Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.177
Peer-review started: March 7, 2014
First decision: April 2, 2014
Revised: May 11, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 7, 2015
Processing time: 306 Days and 2.2 Hours
AIM: To investigate the effect of a fat rich diet on non-steroidal anti-inflammatory drug (NSAID)-induced mucosal damage in the murine small intestine.
METHODS: C57BL6 mice were fed 4 types of diets with or without indomethacin. One group was fed standard laboratory chow. The other groups were fed a fat diet consisting of 8% w/w fat, beef tallow (rich in SFA), fish oil, (rich in omega-3 PUFA), or safflower oil (rich in omega-6 PUFA). Indomethacin (3 mg/kg) was injected intraperitoneally from day 8 to day 10. On day 11, intestines and adhesions to submucosal microvessels were examined.
RESULTS: In the indomethacin-treated groups, mucosal damage was exacerbated by diets containing beef tallow and fish oil, and was accompanied by leukocyte infiltration (P < 0.05). The mucosal damage induced by indomethacin was significantly lower in mice fed the safflower oil diet than in mice fed the beef tallow or fish oil diet (P < 0.05). Indomethacin increased monocyte and platelet migration to the intestinal mucosa, whereas safflower oil significantly decreased monocyte and platelet recruitment (P < 0.05).
CONCLUSION: A diet rich in SFA and omega-3 PUFA exacerbated NSAID-induced small intestinal damage via increased leukocyte infiltration. Importantly, a diet rich in omega-6-PUFA did not aggravate inflammation as monocyte migration was blocked.
Core tip: Non-steroidal anti-inflammatory drugs (NSAIDs) frequently induce mucosal damage in the gastrointestinal tract. The recently developed techniques of capsule enteroscopy and double balloon enteroscopy have shown that NSAIDs cause ulcers in the small intestine (68%) more frequently than previously thought. Although proton pump inhibitors are key drugs for NSAIDs-induced gastropathy, proton pump inhibitors have no effect on NSAIDs-induced intestinal lesions and no drugs are currently available for the prevention and treatment of NSAIDs-induced intestinal lesions. In the present study, we showed the beneficial effect of an omega-6 PUFA-rich diet in NSAID-induced mucosal damage in the murine small intestine. This is a completely novel finding and is important not only in the clinical field, but also in preventive medicine.
- Citation: Ueda T, Hokari R, Higashiyama M, Yasutake Y, Maruta K, Kurihara C, Tomita K, Komoto S, Okada Y, Watanabe C, Usui S, Nagao S, Miura S. Beneficial effect of an omega-6 PUFA-rich diet in non-steroidal anti-inflammatory drug-induced mucosal damage in the murine small intestine. World J Gastroenterol 2015; 21(1): 177-186
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.177
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most frequently used drugs worldwide to control pain or inflammation and are known to cause gastrointestinal tract damage as an adverse effect. The recently developed techniques of capsule enteroscopy and double balloon enteroscopy have shown that NSAIDs cause ulcers in the small intestine more frequently than previously thought[1]. According to a recent study, gross damage was observed in 68% of volunteers who were administered 75 mg of diclofenac for 2 wk[2]. Several factors have been postulated as the pathogenic element of NSAIDs-induced small intestinal lesions. NSAIDs inhibit the activity of cyclooxygenase, a key enzyme, and the resulting prostaglandin deficiency is thought to cause small intestinal damage as well as the development of gastric ulcers[3]. Antibiotic treatments affect NSAIDs-induced small intestinal lesions[4], and probiotic treatments modulate these lesions[5,6]. These reports have suggested that the luminal flora is involved. Involvement of the Toll-like receptor family in the development of lesions suggests that the intestinal immune system plays a significant role in the pathophysiology[4]. Indeed, the amelioration of NSAIDs-induced small intestinal lesions via the depletion of leukocytes suggests that leukocytes contribute to the development of intestinal lesions[7]. Platelets are involved in intestinal inflammation by modulating leukocyte migration to small intestinal microvessels[8,9]. Indeed anti-platelet drugs ameliorate murine NSAIDs-induced small intestinal lesions[10]. Although there are numerous reports on this topic, suitable drugs for the prevention and treatment of these lesions are not currently clinically available, except for prostaglandin analogs[11].
Dietary fat consumption modulates immune function in the intestine by regulating various processes such as leukocyte recruitment to the intestinal mucosa[12], cytokine expression by intraepithelial lymphocytes[13] or residual macrophages[14], and expression of anti-inflammatory agents such as adiponectin[15]. In addition, dietary fat consumption exacerbates intestinal diseases including inflammatory bowel disease[16]. Luminal antigens and food affect the small intestine to a greater extent than the colon when the small intestine is directly exposed. We previously reported that dietary fat intake enhanced leukocyte recruitment to the intestinal mucosa, leading to the enhancement of intestinal inflammation[12]. Fat intake aggravates lifestyle-related diseases such as atherosclerosis by enhancement of leukocyte recruitment, especially monocytes/macrophages[17-19]. On the basis of these findings, we hypothesized that excessive dietary fat intake exacerbates NSAIDs-induced small intestinal lesions by enhancing leukocyte recruitment to the intestinal mucosa. However, no studies have reported the relationship between dietary fat intake and NSAIDs-induced small intestinal lesions.
In this study, we used a murine model to determine whether (1) dietary fat intake affects the severity of NSAIDs-induced small intestinal lesions as assessed by the area of small intestinal lesions; (2) whether recruitment of monocytes/macrophages to the intestinal mucosa is involved in NSAIDs-induced small intestinal lesions; and (3) whether dietary fat intake affects the recruitment of monocytes/macrophages to the inflamed intestinal mucosa.
Eight-week-old, male C57B6/J mice weighing 20.6 ± 0.16 g were maintained on CE-2 as standard laboratory chow (Clea, Tokyo, Japan). The care and use of the laboratory animals were in accordance with the guidelines of the animal facility at the National Defense Medical College (NDMC). The experimental protocol was approved by the Animal Research Committee of NDMC (No. 09016). Indomethacin (IND) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved in DMSO (20 mg/mL) and stored at -20 °C until used in the experiments. To induce small intestinal injury, 3 mg/kg IND was administered i.p. for 3 d (n = 8 in each group). Evans blue (Wako Pure Chemical Industries, Ltd. Tokyo, Japan) was injected intravenously (i.v.) 30 min before sacrifice, after which the small intestine was removed.
The small intestine was then opened along the anti-mesenteric attachment and examined for injury. Blue-stained depressive areas were diagnosed as small intestinal lesions induced by IND. The small intestine was placed on a grid sheet, and the area of the intestinal lesions was calculated after photographs were taken.
Dietary fat administration was commenced 1 wk prior to the administration of IND. Each fat-containing diet consisted of a fat-free AIN76 (Clea Japan, Tokyo) diet and 3 different types of 8% w/w fatty acids. We chose serum bovine tallow (BT) (Clea Japan, Tokyo) as an SFA, fish oil (FO) (Sigma, MO, United States) as a source of omega-3 PUFA, and safflower seed oil (SO) (Sigma, MO, United States) as a source of omega-6 PUFA. CE-2 was given as a reference diet (R). The feeds were stored at 4 °C to prevent fatty acid oxidation[20]. The lipid composition of each diet and approximate fatty acid profile are shown in Tables 1 and 2.
| Ingredient | Fat diet | Nutrients | CE-2® |
| Casein | 19.3% | Water | 8.9% |
| d,l-methionine | 0.3% | Crude protein | 25.4% |
| Sucrose | 48.5% | Crude fat | 4.4% |
| Corn starch | 14.5% | Crude fiber | 4.1% |
| Alphacel, Nonnutritive bulk | 4.8% | Crude ash | 6.9% |
| Choline bitartate | 0.2% | Nitrogen-free extract | 50.3% |
| Mineral mix | 3.4% | ||
| Vitamin mix | 1.0% | ||
| Fat1 | 8.0% |
| Beef tallow | Fish oil | Safflower oil | |
| Myristic (14:0) | 3 | ||
| Palmitic acid (16:0) | 26 | 25.1 | 7.1 |
| Stearic acid (18:0) | 14 | 4.4 | 2.5 |
| Oleic acid (18:1) | 47 | 12.9 | 13.3 |
| Palmitoleic acid (16:1) | 3 | 14.9 | |
| Linoleic acid (18:2) | 3 | 2.4 | 76.0 |
| Linolenic acid (18:3) | 1 | 1.2 | 0.3 |
| Eicosapentaenoic acid (20:5) | 15.6 | ||
| Docosahexaenoic acid (22:6) | 8.8 | ||
| Others | 3 | 1.5 | 0.8 |
| Omega-3/omega-6 ratio | 1:3 | 10.6:1 | 1:255 |
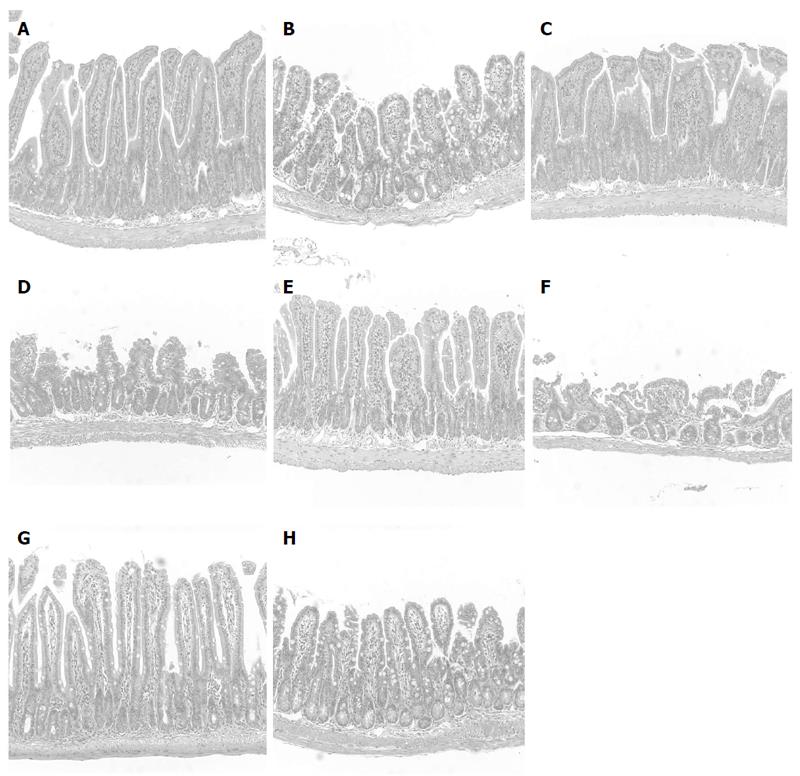
We examined the small intestinal damage histologically. The ileum was fixed in 10% buffered formalin. Tissues were embedded in paraffin and stained with HE. Histological damage was examined in a blinded fashion according to the villous height and the histopathological scale previously described[21,22]. Briefly, the tissue damage was graded from 0 to 5 according to the following criteria: grade 0, normal villi structure; grade 1, development of a small subepithelial space at the villous apex; grade 2, enlarged subepithelial space, but no change in villous length and width; grade 3, few shortened villi and the presence of cells in the lumen; grade 4, most villi are shortened and widened with crypt hyperplasia and cells in the lumen; and grade 5, blunting of all villi with elongated crypts and a high number of cells in the lumen. The number of infiltrating cells was calculated as the number of cells per millimeter of muscularis mucosa and graded as follows: grade 1, less than 30 cells/mm; grade 2, 30-59 cells/mm; grade 3, 60-89 cells/mm; and grade 4, over 90 cells/mm.
The intestinal mucosa was removed after the mice were sacrificed. We separated the small intestine into 24 pieces and numbered them. We excluded the pieces that included deep ulcers from the qRT-PCR analysis as the evaluation of these pieces would be completely different. To eliminate selection bias, the same numbered pieces were also excluded from the control group. Total mRNA was extracted using the RNeasy Mini isolation kit (Qiagen, CA, United States). TaqMan RT-PCR was performed in triplicate for each sample using the ABI PRISM 7700 Sequence Detector (Applied Biosystems, CA, United States). The primer and probes used in this study [MAdCAM-1 (Mm00522088), ICAM-1 (Mm00516023), and VCAM-1 (Mm00449197)] were purchased from Applied Biosystems.
Monocytes were isolated from the bone marrow of C57B6/J mice by magnetic cell sorting (MACS, Miltenyi Biotec, CA, United States) with a bead-conjugated anti-rabbit CD11b polyclonal antibody (Miltenyi Biotec) as described previously[8]. Monocytes were stained with carboxyfluorescein diacetate succinimidyl ester (CFDSE, Molecular Probes, Eugene, OR, United States) solution[8].
Platelets were isolated from the blood of donor mice by centrifugation, as described previously[8]. Platelets were stained with carboxyfluorescein diacetate succinimidyl ester (CFDSE, Molecular Probes, Eugene, OR, United States) solution[8].
For the migration studies a murine ileal segment 1-3 cm in length was selected for observation. The movement and interaction of monocytes in submucosal venules were observed from the serosal side using an intravital microscope through a silicon-intensified target image tube system using a previously described method, and recorded on a digital hard disk recorder[8]. The movement and interaction of platelets were observed using the same method[8]. Cells were counted offline from digital video disk images for analysis, as described previously[8].
All results are expressed as the mean ± SEM. Differences between groups were examined for statistical significance using 2-way factorial ANOVA followed by a post hoc test in the IND-administered animal study and a Kruskal-Wallis test in the microscopic study. Statistical significance was defined as P < 0.05.
IND caused the development of hemorrhagic lesions in the small intestine, mostly in the jejunum and ileum. The area of ulceration in the small intestine was 21.8 ± 2.3 mm2 in the control diet group (Table 3). The results of the histological examination are shown in Figure 1. IND caused a significant increase in the number of cells infiltrating the intestinal mucosa (Table 4).
Next, we investigated whether the administration of dietary fat influenced the severity of intestinal lesions and whether inflammatory cell infiltration was involved in this mechanism. We evaluated the effects of various fat-containing diets (such as those including BT as an SFA, FO as a source of omega-3 PUFA, and SO as a source of omega-6 PUFA) on the degree of inflammation in NSAID-induced intestinal lesions. Table 3 shows the area of ulceration in the small intestine. Treatment of mice with SFA (BT) or omega-3 PUFA (FO) aggravated the small intestinal lesions. However, treatment with omega-6 PUFA (SO) did not increase the area of small intestinal lesions, histological scores or the number of infiltrating cells when compared with mice fed the control diet. The area of small intestinal lesions was significantly lower in the omega-6 PUFA (SO) diet group than in the SFA (BT) or omega-3 PUFA (FO) diet group (P < 0.05). Figure 1A shows the histological scores, which were significantly lower in the omega-6 PUFA (SO) diet group than in the SFA (BT) or omega-3 PUFA (FO) diet group (P < 0.05). Furthermore, we calculated the number of infiltrating cells in the sections stained with HE, as leukocyte infiltration is involved in pathogenesis of NSAIDs-induced small intestinal lesions[10]. The increase in the number of cells infiltrating the intestinal mucosa following the administration of IND was further increased by SFA or omega-3 PUFA, but not by omega-6 PUFA, which corresponded to a reduced area of intestinal ulceration (Table 5). Significantly fewer infiltrating cells were observed in the omega-6 PUFA (SO) group than in the SFA (BT) or omega-3 PUFA (FO) group (P < 0.05). Collectively, the omega-6-PUFA diet resulted in significantly less intestinal damage than the SFA or omega-3 PUFA diet, with decreased infiltration of inflammatory cells. From these observations, it is suggested that treatment with a diet rich in fat affects the severity of small intestinal lesions by modulating leukocyte infiltration.
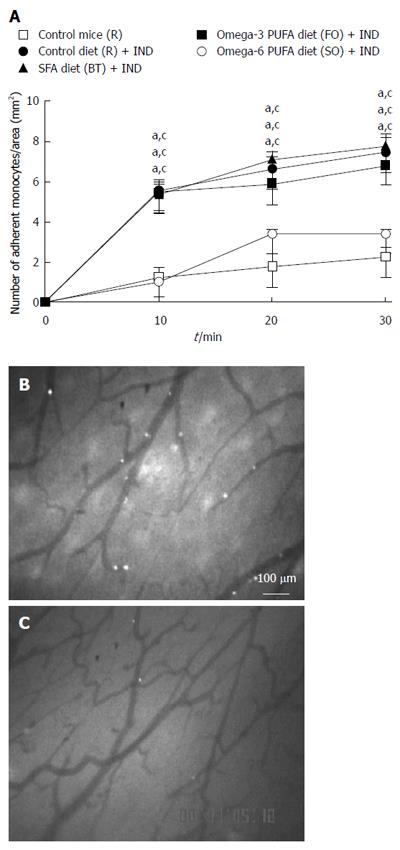
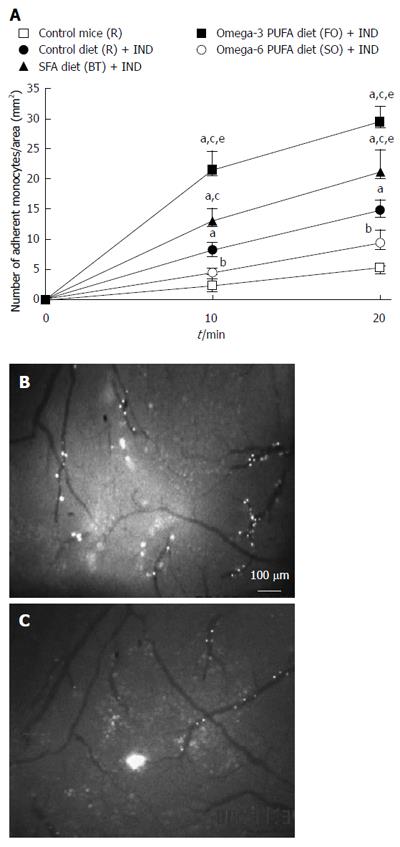
In order to clarify whether a diet rich in fat affects leukocyte recruitment from the blood stream to the intestinal mucosa directly, we studied the migration of leukocytes to NSAID-induced inflamed intestinal mucosa using intravital microscopy in vivo. We focused on the recruitment of monocytes for the following reasons; (1) macrophages in intestinal mucosa play significant roles through the TLR4 receptor in NSAIDs-induced small intestinal lesions[4]; (2) we observed infiltration of P-selectin glycoprotein ligand-1 (PSGL-1) positive cells, which were mainly expressed on macrophages/monocytes, in NSAIDs-induced inflamed intestinal mucosa in our preliminary study (data not shown); and (3) anti-PSGL-1 antibody ameliorated NSAIDs-induced small intestinal lesions[10]. We isolated monocytes from donor mice and injected them into the jugular vein of recipient mice. Figure 2A shows the time-course of monocyte adherence to intestinal microvessels. NSAID treatment increased the adherence of monocytes to intestinal microvessels (P < 0.05, Figure 2B). Significantly fewer adherent cells were induced by NSAID in the omega-6 PUFA (SO) diet group compared with the SFA (BT) or omega-3 PUFA (FO) group (P < 0.05, Figure 2C). Figure 3 shows the time-course of monocyte adherence to postcapillary venules in Peyer’s patches. NSAID administration increased the adherence of monocytes to postcapillary venules (P < 0.05). Treatment with the diet rich in omega-6 PUFA (SO) decreased the enhanced leukocyte adherence induced by NSAID, and the number of adherent leukocytes was significantly less than that in the BT and FO group (P < 0.05, Figure 3C).
We investigated the mRNA expression of ICAM-1, VCAM-1, and MAdCAM-1, which are involved in the pathophysiology of many intestinal diseases, using qRT-PCR. The level of ICAM-1 and VCAM-1 mRNA did not increase after NSAID administration. NSAID significantly increased the expression of MAdCAM-1 mRNA (P < 0.05), but the addition of dietary fat did not increase the expression of MAdCAM-1 mRNA, suggesting that changes in the expression of adhesion molecules were not involved in the dietary fat-induced modification of NSAID-induced small intestinal lesions (data not shown).
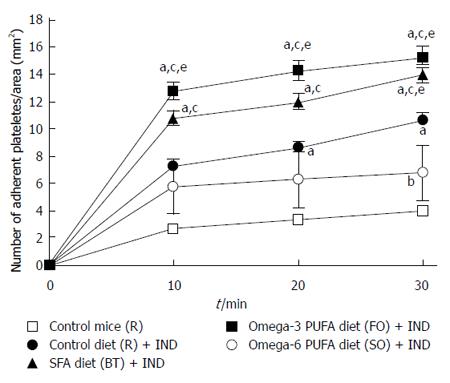
Recently, it was reported that platelet adherence to the vascular endothelium plays a significant role in the adherence of leukocytes, especially monocytes, by crosslinking between the endothelium and monocytes[8]. We reported that inhibition of the interaction between platelets and monocytes following treatment with an anti-platelet drug ameliorated NSAIDs-induced small intestinal lesions[10]. We studied whether dietary fat modulated platelet adherence to the endothelium, thereby affecting small intestinal lesions. We isolated platelets from donor mice and injected them into the jugular vein of recipient mice. NSAID treatment increased the adherence of platelets to intestinal microvessels (P < 0.05) (Figure 4). Treatment with diets rich in SFA and omega-3 PUFA further increased the adherence of platelets to intestinal microvessels. Treatment with the omega-6 PUFA-rich diet ameliorated the increased platelet adherence induced by the NSAID, and the number of adherent platelets was significantly lower in this group than that in the SFA (BT) and omega-3 PUFA (FO) group (P < 0.05). These results are consistent with the effects observed for the area of NSAID-induced small intestinal lesions and for the number of monocytes adhering to intestinal microvessels.
In this study, we showed the following: (1) treatment with a diet rich in SFA, omega-3 PUFA, or omega-6 PUFA without NSAID did not cause small intestinal injury[2]; (2) treatment with a diet rich in SFA aggravated NSAID-induced small intestinal lesions; (3) NSAID-induced small intestinal lesions were significantly smaller in the omega-6 PUFA group than in the SFA and omega-3 PUFA group; and (4) treatment with the omega-6 PUFA-rich diet significantly decreased the enhanced leukocyte infiltration to intestinal mucosa caused by NSAID administration. From these observations, we conclude that NSAID-induced small intestinal lesions were affected by dietary fat and that an omega-6 PUFA-containing diet is preferable for the prevention of NSAID-induced small intestinal lesions than a diet rich in omega-3 PUFA or SFA.
The intestinal lesions were significantly smaller in the omega-6 PUFA group than in the SFA and omega-3 PUFA group, and omega-6 PUFA decreased NSAID-induced leukocyte recruitment to the small intestine. Therefore, we performed an additional study to determine the mechanisms by which omega-6 PUFA induced the decrease in the number of infiltrating leukocytes. We observed that the omega-6 PUFA-rich diet decreased monocyte migration to intestinal microvessels using intravital microscopy. Recently, platelets were postulated to be involved in the recruitment of leukocytes, and the migration of platelets was postulated to be involved in the pathophysiology of a murine chronic ileus model[8,23,24]. We previously reported the involvement of platelets in the pathophysiology of NSAIDs-induced small intestinal damage due to the following observations: (1) NSAID administration enhanced leukocyte migration with an increase in leukocyte-platelet interactions; and (2) cilostazol, an anti-platelet drug, ameliorated NSAIDs-induced small intestinal damage through the inhibition of leukocyte recruitment by blocking leukocyte-platelet interactions[10]. In the present study, adherence of platelets to intestinal microvessels was increased following NSAID administration and was further increased by the SFA or omega-3 PUFA diet and decreased by the omega-6 PUFA diet. These results suggested that dietary fat modulates the severity of NSAID-induced small intestinal lesions by monocyte recruitment to intestinal microvessels by changing platelet migration. Because the side effects of anti-platelet drugs are apparent despite their anti-inflammatory role, omega-6 PUFA is advantageous in terms of its safety.
The expression of adhesion molecules plays an important role in leukocyte recruitment, and fatty acids, especially omega-3 PUFA, are known to modulate the expression of ICAM-1 and VCAM-1 on vascular endothelium[25]. Recently, it was reported that treatment with omega-3 PUFA decreased VCAM-1 expression on IL-1β-activated human intestinal microvascular endothelial cells in vitro. In addition, omega-3 PUFA treatment ameliorated the increased VCAM-1 expression on the large intestinal endothelium of rats caused by trinitrobenzene sulfonic acid (TNBS) treatment[25]. We investigated the expression of ICAM-1 and VCAM-1 in the NSAID-treated small intestine; however, we found that their expression did not increase after NSAID administration. The expression of MAdCAM-1 increased in NSAID-induced intestinal lesions, but did not change after administration of the fat-rich diet. Thus, it is unlikely that a fat-rich diet affects NSAID-induced lesions via the modulation of adhesion molecules expression on the vascular endothelium. These discrepancies between the results of our study and those of a previous study are likely the result of different causes of inflammation. In the previous study, IL-1β-induced inflammation and TNBS-induced inflammation were accompanied by increased expression of cyclooxygenase and PGE2, which are considered exacerbating factors for inflammation. The inhibitory effect of omega-3 PUFA treatment on VCAM-1 expression was accompanied by a decrease in PGE2 both in vitro and in vivo[26]. In contrast, the NSAIDs-induced small intestinal injury was characterized by a decrease rather than an increase in PGE2. This contrasting involvement of PGE2 in different pathophysiological mechanisms may account for the difference in the effect of omega-3 PUFA treatment on VCAM-1 expression between NSAIDs-induced inflammation and TNBS- and IL-1β-induced inflammation.
The adverse effects of SFA on intestinal inflammation have been reported by other groups[27,28]. In particular, SFA activates the expression of pro-inflammatory cytokines in inflammatory cells such as macrophages through the TLR4-Myd88 pathway[27]. Recently, it was reported that consumption of diets high in fat causes changes in the luminal flora[28]. Because the luminal flora is involved in the development of NSAIDs-induced small intestinal lesions[5,6], it is also possible that the addition of a fat-containing diet to the NSAIDs treatment modifies the intestinal injury by changing the luminal microflora.
We did not expect to find that omega-3 PUFA aggravated NSAID-induced intestinal lesions as the anti-inflammatory role of omega-3 PUFA has been reported in many inflammatory diseases such as ischemic heart disease and rheumatoid arthritis[17,29]. However, the effects of omega-3 PUFA on intestinal inflammatory diseases have been recognized as equivocal[30,31]. In a murine model of inflammatory bowel disease, omega-3 PUFA aggravated dextran sodium sulfate-induced colitis[15], but ameliorated spontaneously developed ileitis[9], which suggests that the role of omega-3 PUFA differs according to the pathogenesis of inflammation. We consider that the unexpected findings in this study were a result of the dual role of PGE2 in inflammation. The anti-inflammatory effects of omega-3 PUFA on cytokine expression have been established in leukocytes activated by pro-inflammatory cytokines[32]. In cytokine-induced inflammation, PGE2 derived from COX-2 functions as a pro-inflammatory factor and cyclooxygenase inhibitors such as NSAIDs function as anti-inflammatory agents. Fatty acids are involved in PGE2 synthesis, and cyclooxygenase is involved in PGE2 production from omega-6 PUFA[33]. The anti-inflammatory role of omega-3 PUFA is based on the concept that omega-3 PUFA antagonizes omega-6 PUFA by downregulating the arachidonic acid cascade, which results in a decrease in PGE[34]. Thus, the effect of omega-3 PUFA resembles that of NSAIDs with regard to PG metabolism. In contrast, the opposite pattern was observed in this study, especially with regard to PGE2 concentration. A low concentration of PGE2 derived from constitutively expressed COX-1 functions to maintain homeostasis in tissues such as vascular endothelium[35]. The pathogenesis of NSAIDs-induced intestinal injury can thus be explained in part by a decrease in PGE2, which results in disruption of homeostasis. In this vein, the supplementation of PGE1 ameliorates NSAIDs-induced intestinal injury[11]. Because insufficiency of PGE is involved in the pathogenesis of NSAIDs-induced intestinal injury, it is possible that omega-6 PUFA treatment plays a protective role by supplementing PGE2 after metabolism by cyclooxygenase[36] and that omega-3 PUFA treatment has a deleterious effect resulting in a further decrease in PG concentration. It is difficult to measure the net effect of omega-3 PUFA on pro-inflammation and anti-inflammation in vivo. However, our results suggest that PGE2 is involved in NSAID-induced intestinal injury and the pro-inflammatory role of omega-3 PUFA is more dominant than its anti-inflammatory role.
Although this study has some limitations: (1) this was an animal study; and (2) the mechanism of action was not completely elucidated, the results provide important information for medical practice. A clinical study to determine whether omega-6 PUFA capsules are effective in the prevention or treatment of NSAIDs-induced small intestinal lesions should be performed.
Although non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in daily practice, few drugs have showed satisfactory effects on NSAIDs-induced enteropathy. Dietary fat consumption modulates immune function in the intestine by regulating various processes such as leukocyte recruitment to the intestinal mucosa. The aim of this study was to demonstrate that dietary fat intake affects the severity of NSAIDs-induced small intestinal lesions.
Several studies have focused on the major role of leukocyte migration to intestinal mucosa which results in mucosal damage. In this study, the authors evaluated the effect of dietary fat on leukocyte migration using intravital microscopy.
The present study indicates the beneficial role of an omega-6 PUFA-rich diet in NSAID-induced mucosal damage in the murine small intestine.
The safety and effectiveness of dietary treatment confirm that an omega-6 PUFA-rich diet may be helpful in healing NSAIDs-induced enteropathy and in preventing this condition.
The main hypothesis of the paper for management of NSAIDs-induced enteropathy is valuable. The experimental design is reasonable and the findings are interesting.
P- Reviewer: Danielsen EM, Jeung EB S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S
| 1. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. [PubMed] |
| 4. | Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Kamil R, Geier MS, Butler RN, Howarth GS. Lactobacillus rhamnosus GG exacerbates intestinal ulceration in a model of indomethacin-induced enteropathy. Dig Dis Sci. 2007;52:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Asahara T. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506-G513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Miura S, Suematsu M, Tanaka S, Nagata H, Houzawa S, Suzuki M, Kurose I, Serizawa H, Tsuchiya M. Microcirculatory disturbance in indomethacin-induced intestinal ulcer. Am J Physiol. 1991;261:G213-G219. [PubMed] |
| 8. | Higashiyama M, Hokari R, Matsunaga H, Takebayashi K, Watanabe C, Komoto S, Okada Y, Kurihara C, Kawaguchi A, Nagao S. P-selectin-dependent monocyte recruitment through platelet interaction in intestinal microvessels of LPS-treated mice. Microcirculation. 2008;15:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y. Omega-3 polyunsaturated fatty acids ameliorate the severity of ileitis in the senescence accelerated mice (SAM)P1/Yit mice model. Clin Exp Immunol. 2009;158:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Higashiyama M, Hokari R, Kurihara C, Ueda T, Watanabe C, Tomita K, Komoto S, Okada Y, Kawaguchi A, Nagao S. Indomethacin-induced small intestinal injury is ameliorated by cilostazol, a specific PDE-3 inhibitor. Scand J Gastroenterol. 2012;47:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Watanabe T, Sugimori S, Kameda N, Machida H, Okazaki H, Tanigawa T, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Fujiyama Y, Hokari R, Miura S, Watanabe C, Komoto S, Oyama T, Kurihara C, Nagata H, Hibi T. Butter feeding enhances TNF-alpha production from macrophages and lymphocyte adherence in murine small intestinal microvessels. J Gastroenterol Hepatol. 2007;22:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Hara Y, Miura S, Komoto S, Inamura T, Koseki S, Watanabe C, Hokari R, Tsuzuki Y, Ogino T, Nagata H. Exposure to fatty acids modulates interferon production by intraepithelial lymphocytes. Immunol Lett. 2003;86:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Inamura T, Miura S, Tsuzuki Y, Hara Y, Hokari R, Ogawa T, Teramoto K, Watanabe C, Kobayashi H, Nagata H. Alteration of intestinal intraepithelial lymphocytes and increased bacterial translocation in a murine model of cirrhosis. Immunol Lett. 2003;90:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2008;14:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741-745. [PubMed] |
| 17. | Shaper AG. Environmental factors in coronary heart disease: diet. Eur Heart J. 1987;8 Suppl E:31-38. [PubMed] |
| 18. | Wahle KW, Rotondo D. Fatty acids and endothelial cell function: regulation of adhesion molecule and redox enzyme expression. Curr Opin Clin Nutr Metab Care. 1999;2:109-115. [PubMed] |
| 19. | Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006-1014. [PubMed] |
| 20. | Umezawa M, Tatematsu K, Korenaga T, Fu X, Matushita T, Okuyama H, Hosokawa M, Takeda T, Higuchi K. Dietary fat modulation of apoA-II metabolism and prevention of senile amyloidosis in the senescence- accelerated mouse. J Lipid Res. 2003;44:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483 [. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1427] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 22. | Wu CC, Lu YZ, Wu LL, Yu LC. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol. 2010;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 2011;6:e25595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Matsunaga H, Hokari R, Higashiyama M, Kurihara C, Okada Y, Watanabe C, Komoto S, Nakamura M, Kawaguchi A, Nagao S. Cilostazol, a specific PDE-3 inhibitor, ameliorates chronic ileitis via suppression of interaction of platelets with monocytes. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1077-G1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | De Caterina R, Libby P. Control of endothelial leukocyte adhesion molecules by fatty acids. Lipids. 1996;31 Suppl:S57-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Ibrahim A, Mbodji K, Hassan A, Aziz M, Boukhettala N, Coëffier M, Savoye G, Déchelotte P, Marion-Letellier R. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. 2011;30:678-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041-37051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440-G448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 29. | Adam O, Beringer C, Kless T, Lemmen C, Adam A, Wiseman M, Adam P, Klimmek R, Forth W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. 2003;23:27-36. [PubMed] |
| 30. | Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D’Haens G, Bradette M, Cohen A, Dallaire C, Ponich TP, McDonald JW. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA. 2008;299:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Belluzzi A, Boschi S, Brignola C, Munarini A, Cariani G, Miglio F. Polyunsaturated fatty acids and inflammatory bowel disease. Am J Clin Nutr. 2000;71:339S-342S. [PubMed] |
| 32. | Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Kuehl FA, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978-984. [PubMed] |
| 34. | Harbige LS. Fatty acids, the immune response, and autoim-munity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323-341. [PubMed] |
| 35. | Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247-258. [PubMed] |
| 36. | Kremmyda LS, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease: a review. part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:195-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |