Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2403
Revised: December 31, 2013
Accepted: January 19, 2014
Published online: March 7, 2014
Processing time: 168 Days and 3.5 Hours
AIM: To determine the prognostic value of circulating indicators of cell death in acute-on-chronic liver failure (ACLF) patients with chronic hepatitis B virus (HBV) infection as the single etiology.
METHODS: Full length and caspase cleaved cytokeratin 18 (detected as M65 and M30 antigens) represent circulating indicators of necrosis and apoptosis. M65 and M30 were identified by enzyme-linked immunosorbent assay in 169 subjects including healthy controls (n = 33), patients with chronic hepatitis B (CHB, n = 55) and patients with ACLF (n = 81). According to the 3-mo survival period, ACLF patients were defined as having spontaneous recovery (n = 33) and non-spontaneous recovery which included deceased patients and those who required liver transplantation (n = 48).
RESULTS: Both biomarker levels significantly increased gradually as liver disease progressed (for M65: P < 0.001 for all; for M30: control vs CHB, P = 0.072; others: P < 0.001 for all). In contrast, the M30/M65 ratio was significantly higher in controls compared with CHB patients (P = 0.010) or ACLF patients (P < 0.001). In addition, the area under receiver operating characteristic curve (AUC) analysis demonstrated that both biomarkers had diagnostic value (AUC ≥ 0.80) in identifying ACLF from CHB patients. Interestingly, it is worth noting that the M30/M65 ratio was significantly different between spontaneous and non-spontaneous recovery in ACLF patients (P = 0.032). The prognostic value of the M30/M65 ratio was compared with the Model for End-Stage Liver Disease (MELD) and Child-Pugh scores at the 3-mo survival period, the AUC of the M30/M65 ratio was 0.66 with a sensitivity of 52.9% and the highest specificity of 92.6% (MELD:AUC = 0.71; sensitivity, 79.4%; specificity, 63.0%; Child-Pugh: AUC = 0.77; sensitivity, 61.8%; specificity, 88.9%).
CONCLUSION: M65 and M30 are strongly associated with liver disease severity. The M30/M65 ratio may be a potential prognostic marker for spontaneous recovery in patients with HBV-related ACLF.
Core tip: Massive hepatic cell death is a key characteristic of liver failure. Enzyme-linked immunosorbent assay was used to measure M65 and M30 in a chronic hepatitis B (CHB) infection cohort which included healthy controls, CHB and acute-on-chronic liver failure (ACLF) patients. Elevated M65 and M30 differentiated CHB or ACLF patients from healthy controls and gradually increased with disease severity. M30/M65 was significantly increased in ACLF patients with spontaneous recovery (P = 0.032), and the AUC of this ratio at the 3-mo survival period was 0.661 (sensitivity: 52.9%) with a high specificity (92.6%) compared with the Model for End-Stage Liver Disease and Child-Pugh scores.
- Citation: Zheng SJ, Liu S, Liu M, McCrae MA, Li JF, Han YP, Xu CH, Ren F, Chen Y, Duan ZP. Prognostic value of M30/M65 for outcome of hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol 2014; 20(9): 2403-2411
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2403
Chronic hepatitis B virus (HBV) infection is one of the leading causes of liver-associated death and disease in China[1,2], and accounts for more than 83% of overall liver failure and over 90% of acute-on-chronic liver failure (ACLF) cases[3-5]. Recently, ACLF has been found to be an increasingly recognized entity encompassing acute deterioration of liver function in patients with chronic liver disease worldwide[6-8]. Prompt and accurate prediction of outcome and correct medical decision making can improve outcome in ACLF patients. The currently used prognostic models, such as the Model for End-Stage Liver Disease (MELD) and Child-Pugh scores, were not specifically designed for patients with HBV-related ACLF, therefore, it is worth exploring new potential biomarkers which might improve the prognostic model for HBV-related ACLF patients.
Dysregulation of hepatocyte apoptosis plays an important role in liver disorders[9]. M30-antigen, the caspase-cleaved cytokeratin-18, is used to detect apoptosis[10-13]. Uncleaved cytokeratin-18, detectable as M65-antigen[14], is also released from dying cells (due to both apoptosis and necrosis).
An elevation in M30 was identified in patients with liver disorders, including chronic hepatitis C (CHC)[15-17]. A previous study reported that elevated serum M30 was positively associated with a higher degree of fibrosis in CHC patients, and it was suggested that elevated M30 may be more sensitive than aminotransferase for identifying liver injury, particularly in CHC patients with normal aminotransferase profiles[18]. Another study showed that the measurement of serum M30 or M65 can accurately differentiate nonalcoholic steatohepatitis (NASH) or simple steatosis from controls, with M65 being a better diagnostic indicator than M30[19]. In all of these reports, an elevation in serum M30 showed some correlation with disease severity in the early stage of liver injury. In addition, M30 showed clinical significance as a potential prognostic marker in end-stage liver disorders, however, these findings are still controversial. Rutherford et al[20] reported that the median levels of tumor necrosis factor-alpha and M30 were at least 10-fold greater in acute liver failure (ALF) than in either CHC or normal controls. Based on the serum level of M30 and commonly measured clinical variables, the same group developed a prognostic index for ALF which was validated in 250 patients, and found that M30 and clinical variables at study entry most accurately identified patients who would require liver transplantation (LT) or would die[21]. In contrast, Volkmann et al[22] found that patients who spontaneously recovered from ALF had a significantly higher level of serum M30 than patients who required LT or who died. Several additional reports have evaluated the role of overall cell death in NASH, hepatitis C virus infection and liver failure[23-25], and controversies still exist regarding which mode of cell death predominates in various disease stages[9].
However, as potential prognostic biomarkers for clinical outcome of ACLF, an investigation of M30 and M65 in patients with HBV infection as the single etiology has not been reported. In this study, we first investigated the profile of cell death at different disease stages, and in order to determine if there was a correlation with liver damage, we then investigated the association between M30 and M65 with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) in cohort patients. Finally, we evaluated these cell death biomarkers as predictive markers of outcome in ACLF patients at the 3 mo-survival period, and further compared these biomarkers with the MELD and Child-Pugh scores.
From July 2008 to October 2011, 136 subjects including patients with CHB (n = 55), patients with ACLF (n = 81), and 33 healthy individuals were enrolled. All subjects underwent a physical examination, biochemical screening, a blood coagulation test and a liver function test.
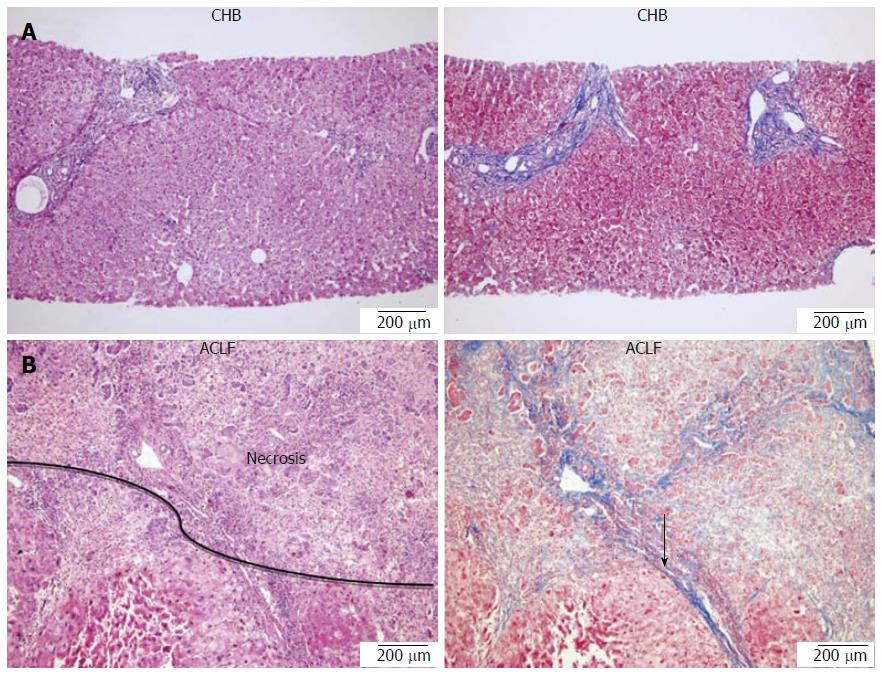
All patients had a history of chronic HBV infection and were either hepatitis B surface antigen (HBsAg) or HBV DNA positive (detected by real-time polymerase chain reaction assay) for more than 6 mo before enrollment. Patients with CHB had persistent ALT elevation or had repeated elevation of ALT and/or inflammation as seen on histological examination of liver biopsy samples[26]. Patients with ACLF fulfilled the following criteria: presented with evidence of acute hepatic insult manifesting as jaundice and coagulopathy, complicated quickly by ascites and/or encephalopathy and with previously diagnosed or undiagnosed chronic liver disease. In addition, serum total bilirubin was ≥ 171 μmol/L, prothrombin time increased significantly and the percentage of prothrombin activity (PTA) was ≤ 40%[6]. Thirty-six of 55 CHB patients underwent liver biopsy, and a pathological analysis was conducted. The liver tissues from 19 ACLF patients who underwent LT were also analyzed by the same experienced pathologist (Figure 1).
All patients with ACLF were followed for at least 3 mo to determine the 3-mo survival period. The patients who died or underwent LT during the admission period were recorded, and patients who were discharged before the end of the follow-up period were monitored via telephone. The spontaneous recovery group was defined as patients with ACLF who survived for more than 3 mo, whereas the non-spontaneous recovery group was defined as patients with ACLF who died within 3 mo or received a liver transplant during this time. The 3-mo start point was set as the day when the serum was collected.
Patients presenting with other viral hepatitis, hemochromatosis, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, biliary obstruction, alpha-1 antitrypsin deficiency, or malignancies were excluded from the study cohort.
Peripheral fasting blood samples were collected from patients on the morning of the second day after hospitalization, centrifuged at 1000 g for 15 min at 4 °C and the serum collected. Aliquoted serum was then immediately stored at -80 °C until used in the enzyme-linked immunosorbent assay (ELISA) analysis.
The study protocol was reviewed and approved by the Institutional Review Board of Beijing Youan Hospital, Capital Medical University, Beijing, China. Written informed consent was obtained from each participant before initiation of the study. The study was carried out according to the Declaration of Helsinki and the guidelines of the International Conference on Harmonization for Good Clinical Practice.
All serum samples collected were first blinded and then tested in duplicate. Total cytokeratin 18 and caspase-cleaved cytokeratin 18 fragments were measured using the M65 and M30 Apoptosense ELISA kits purchased from PEVIVA (Bromma, Sweden) according to the manufacturer’s recommended procedure[27]. In brief, serum samples were first placed into 96-well plates which had been coated with a mouse monoclonal antibody as a catcher. Following incubation for 4 h at room temperature, the plate was washed five times with phosphate buffered saline. A horseradish peroxidase conjugated antibody (against either M65 or M30) was then used to detect the presence and concentration of M65 or M30. The absorbance at 450 nm was determined in a microplate reader within 30 min of setting up the assay.
Statistical analysis was performed using SPSS 16.0 for Windows, and the results are expressed as mean ± SD unless otherwise stated. The continuous variables among multigroups were analyzed by one way ANOVA or the Kruskal-Wallis test depending on data distribution, and pairwise comparisons between groups were performed using the LSD-t or Mann-Whitney test. In addition, the independent-samples t test or Mann-Whitney test was employed to compare the spontaneous recovery group with the non-spontaneous recovery group in ACLF patients. Categoric variables were analyzed by the χ2 test. Correlation analysis was performed by Spearman’s correlation. The diagnostic value of the index with significant difference was assessed by the area under the receiver operating characteristic (ROC) curves. A two-sided P value < 0.05 was considered statistically significant.
In this patient population, 84% of patients were male (114/136) and 16% were female (22/136), and the age range was 19-65 years with an average age of 41.13 ± 11.66 years. In ACLF patients, 16.05% were female (13/81) and 83.95% were male (68/81) with an average age of 42.84 ± 11.12 years. Previous studies repeatedly reported that male Chinese patients developed HBV-related ACLF more frequently than female ones[3-5]. Therefore, in the present study, gender-matched healthy individuals and CHB patients were enrolled. The demographic and clinical characteristics of the subjects are summarized in Table 1.
| Health control (n = 33) | CHB (n = 55) | ACLF (n = 81) | P value | |
| Male | 26 (78.79) | 46 (83.64) | 68 (83.95) | 0.788 |
| Age (yr) | 39.73 ± 7.12 | 38.62 ± 12.08 | 42.84 ± 11.12 | 0.075 |
| HBV DNA viral load (Log) | - | 6.66 ± 1.49 | 5.76 ± 1.73 | 0.000 |
| ALT (U/L) | 18.71 ± 9.33 | 212.07 ± 290.77 | 527.06 ± 790.14 | < 0.0001 |
| AST (U/L) | 21.70 ± 11.84 | 109.85 ± 158.32 | 401.72 ± 571.31 | < 0.0001 |
| Total bilirubin (μmol/L) | 11.07 ± 3.47 | 28.43 ± 30.72 | 394.98 ± 200.16 | < 0.0001 |
| Albumin (g/L) | 46.17 ± 2.05 | 40.02 ± 3.73 | 31.06 ± 3.99 | < 0.0001 |
| Crea (μmol/L) | 66.72 ± 16.14 | 72.10 ± 12.66 | 80.96 ± 50.26 | 0.1768 |
| International normalized ratio | - | 1.03 ± 0.11 | 2.40 ± 1.00 | < 0.0001 |
| Prothrombin activity | - | 98.86% ± 17.34% | 31.34% ± 11.24% | < 0.0001 |
| White blood cell (× 109/L) | 5.85 ± 1.22 | 5.23 ± 1.30 | 8.19 ± 3.79 | < 0.0001 |
| Platelet (× 1012/L) | 223.55 ± 22.09 | 170.72 ± 56.45 | 105.90 ± 58.13 | < 0.0001 |
In ACLF patients, approximately 40% (33/81) fulfilled the criteria for spontaneous recovery, and 59% (48/81) either required LT (n = 19) or died (n = 29) and fulfilled the criteria for non-spontaneous recovery. The clinical features of ACLF patients with spontaneous recovery or non-spontaneous recovery are summarized in Table 2.
| Features | Spontaneous recovery (n = 33) | Non spontaneous recovery (n = 48) | P value |
| Age (yr) | 41.06 ± 12.48 | 44.06 ± 10.04 | 0.235 |
| Male | 29 (87.88) | 38 (79.17) | 0.308 |
| HBV DNA viral load (Log) | 5.78 ± 1.62 | 5.29 ± 2.31 | 0.487 |
| ALT (U/L) | 540.98 ± 555.99 | 525.15 ± 920.58 | 0.098 |
| AST (U/L) | 412.38 ± 525.00 | 397.69 ± 605.02 | 0.645 |
| Total bilirubin (μmol/L) | 371.38 ± 199.37 | 407.99 ± 211.36 | 0.435 |
| Albumin (g/L) | 31.67 ± 3.58 | 30.82 ± 4.24 | 0.492 |
| Crea (μmol/L) | 68.20 ± 31.59 | 88.34 ± 59.38 | 0.094 |
| Prothrombin activity | 35.89% ± 10.62% | 28.45% ± 10.73% | 0.003 |
| International normalized ratio | 2.04 ± 0.49 | 2.67 ± 1.18 | 0.003 |
| White blood cell (× 109/L) | 8.40 ± 3.66 | 8.20 ± 4.10 | 0.571 |
| Platelet (× 1012/L) | 119.44 ± 51.17 | 95.60 ± 61.65 | 0.017 |
| M30 (U/L) | 891.35 ± 741.27 | 695.24 ± 385.15 | 0.663 |
| M65 (U/L) | 2477.23 ± 1671.10 | 2497.77 ± 1503.84 | 0.488 |
| M30/M65 | 0.38 ± 0.13 | 0.32 ± 0.10 | 0.032 |
| MELD score | 23.07 ± 4.89 | 27.59 ± 6.55 | 0.002 |
| Child-Pugh score | 11.42 ± 1.23 | 12.49 ± 1.14 | 0.000 |
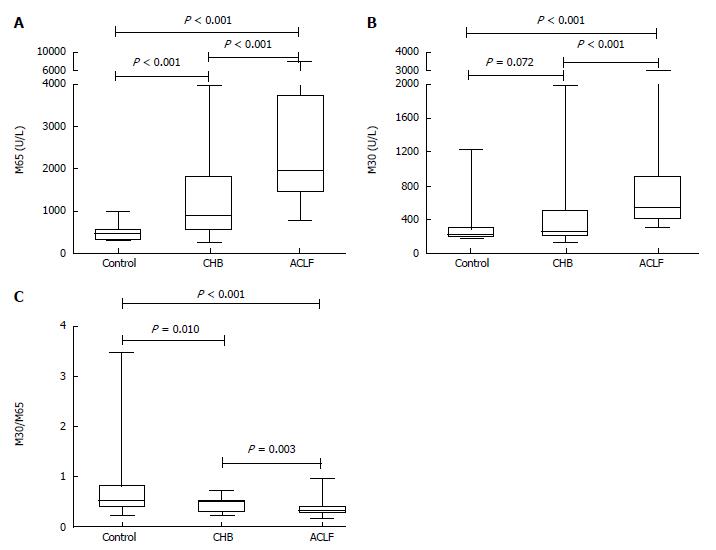
Using ELISA, we detected M30 and M65 levels in patient sera and found that both M65 and M30 levels significantly increased gradually as liver disease progressed (for M65: P < 0.001 for all between different groups; for M30: control vs CHB, P = 0.072; others: P < 0.001 for all). The median M65 level was 458.40 (range: 270.80-958.40) U/L in healthy controls, 876.60 (range: 268.20-4000.00) U/L in CHB patients and 1980.55 (range: 770.80-8020.00) U/L in ACLF patients, and for M30, the median level was 218.80 (range: 168.76-1234.40) U/L in healthy controls, 248.40 (range: 146.88-2000.00) U/L in CHB patients and 537.60 (range: 295.32-3000.00) U/L in ACLF patients (Figure 2A and B). Therefore, our analysis revealed that disease severity was associated with more hepatocyte necrosis and apoptosis.
Measurement of the M30/M65 ratio by ELISA has been reported to be useful in reflecting the balance between hepatocyte necrosis and apoptosis in early stage liver disorders[19,23]. However, few reports on the late stage of liver disorders are available. Therefore, we analyzed the M30/M65 ratio in our cohort. Compared with the control group [0.54 (range: 0.23-3.49)], the median M30/M65 ratio gradually decreased in the CHB group [0.46 (range: 0.20-0.73)] and was lowest in the ACLF group [0.33 (range: 0.15-0.95)], and the differences were statistically significant between the healthy controls and each disease stage (P < 0.05, respectively). The differences between healthy controls and CHB patients and between ACLF patients and CHB patients were statistically significant (P = 0.01, 0.003) (Figure 2C). These results demonstrated that more hepatocyte necrosis than apoptosis occurred during the development of liver disease deterioration.
In order to confirm overall hepatic cell death levels in the corresponding liver tissues, representative liver tissues from CHB and ACLF patients were analyzed pathologically. It was observed that the severity of liver cell necrosis increased from CHB to ACLF, and massive necrosis of hepatic parenchyma was observed in ACLF (Figure 1).
To date, the most convenient and economic indicator of liver damage is the measurement of serum ALT[28]. Interestingly, in the present study, there was also a strong positive correlation between M65 level and ALT and AST in ACLF patients (for ALT: r = 0.43; for AST: r = 0.42, P = 0.000 for all) and CHB patients (for ALT: r = 0.53; for AST: r = 0.64, P = 0.000 for all).
Similar to M65, M30 levels were significantly correlated with ALT and AST, in both ACLF patients (for ALT: r = 0.34, P = 0.007; for AST: r = 0.32, P = 0.013) and CHB patients (for ALT: r = 0.64, P = 0.000; for AST: r = 0.63, P = 0.000).
In aggregated patients including both CHB and ACLF, as expected, strong and significant positive correlations were observed between M65 or M30 and ALT (M65: r = 0.54, P = 0.000; M30: r = 0.53, P = 0.000), and AST (M65: r = 0.64, r = 0.55, both P = 0.000). These results revealed that both serum M65 and M30 had diagnostic value in reflecting liver injury.
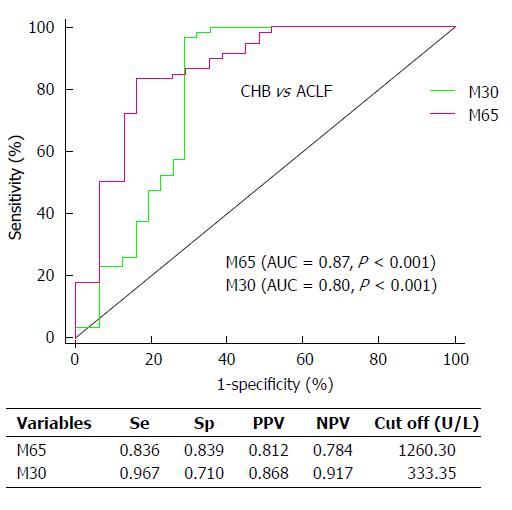
Good diagnostic indicators able to identify patients suffering from end-stage liver diseases who are at high risk of dying are essential for good clinical management. The ROC analysis showed that both serum M65 and M30 levels had significant diagnostic value for identifying ACLF patients [M65: cutoff: 1260.30 U/L, AUC = 0.87 (95%CI: 0.78-0.95); sensitivity, 83.6%; specificity, 83.9%; M30: cutoff: 333.35 U/L, AUC= 0.80 (95%CI: 0.68-0.92); sensitivity, 96.7%; specificity, 71.0%) (Figure 3).
In order to investigate the possible prognostic value of cell death biomarkers, based on 3-mo survival time in ACLF patients, they were defined as the spontaneous recovery group and the non-spontaneous recovery group. We investigated whether there were possible associations between M65 or M30 and outcome, however, we did not find significant differences between them. We then analyzed a possible association between the M30/M65 ratio and outcome, and, for the first time, a significant difference was found between the M30/M65 ratio and the 3-mo survival period (P = 0.032) in patients with HBV-related ACLF. These data are shown in Table 2.
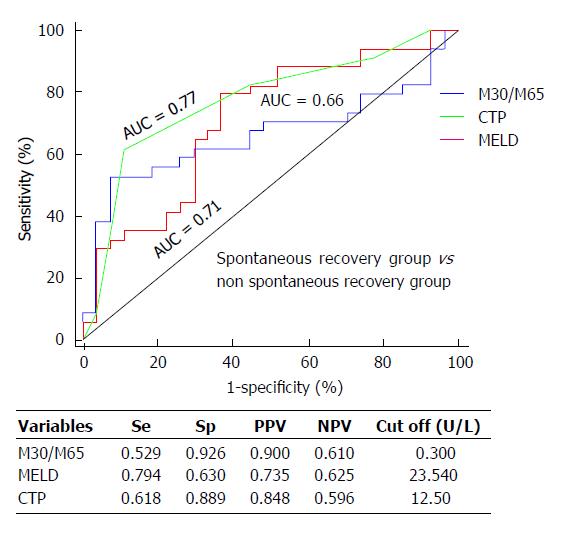
We then conducted an AUC analysis to compare the prognostic performance of the M30/M65 ratio, MELD and Child-Pugh scores. Although the AUC of the M30/M65 ratio was not as high as those of the MELD and Child-Pugh scores, the AUC (95%CI) of the M30/M65 ratio (cut off value: 0.3) was 0.66 (0.52-0.80) with a sensitivity of 52.9% and the highest specificity of 92.6% compared with the MELD and Child-Pugh scores [MELD: AUC = 0.71 (95%CI: 0.58-0.84); sensitivity, 79.4%; specificity, 63.0%; Child-Pugh: AUC = 0.77 (95%CI: 0.65-0.89); sensitivity, 61.8%; specificity, 88.9%) (Figure 4).
A fuller understanding of the mechanism(s) underpinning the different disease stages leading to better judgment of liver disease severity, coupled with additional biomarker-guided individualized therapeutic strategies, is crucial for the improved management of patients with end-stage liver disease. Currently available biomarkers, such as aminotransferase levels, PTA and bilirubin, are representative indicators of the outcome of liver damage. Hepatocyte death plays a fundamental role in disease staging and severity, and consequently the identification of new biomarkers reflecting basic hepatocyte necrosis or apoptosis could provide useful insights into disease development and clinical outcomes. In this context, the measurement of serum M65 and M30 levels has allowed the investigation of both overall cell death and analysis of the cell death profile at different disease severities resulting from chronic HBV infection, and evaluation of the potential prognostic value of these biomarkers in ACLF patients.
This is the first study carried out in a Chinese cohort with a single etiology (chronic HBV infection, a major disease in China) to demonstrate that elevated M65 and M30 levels can differentiate CHB or ACLF patients from healthy controls. In addition, the gradual increase in the levels of M65 and M30 and the associated progression through disease staging allowed a correlation between these biomarkers and ALT or AST to be established. Furthermore, ROC-area under curve (ROC-AUC) analysis demonstrated that both biomarkers showed diagnostic value (AUC ≥ 0.80) in identifying ACLF patients from CHB patients. Interestingly, it is worth noting that there was a significant difference in the M30/M65 ratio between ACLF patients with spontaneous recovery and those with non-spontaneous recovery which included those who required LT or who died (P = 0.032) at the 3-mo survival period. The prognostic value of the M30/M65 ratio was compared to the MELD and Child-Pugh scores at the 3-mo survival period, and the AUC for the M30/M65 ratio was 0.66 with the highest specificity of 92.6%. Our study demonstrated that the M30/M65 ratio may be a potential prognostic marker for clinical outcome in ACLF patients.
Measurement of serum ALT is widely accepted as the most sensitive indicator of hepatic injury. The ultimate outcome of hepatic damage is increased hepatic cell death (necrosis and apoptosis), thus, in this study M65 and M30 levels were found to be strongly correlated with ALT and AST in the whole patient group and in each subgroup. Similar results have been reported previously in earlier stages of liver disorders including steatosis, CHC, and acute liver failure[18,20,29].
Disease-associated cell death typically follows one of two patterns, apoptosis or necrosis[30]. The majority of earlier studies in patients with NASH, hepatitis C virus infection, liver failure[22-24], and acetaminophen hepatotoxicity[31] focused primarily on apoptosis, and as a result, there is some controversy regarding which mode of cell death predominates in the various forms of liver disease, and the severity of overall liver damage that results from each[9]. The present results have shown, for the first time in chronic HBV infection, that although both patterns of cell death are important at different stages of liver disease, hepatocyte apoptosis predominates at earlier stages of disease. As the disease stage progresses there is a gradual switch to necrosis, and eventually necrosis predominates in the late stage of liver disease. The ratio of apoptosis vs necrosis found in control samples was 54% vs 46%. However, in CHB this ratio changed to 46% vs 54%, and was 33% vs 67% in ACLF. Our investigation revealed that hepatic cell necrosis has a stronger role than apoptosis in the progression of chronic HBV infection. Although at present it is unclear which specific factors contribute to the switch, this phenomenon was observed and its clinical significance should be addressed.
We then analyzed the diagnostic value of M65 and M30 in identifying ACLF patients, as expected the ROC analysis showed that the AUC for both biomarkers reached 0.867 (AUC for M65: 0.87; M30: 0.80) in identifying ACLF patients in the patient cohort.
A potential association between hepatic cell death biomarkers and the 3-mo survival period in ACLF patients was further evaluated. Neither M65 nor M30 was significantly different between the spontaneous recovery group and the non-spontaneous recovery group during the 3-mo survival period; however, a decrease in the ratio of M30/M65 was significantly associated with poor prognosis. In order to demonstrate the possible prognostic performance of the M30/M65 ratio, we analyzed and compared its prognostic power with that of the MELD and Child-Pugh score. Although the prognostic performance of the M30/M65 ratio was not as good as that of the MELD or Child-Pugh score, it still showed some prognostic power with the highest specificity of 92.6% (MELD: 63.0%; Child-Pugh: 88.9%). This is the first report on HBV-related ACLF to show that the M30/M65 ratio has prognostic value for predicting the clinical outcome of ACLF patients at the 3-mo survival period.
It is widely accepted that the development of liver failure is complicated and that multiple organs are involved. In HBV-related ACLF, the pathogenesis is more complex, and we consider it impossible that any single parameter can provide sufficient prognostic value. Only a model with a combination of several major and fundamental clinical parameters can have the power to provide more comprehensive and accurate prognostic values for the clinical outcome of liver failure, such as the classic MELD or Child-Pugh scores. Serum M65 has been evaluated and served as a major parameter in a modified MELD score for predicting spontaneous recovery in ALF[21,32].
However, evidence on the predictive value of cell death biomarkers was not consistent for liver disorders in previous reports. In an ALF cohort study, neither apoptotic nor necrotic cell death markers accurately predicted survival in ALF patients[33]. However, conflicting positive associations have also been reported in ALF patients[20-22]. Most previous reports revealed that either M30 or M65 had prognostic value, however, our investigation demonstrated that only the M30/M65 ratio has prognostic value and not each biomarker alone. We consider that the M30/M65 ratio represents the overall hepatic cell death profile, not like each cell death biomarker which reflects either cell necrosis or apoptosis. However, further well-designed studies with larger cohorts are needed to re-evaluate these possible predictive values.
In addition to the above findings, a previous study found that the M30/M65 ratio was significantly decreased in congestive heart failure-induced ALF compared with HBV-related ALF[32]. However, the number of patients with HBV as the single cause of ALF was limited in this study[32]. Thus, a study on the cell death profile in liver failure induced by different etiologies may provide important clinical results. To date, our investigation is the first to include 81 ACLF patients with chronic HBV infection as the single cause.
This study has some limitations. Our study demonstrated, for the first time, that the M30/M65 ratio showed prognostic value in ACLF patients at the 3-mo survival period, however, further research will be required to combine this ratio into the classic model or establish a new and more accurate prognostic model. The present study was a single center investigation; the findings need to be confirmed in larger multi-center studies.
In summary, this study confirmed, for the first time in a Chinese cohort with a single etiology (chronic HBV infection), that a decrease in the M30/M65 ratio is associated with poorer clinical outcomes and that the M30/M65 ratio has prognostic significance for ACLF patients at the 3-mo survival period.
We thank Professor Fengmin Lu at Peking University Health Science Center for providing the helpful information and comments on the manuscript.
In China, over 90% of acute-on-chronic liver failure (ACLF) patients have chronic hepatitis B virus (HBV) infection. Prompt and accurate prediction of prognosis can improve the outcome of HBV-ACLF patients. To date, there are no prognostic models specifically designed for HBV-ACLF patients. Massive hepatic cell death is a fundamental characteristic of liver failure. Using enzyme-linked immunosorbent assay (ELISA) to measure serum M65 and M30, hepatocyte apoptosis and necrosis can be determined. Although a previous study demonstrated that M30 or M65 had prognostic value in patients with acute liver failure due to various etiologies, the prognostic value of M30 and M65 in patients with HBV-ACLF has not been reported.
Some studies have demonstrated that serum levels of M30 or M65 are associated with liver disease severity. Although numerous studies found that serum M30 or M65 showed clinical significance as potential prognostic markers in acute liver failure, these studies had a limited sample size and conflicting results, and no reports in patients with HBV-related ACLF are available.
The measurement of circulating indicators of cell death can reflect hepatocyte apoptosis and necrosis. A previous study reported that an elevation in serum M30 or M65 was correlated with liver disease severity. Although M30 or M65 showed clinical significance as potential prognostic markers in acute liver failure due to various etiologies, these findings are controversial. In order to study their prognostic value in patients with chronic HBV infection as the single etiology, M30 and M65 were identified by ELISA in 169 subjects including healthy controls (n = 33), chronic hepatitis B (CHB) patients (n = 55) and ACLF patients (n = 81). Both biomarker levels significantly increased gradually as liver disease progressed. In contrast, the M30/M65 ratio was significantly higher in controls compared with CHB or ACLF patients. receiver operating characteristic-area under the curve (ROC-AUC) analysis demonstrated that both biomarkers had diagnostic value (AUC ≥ 0.80) in identifying ACLF patients from CHB patients. Moreover, a significant difference in the M30/M65 ratio in ACLF patients was found at the 3-mo survival period. The prognostic value of the M30/M65 ratio was compared to the MELD and Child-Pugh scores at the 3-mo survival period, and its AUC was 0.66 with the highest specificity of 92.6%.
The present study demonstrated that both M30 and M65 were strongly associated with liver disease severity. The M30/M65 ratio may be a potential prognostic marker for spontaneous recovery in patients with HBV-related ACLF.
M65: The M65 ELISA detects a common epitope present in the full-length cytokeratin 18 as well as in the caspase-cleaved fragment and is released from dying cells (due to both apoptosis and necrosis). M30: The M30 detection antibody recognizes a neo-epitope mapped to positions 387 to 396 of cytokeratin 18, which is only revealed after caspase cleavage of the protein and is considered a selective biomarker of apoptosis.
This is the first report in HBV related ACLF that M30/M65 ratio has prognostic value for predicting ACLF patient clinical outcomes at 3-mo survival period. The results are interesting and suggest that serum M65 and M30 are strongly associated with liver disease severity. M30/M65 ratio might be a potential prognostic marker for spontaneous recovery in HBV-related ACLF patients.
P- Reviewer: Hashimoto N S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263-267. [PubMed] |
| 2. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 452] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Wang RB, Zhou GQ, Jiang YY, Sun FX, Wu YZ, Sun JY, Meng PP, Niu SM. An analysis of the relationship between HBV DNA and HBeAg expression and mortality in 799 severe hepatitis patients. Zhonghua Gan Zang Bing Za Zhi. 2006;14:655-657. [PubMed] |
| 4. | Zou Z, Chen J, Xin S, Xing H, Li B, Li J, Shen H, Liu Y. Single factor study of prognosis from 520 cases with chronic severe hepatitis. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2002;16:246-248. [PubMed] |
| 5. | Liu XY, Hu JH, Wang HF, Chen JM. Etiological analysis of 1977 patients with acute liver failure, subacute liver failure and acute-on-chronic liver failure. Zhonghua Gan Zang Bing Za Zhi. 2008;16:772-775. [PubMed] |
| 6. | Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi. 2006;14:643-646. [PubMed] |
| 7. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 8. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-e9. [PubMed] |
| 9. | Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths. Hepatology. 2006;43:S31-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Caulín C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379-1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 478] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Ku NO, Liao J, Omary MB. Apoptosis generates stable fragments of human type I keratins. J Biol Chem. 1997;272:33197-33203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | MacFarlane M, Merrison W, Dinsdale D, Cohen GM. Active caspases and cleaved cytokeratins are sequestered into cytoplasmic inclusions in TRAIL-induced apoptosis. J Cell Biol. 2000;148:1239-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005;59 Suppl 2:S359-S362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 16. | Bantel H, Schulze-Osthoff K. Apoptosis in hepatitis C virus infection. Cell Death Differ. 2003;10 Suppl 1:S48-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Bantel H, Ruck P, Gregor M, Schulze-Osthoff K. Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol. 2001;80:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Bantel H, Lügering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Joka D, Wahl K, Moeller S, Schlue J, Vaske B, Bahr MJ, Manns MP, Schulze-Osthoff K, Bantel H. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Rutherford AE, Hynan LS, Borges CB, Forcione DG, Blackard JT, Lin W, Gorman AR, Shaikh OS, Reuben A, Harrison E. Serum apoptosis markers in acute liver failure: a pilot study. Clin Gastroenterol Hepatol. 2007;5:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Volkmann X, Anstaett M, Hadem J, Stiefel P, Bahr MJ, Lehner F, Manns MP, Schulze-Osthoff K, Bantel H. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology. 2008;47:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 24. | Feldstein AE, Gores GJ. An apoptosis biomarker goes to the HCV clinic. Hepatology. 2004;40:1044-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 26. | Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005). Chin Med J (Engl). 2007;120:2159-2173. [PubMed] |
| 27. | Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050-2068. [PubMed] |
| 29. | Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gurel S, Gulten M. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;13:837-844. [PubMed] |
| 30. | Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3-15. [PubMed] |
| 31. | Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713-716. [PubMed] |
| 32. | Bechmann LP, Jochum C, Kocabayoglu P, Sowa JP, Kassalik M, Gieseler RK, Saner F, Paul A, Trautwein C, Gerken G. Cytokeratin 18-based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J Hepatol. 2010;53:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Craig DG, Lee P, Pryde EA, Masterton GS, Hayes PC, Simpson KJ. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |