in this review.
Much effort has focused to find evidence for causative risk factors but reasonably few are widely established. Tobacco smoking, obesity, longstanding diabetes, family history of PDAC and chronic pancreatitis are risk factors[3]. Overweight (BMI > 25) during adult life is associated with increased risk and earlier onset of the disease[4]. Diabetes mellitus has recently been presented as an independent risk factor and increased risk associated with disease duration, although metformin reduces this risk[5]. Still this connection is somewhat complicated since studies have reported that diabetes might be an early indication of underlying PDAC[6]. Several studies have also provided evidence for the connection between chronic pancreatitis and PDAC. This usually occurs with a delay of one or two decades between the chronic pancreatitis and PDAC[7].
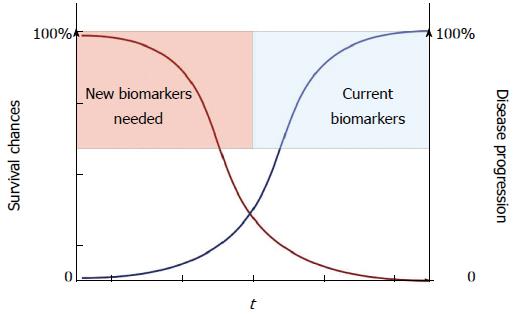
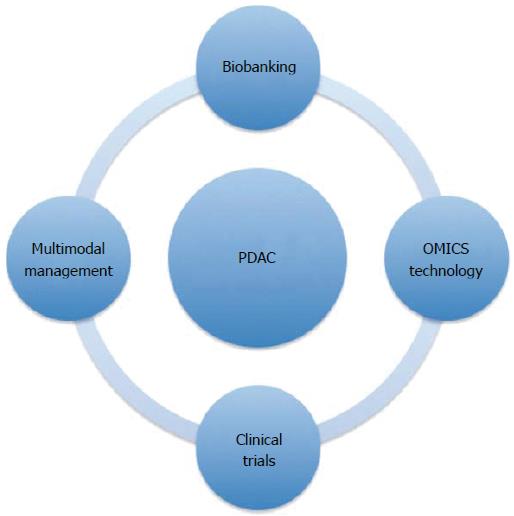
Lack of early biomarkers together with the silent nature of the disease, consequently rendering diagnosis in late, unresectable stages, contributes to the high death rate (Figure 1). To make progress and improve long-term survival, advances in several areas such as biomarkers for early diagnosis, optimized surgical strategies, and new types of systemic therapy are required (Figure 2). This review aims to update and comment on recent knowledge concerning PDAC management. PDAC is a complex disease with different clinical and pathological phenotypes and hence requires multiple modes of intervention to control disease progression.
In 1976 it was suggested that ductal papillary hyperplasia, atypia and carcinoma in situ morphologically appear as precursor lesions. This was based on examinations of histological samples from 227 cases of human PDAC[8]. Numerous studies have since observed and graded histological tissue of these lesions, called pancreatic intraepithelial neoplasia (PanIN) and provided confirmation for a common pathogenesis with similar profiles and progress leading to PDAC[9]. Transformation from a dysplastic epithelium to dysplasia and ultimately invasive carcinoma is parallel with the accumulation of mutations[10]. There have been focused efforts to genetically map out PanINs and to discover collective mutations in order to find clinical useful biomarkers. Commonly found mutations include activation of KRAS oncogene, inactivation of tumor suppressor genes CDKN2A/INK4A, TP53 and SMAD4[11].
KRAS mutation is a critical event and is the most frequently found oncogene in PDAC[12]. This mutation induces degradation of the tumor suppressor protein p53-SNAIL complex and is present in a higher degree in more advanced stages of PanIN[13]. KRAS has been considered for diagnostic purposes, but has a lack in both sensitivity and specificity[14]. The inactivation of CDKN2A results in loss of the p16 protein, a cell cycle regulator which generates increased cellular proliferation[10]. This mutation occurs in PDAC, but is also seen in several other malignancies. Abnormal TP53 adds genomic instability by permitting cells to bypass DNA checkpoints and this way avoid apoptotic signals. This mutation occurs in approximately 50%-75% of PDAC[10]. Tumor suppressor gene SMAD4 is mutated in about 55% of PDAC[15]. SMAD4 occurs in various cancers but with a higher sensitivity and specificity in PDAC. It often appears in late stages and loss of SMAD4 expression correlates with a poorer prognosis and potential metastasis[16]. However almost all PDAC contain one of these four mentioned mutations[17].
The stroma, a hallmark for PDAC, is the structural framework surrounding the tumor. The PDAC cells interact with the stromal cells in order to create a unique microenvironment to facilitate tumor progression by promoting tumor growth, local hypoxia and preventing the effects of chemotherapy[18]. Signals to stromal cells, from PDAC cells, modify the structure of the extracellular matrix (ECM) composed of collagens, noncollagen glycoproteins, glycosaminoglycans, growth factors and proteoglycans. These signals (mostly being proinflammatory) increase the recruitment of inflammatory cells, but also stimulate proliferation of fibroblasts, specifically pancreatic stellate cells (PSCs)[19]. PSCs contribute to the excess fibrosis formation around the tumor and promote tumor progression[20]. PSCs seem to facilitate immune evasion, chemoresistance and the recurrence of PDAC, as verified both by in vitro and in vivo studies[21].
Currently, carbohydrate antigen 19-9 (CA19-9) is the only biomarker used in the routine management of PDAC. CA19-9 is a sialylated Lewis blood group antigen that can be quantitatively measured in serum, but its use is limited to monitoring response to therapy, not as a diagnostic marker. The sensitivity and specificity of CA19-9 is about 80% for PDAC diagnosis[22]. CA19-9 is elevated in benign conditions, such as cholestasis and chronic pancreatitis, which hampers its specificity for PDAC. Moreover, approximately 5%-10% of the population do not express Lewis antigens. Thus, there is great interest in identifying and developing new markers for PDAC to help detect early stage PDAC and its precursors. The development of useful biomarkers requires well-designed studies that evaluate marker performance in the appropriate clinical setting where the marker will be required. Because blood is more accessible and less invasive to achieve than tissue, the optimal screening strategy for PDAC will likely involve the development of highly accurate and inexpensive blood biomarkers, followed by a second-level, imaging-based test to confirm a positive biomarker result. The recent developments in the detection of biomarkers, such as point mutations, DNA methylation patterns, microRNAs (miR), and proteins in blood from patients with PDAC, have opened up new avenues of research for the early diagnosis and treatment of this lethal disease.
Detecting resectable disease is the first step in the fight against PDAC[23]. Biomarkers that could increase the proportion of patients that are candidates for surgery could potentially improve survival rates. Of the entire PDAC cohort, only 10%-15% have localized disease. According to the American Cancer Society[24], the 5-year survival is 24% for resectable PDAC, 9% for locally advanced PDAC, and 2% for metastatic PDAC. However, the 5-year survival rate in resectable PDAC is about 50% in tumors < 2 cm and close to 100% in tumors < 1 cm[25], indicating that even earlier detection can improve survival. A recent study[26] modeled the benefits of early diagnosis of PDAC using a blood-based biomarker signature. It was found that the cost-effectiveness depended on the incidence of PDAC within the population and that certain high-risk groups, such as familial cases and new-onset diabetes mellitus, could be screened at an acceptable cost for the society.
The genetic alterations occurring during PDAC development have been extensively studied. A comprehensive genetic analysis of 24 PDAC found an average of 63 genetic alterations, the majority of which are point mutations[27]. Mathematical analyses of tumor DNA sequence data suggest a broad time window of opportunity for early detection to prevent deaths from metastatic PDAC[28]. One recent study showed that mutations in KRAS and TP53 can be detected using genomic DNA from exosomes derived from serum from patients with PDAC, providing a novel diagnostic tool for PDAC[29].
PDAC is an epigenetic disease in addition to being a genetic disease. Epigenetic biomarkers, such as DNA methylation and microRNAs, may be utilized for diagnosis of PDAC. One study identified two novel genes, BNC1 and ADAMTS1, that showed a high frequency of methylation in PDAC, up to 100% in PanIN-3 and 97% in stage I invasive cancers[30]. These alterations could be detected in serum samples from patients with PDAC. The sensitivity was 79% for BNC1 and 48% for ADAMTS1, whereas specificity was 89% for BNC1 and 92% for ADAMTS1. Overall sensitivity using both markers was 81% and specificity was 85% for PDAC diagnosis. MicroRNAs are small non-coding RNA molecules, about 22 nucleotides, that regulate stability and translation of messenger RNAs. One study investigated differences in microRNA expression in whole blood between patients with PDAC, chronic pancreatitis, and healthy participants[31]. A total of 38 microRNAs were significantly dysregulated in patients with PDAC as compared to controls. Two diagnostic panels were constructed comprising 4 microRNAs in index I (miR-145, miR-150, miR-223, miR-636) and 10 in index II (miR-26b, miR-34a, miR-122, miR-126*, miR-145, miR-150, miR-223, miR-505, miR-636, miR-885.5p). The performance of the panels was validated in patients with stage IA-IIB PDAC, with an index I area under the curve (AUC) of 0.80; index I and CA19-9 AUC of 0.83; index II AUC of 0.91; and index II and CA19-9 AUC of 0.91.
Proteomics is defined as the large-scale study of proteins, particularly their abundances, functions, structures, and interacting partners. In the field of proteomics, antibody-based technologies and mass spectrometry are the most common techniques used for biomarker discovery. One study used a recombinant antibody microarray platform, targeting mainly immunoregulatory proteins, in sera from patients with resectable PDAC, chronic pancreatitis, autoimmune pancreatitis, and healthy controls[32]. The results identified serum portraits distinguishing PDAC from chronic pancreatitis (AUC: 0.86), autoimmune pancreatitis (AUC: 0.99) and healthy controls (AUC: 0.95). A 25-serum biomarker signature discriminating PDAC from the combined group of chronic pancreatitis, autoimmune pancreatitis, and healthy controls was determined that had a high diagnostic yield with an AUC of 0.88. Another study applied protein deep sequencing using high-definition mass spectrometry (HDMSE) to serum samples from patients with resectable PDAC, benign pancreatic disease, and healthy controls[33]. A global protein expression comparison of the three study groups was made using label-free quantification and bioinformatic analyses. More than 71000 features were detected within the data revealing 715 unique proteins. Two-way unsupervised hierarchical clustering identified 134 proteins that successfully classified PDAC patients from the controls, and found 40 proteins that showed a significant up-regulation in the PDAC group. This discrimination reliability was further confirmed by principal component analysis. Disease link associations could be made for BAZ2A, CDK13, DAPK1, DST, EXOSC3, INHBE, KAT2B, KIF20B, SMC1B, and SPAG5, by pathway network linkages to TP53, the most frequently altered tumor suppressor in PDAC.
PDAC is generally diagnosed when the disease is at an advanced stage. As a consequence, the majority of samples available for biomarker discovery come from patients with advanced disease. One potential solution is to take advantage of prospective cohort studies, such as European Prospective Investigation into Cancer and Nutrition. In such cohorts, the performance of the biomarkers in the months or even years prior to PDAC diagnosis can be evaluated. Obstructive jaundice is a common complication of PDAC, but few studies include patients with benign causes of obstructive jaundice in their evaluation of tumor markers. Furthermore, many candidate markers of PDAC have been found to clearly distinguish PDAC from healthy controls but fail to distinguish them from chronic pancreatitis. This may be due to the fact that there is an inflammatory component in PDAC and several markers may be shared by both conditions. These observations suggest that choice of adequate controls is important identify the most cancer-specific biomarkers.
Surgical resection with radical intent remains the only potential curative treatment option today. PDAC is staged according to the tumor-node-metastasis classification, which categorizes patients into 3 stages: resectable, locally advanced, and metastatic disease[34]. Contrast-enhanced computed tomographyis an established method for staging and provides around 80% accuracy concerning resectability[35]. Magnetic resonance imaging, and endoscopic ultrasound are valuable modalities if diagnostic difficulties persist after[36]. Tumor size, vascular involvement, age and comorbidity are to be considered in the preoperative staging and decision to proceed with surgical resection[37]. A pancreatoduodenectomy (Whipple), distal pancreatectomy or a total pancreatectomy is usually performed, depending on tumor location and type. The hospital mortality rate following surgical resection may be below 2%, while overall morbidity remains up to 60%[38]. Complications include delayed gastric emptying, wound infections, abdominal abscess, and not at least pancreatic fistulas where grades B and C are most problematic[39-41]. It should be emphasized that an uncomplicated postoperative course is associated with a better long-term survival[42]. With regard to endocrine status, progression of disease has a greater impact than the surgical intervention, and diabetes mellitus (especially new-onset) may often be resolved by resection of the pancreatic tumor[43,44].
Surgical results tend to correlate strongly with both institutional and surgical volumes. Several studies have demonstrated significantly decreased mortality and morbidity at high volume centers[38,45,46]. This association also applies to long-term survival even after corrected perioperative mortality[47]. This association between hospital volume and improved results is believed to be multifactorial. One suggested explanation is more experienced medical staff with ability to detect and treat complications at an earlier stage, which may improve the outcome[47]. Superior surgical performance due to higher operation frequency may improve both short- and long-term outcome. Since the treatment is multimodal and also includes adjuvant therapy, the oncologists are likely to improve their results with increased experience as well. Following the implementation of centralization, the 2-year survival among resected patients has increased with over 10% and is considered a great way to improve surgical outcome[46].
The fast-track (FT) concept is a standardized and coordinated perioperative protocol to handle surgical patients. This method intends to reduce surgical stress, accelerate postoperative recovery and improve safety[48]. Several studies have displayed the effect of implementation of the FT concept in pancreaticoduodenectomy[49-51]. It evidently correlates with early recovery and reduces morbidities, such as delayed gastric emptying[52]. The length of hospital stay will also be significantly shortened by 2-6 d[49]. The FT concept is also beneficial from an economical point of view with reduced hospital costs as demonstrated by Kennedy et al[49].
Adjuvant chemotherapy is recommended after pancreatic resection for PDAC based on several randomized controlled trials, including GITSG[53], ESPAC-1[54], ESPAC-3[55], RTOG-9704[56] and CONKO-001[57]. Whether neoadjuvant therapy is superior to adjuvant therapy for PDAC is controversial. The proposed upside of neoadjuvant therapy is early treatment of micrometastases, potential downstaging of borderline resectable tumors, decreasing the percentage of positive lymph nodes, enhanced chemotherapeutic penetrance due to improved vascularization and a higher percentage of accomplished R0 resections[58,59]. It has also been hypothesized that patients receiving neoadjuvant treatment stand a better chance of completing the full multimodal treatment[58]. Another suggested advantage is the selection and the ability to exclude patients developing progressing and metastatic disease, thus avoiding unnecessary surgery[60]. A neoadjuvant treatment requires histological confirmation and this might further increase the detection of patients unlikely to benefit from resection[59,61]. Neoadjuvant therapy is associated with delayed resection, and thus theoretically a possible risk of tumor progression[62]. Previous studies have concluded gemcitabine with radiotherapy as the most effective neoadjuvant treatment with the best effect on overall survival[58,63]. A population based study between 1987-2006 demonstrated a 12 mo survival advantage and lower rate of lymph node positivity between neoadjuvant and adjuvant treatment[59]. Still a recent meta-analysis did not conclude a significant effect on overall survival among resectable patients after receiving neoadjuvant or adjuvant treatments approaches. However, there was a small though not statistically significant effect on survival benefits for neoadjuvant as compared to adjuvant treatment[62]. Thus, with better chemotherapy (see e.g., below) there might be a case for neoadjuvant treatment future on.
Lymph node involvement and resection margin status (R0/R1) remain important prognostic factors after surgical resection for PDAC[64,65]. Artificial neural networks (ANNs) represent a non-linear pattern recognition technique that simulates the analytic learning processes of the human brain. Thus, adapting to changing environment through continuous learning via trial and error, ANN is supplied with information from various sources to detect complicated patterns. The benefit of ANNs is to automatically detect relationships between “inputs” to the network and the “output” by integrating all possible connections between the input variables[66]. One study displayed ANN as a tool in prediction of survival after surgical resection[67]. This was achieved by including clinical risk factors in order to create a survival model. The C-index of ANN was 0.79 compared to Cox regression 0.67 thus, indicating ANNs superior predictive ability. Biomarkers, if available, should also be taken into consideration for prognostic prediction. Unfortunately, there are no validated biomarkers to predict the clinical course[68]. In the future, however promising investigational biomarkers may be incorporated into ANNs in order to improve prognostic performance and help inform clinical decision-making.
Most PDAC patients present with locally advanced or metastatic disease and are consequently not suitable for surgery. Single therapy with gemcitabine has generally been regarded as first-line therapy for advanced PDAC over the almost two last decades[69]. Numerous patients do not respond to gemcitabine therapy due to chemoresistance[70]. The variation in chemoresistance between individuals is partly attributed the human equilibrative nucleoside transporter-1 (hENT-1)[71]. This transporter is responsible for the intracellular uptake of gemcitabine and studies have demonstrated a relationship between longer median survival and high levels of hENT-1[72,73]. Clinical data have revealed that a majority of patients do not have a high hENT-1 expression and thus an expected decreased response to gemcitabine treatment[70]. To determine expression of hENT-1 and individualize gemcitabine treatment strategies could be both cost efficient and avoid unnecessary treatment, but needs further investigations and ethical considerations[74].
Few combinations of gemcitabine with other cytotoxic agents have yet provided any significantly prolonged overall survival rates[75,76]. Gemcitabine treatment combinations also often involved more toxic effects compared with gemcitabine administration as monotherapy[76]. FOLFIRINOX (i.e., a combination of oxaliplatin, irinotecan, fluorouracil, and leucovorin) has been proved to prolong both survival (from 6.8 mo with gemcitabine alone to 11.1 mo with FOLFIRINOX) and progression free time (3.4 to 6.4 mo) for metastatic patients with good performance status (ECOG 0-1). This treatment does, however, also carry significantly more adverse effects and is only applicable in a selected group of patients.
Another interesting treatment regime is albumin-bound paclitaxel (nab-paclitaxel) in combination with gemcitabine. Preclinical studies have showed synergistic effects and increased chemotherapeutic levels in the tumor[77]. SPARC has affinity for albumin, and could thus theoretically facilitate delivery of paclitaxel to the tumor stroma. The stroma is thereby broken down (stromal depletion) and tumor cells close in on each other with resulting increased tumor vascularity[78]. Conversely, a study in mice reported that SPARC deficiency did not alter levels of paclitaxel in the tumors[79]. However, recently published data from a phase 3 study reported improved survival, in patients with advanced PDAC treated with gemcitabine and nab-paclitaxel, compared to gemcitabine as a single therapy[80]. The toxicity profile includes neutropenia and neuropathy, but is not worse when compared to gemcitabine treatment alone. Consequently, nab-paclitaxel has become a therapeutic agent with potential to be part of future PDAC treatments[81].
Secreted protein acidic and rich in cysteine (SPARC) or osteonectin is a calcium-binding glycoprotein frequently expressed by stromal cells surrounding the tumor. The biological role of SPARC involves cell-to-matrix interactions, including cell migration and tissue remodeling, mainly in tissues with high ECM turnover. SPARC is tumor-specific and could act as a tumor suppressor, but also have preinvasive characteristics that possibly increase efficiency of chemotherapy[82]. High SPARC expression, as in the pancreatic tumor stroma, has emerged as a clinical and prognostic factor for PDAC[83,84]. Other strategies to target the tumor stroma involve reverting the activated PSC to its quiescent state. It has been found that PSCs express high levels of the vitamin D and that treatment with calcipotriol, a vitamin receptor D ligand, depletes the stroma and increases intratumoral delivery of gemcitabine in mouse models of PDAC[85]. Other therapies aiming to target and block the interaction between tumor cells and PSCs are also being tested, hopefully further improving the poor prognosis of PDAC[19]. These studies provide novel approaches for targeting the pancreatic tumor stroma and suggest that stromal depletion could become the mainstay of PDAC therapy in the future.
Many new genetic, epigenetic, and proteomic tumor markers are under investigation for the non-invasive diagnosis of early-stage PDAC. For a cost-effective diagnostic test, screening should be applied in high-risk individuals and the test must have high sensitivity and specificity. The use of adequate controls is mandatory during discovery and validation steps in the biomarker development phase. Future biomarkers need to identify patients already in a pre-symptomatic stage. There are strong data that centralization of surgical services improves outcomes after PDAC surgery. Principles for the fast-track concept are now well recognized as both safe and applying best possible available evidence-based and structured care. The benefits of neoadjuvant treatment strategies warrant further investigation before being fully implemented. ESPAC-5, an ongoing study with comparisons between immediate surgical explorations to neoadjuvant therapy, will hopefully further determine the potential role of neoadjuvant therapy. Increased understanding of the stromal compartment of PDAC, both in terms of tumor progression and as a defense barrier against innate immunity and chemotherapy, will be of outmost relevance for the development of future treatments. Even though improved diagnostics and neoadjuvant/preoperative chemotherapy might increase the number of resectable patients, PDAC is a systemic disease already at the time of diagnosis and surgery alone is not enough.
P- Reviewer: Fusai GK S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 3. | Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553-2562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, Ren ZG, Jiang GL. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 7. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690-2698. [PubMed] |
| 9. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 845] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 10. | Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23 Suppl 10:x135-x138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Aho U, Zhao X, Löhr M, Andersson R. Molecular mechanisms of pancreatic cancer and potential targets of treatment. Scand J Gastroenterol. 2007;42:279-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Klimstra DS, Longnecker DS. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol. 1994;145:1547-1550. [PubMed] |
| 13. | Herreros-Villanueva M, Gironella M, Castells A, Bujanda L. Molecular markers in pancreatic cancer diagnosis. Clin Chim Acta. 2013;418:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Herreros-Villanueva M, Rodrigo M, Claver M, Muñiz P, Lastra E, García-Girón C, Coma del Corral MJ. KRAS, BRAF, EGFR and HER2 gene status in a Spanish population of colorectal cancer. Mol Biol Rep. 2011;38:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Iacobuzio-Donahue CA, Song J, Parmiagiani G, Yeo CJ, Hruban RH, Kern SE. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res. 2004;10:1597-1604. [PubMed] |
| 16. | Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Eshleman JR. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674-4679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 17. | Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 18. | Hamada S, Masamune A, Shimosegawa T. Alteration of pancreatic cancer cell functions by tumor-stromal cell interaction. Front Physiol. 2013;4:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett. 2014;343:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Haqq J, Howells LM, Garcea G, Metcalfe MS, Steward WP, Dennison AR. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. Eur J Cancer. 2014;50:2570-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol. 2014;20:11216-11229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 23. | Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 25. | Ansari D, Aronsson L, Sasor A, Welinder C, Rezeli M, Marko-Varga G, Andersson R. The role of quantitative mass spectrometry in the discovery of pancreatic cancer biomarkers for translational science. J Transl Med. 2014;12:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Ghatnekar O, Andersson R, Svensson M, Persson U, Ringdahl U, Zeilon P, Borrebaeck CA. Modelling the benefits of early diagnosis of pancreatic cancer using a biomarker signature. Int J Cancer. 2013;133:2392-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 27. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3026] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 28. | Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2041] [Cited by in RCA: 1944] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 29. | Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 786] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 30. | Yi JM, Guzzetta AA, Bailey VJ, Downing SR, Van Neste L, Chiappinelli KB, Keeley BP, Stark A, Herrera A, Wolfgang C. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin Cancer Res. 2013;19:6544-6555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE, Yilmaz M, Holländer NH. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 32. | Wingren C, Sandström A, Segersvärd R, Carlsson A, Andersson R, Löhr M, Borrebaeck CA. Identification of serum biomarker signatures associated with pancreatic cancer. Cancer Res. 2012;72:2481-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Ansari D, Andersson R, Bauden MP, Andersson B, Connolly JB, Welinder C, Sasor A, Marko-Varga G. Protein deep sequencing applied to biobank samples from patients with pancreatic cancer. J Cancer Res Clin Oncol. 2015;141:369-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2205] [Article Influence: 147.0] [Reference Citation Analysis (2)] |
| 35. | Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Klapman J, Malafa MP. Early detection of pancreatic cancer: why, who, and how to screen. Cancer Control. 2008;15:280-287. [PubMed] |
| 37. | Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 38. | Ansari D, Williamsson C, Tingstedt B, Andersson B, Lindell G, Andersson R. Pancreaticoduodenectomy--the transition from a low- to a high-volume center. Scand J Gastroenterol. 2014;49:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 40. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 41. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210; discussion 1210-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1122] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 42. | Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, Madura JA, Wiebke EA, Lillemoe KD. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338-1345; discussion 1345-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Bartosch-Härlid A, Andersson R. Diabetes mellitus in pancreatic cancer and the need for diagnosis of asymptomatic disease. Pancreatology. 2010;10:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | He XY, Li JF, Yao WY, Yuan YZ. Resolution of new-onset diabetes after radical pancreatic resection predicts long-term survival in patients with pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20:3809-3816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 46. | Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg. 2011;98:1455-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 48. | Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641. [PubMed] |
| 49. | Kennedy EP, Grenda TR, Sauter PK, Rosato EL, Chojnacki KA, Rosato FE, Profeta BC, Doria C, Berger AC, Yeo CJ. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg. 2009;13:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 51. | Kennedy EP, Rosato EL, Sauter PK, Rosenberg LM, Doria C, Marino IR, Chojnacki KA, Berger AC, Yeo CJ. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution--the first step in multidisciplinary team building. J Am Coll Surg. 2007;204:917-923; discussion 923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Pillai SA, Palaniappan R, Pichaimuthu A, Rajendran KK, Sathyanesan J, Govindhan M. Feasibility of implementing fast-track surgery in pancreaticoduodenectomy with pancreaticogastrostomy for reconstruction--a prospective cohort study with historical control. Int J Surg. 2014;12:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903. [PubMed] |
| 54. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1908] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 55. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 56. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 57. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 58. | Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 59. | Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Brunner TB. Neoadjuvant therapy for potentially resectable pancreatic cancer: an emerging paradigm? Curr Oncol Rep. 2013;15:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | He J, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J Gastroenterol. 2014;20:2255-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Xu CP, Xue XJ, Liang N, Xu DG, Liu FJ, Yu XS, Zhang JD. Effect of chemoradiotherapy and neoadjuvant chemoradiotherapy in resectable pancreatic cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:549-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Zhu CP, Shi J, Chen YX, Xie WF, Lin Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: a meta-analysis. Radiother Oncol. 2011;99:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Collins A, Bloomston M. Diagnosis and management of pancreatic cancer. Minerva Gastroenterol Dietol. 2009;55:445-454. [PubMed] |
| 65. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 66. | Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 67. | Ansari D, Nilsson J, Andersson R, Regnér S, Tingstedt B, Andersson B. Artificial neural networks predict survival from pancreatic cancer after radical surgery. Am J Surg. 2013;205:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 69. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 70. | Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Damaraju VL, Damaraju S, Young JD, Baldwin SA, Mackey J, Sawyer MB, Cass CE. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524-7536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 72. | Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, Orlandini C, Boggi U, Campani D, Del Chiaro M. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 73. | Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956-6961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 74. | Ansari D, Tingstedt B, Andersson R. Pancreatic cancer - cost for overtreatment with gemcitabine. Acta Oncol. 2013;52:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review). Oncol Rep. 2010;23:1183-1192. [PubMed] |
| 76. | Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012;18:4944-4958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (2)] |
| 78. | Garber K. Stromal depletion goes on trial in pancreatic cancer. J Natl Cancer Inst. 2010;102:448-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63:974-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 80. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4883] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 81. | Hartlapp I, Müller J, Kenn W, Steger U, Isbert C, Scheurlen M, Germer CT, Einsele H, Kunzmann V. Complete pathological remission of locally advanced, unresectable pancreatic cancer (LAPC) after intensified neoadjuvant chemotherapy. Onkologie. 2013;36:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 84. | Saif MW. Advancements in the management of pancreatic cancer: 2013. JOP. 2013;14:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 85. | Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S. Vitamin d receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 890] [Article Influence: 80.9] [Reference Citation Analysis (0)] |