Published online Feb 14, 2014. doi: 10.3748/wjg.v20.i6.1424
Revised: December 18, 2013
Accepted: January 3, 2014
Published online: February 14, 2014
Processing time: 129 Days and 10 Hours
Helicobacter pylori (H. pylori) infection is the most common bacterial infection worldwide. Persistent infection of the gastric mucosa leads to inflammatory processes and may remain silent for decades or progress causing more severe diseases, such as gastric adenocarcinoma. The clinical consequences of H. pylori infection are determined by multiple factors, including host genetic predisposition, gene regulation, environmental factors and heterogeneity of H. pylori virulence factors. After decades of studies of this successful relationship between pathogen and human host, various mechanisms have been elucidated. In this review, we have made an introduction on H. pylori infection and its virulence factors, and focused mainly on modulation of host immune response triggered by bacteria, changes in the pattern of gene expression in H. pylori-infected gastric mucosa, with activation of gene transcription involved in defense mechanisms, inflammatory and immunological response, cell proliferation and apoptosis. We also highlighted the role of bacteria eradication on gene expression levels. In addition, we addressed the recent involvement of different microRNAs in precancerous lesions, gastric cancer, and inflammatory processes induced by bacteria. New discoveries in this field may allow a better understanding of the role of major factors involved in the pathogenic mechanisms of H. pylori.
Core tip: In this review, we focused some aspects of Helicobacter pylori (H. pylori) infection as bacterial virulence factor and mainly on modulation of host immune response and changes in the pattern of gene expression in H. pylori-infected gastric mucosa, with activation of gene transcription involved in inflammatory and immunological response, cell proliferation and apoptosis. We also highlighted the role of bacteria eradication for the normalization of gene expression levels. In addition, we addressed the recent involvement of different microRNAs in normal gastric mucosa, precancerous lesions, gastric cancer, and inflammatory processes induced by bacteria, showing deregulated expression.
-
Citation: Cadamuro ACT, Rossi AFT, Maniezzo NM, Silva AE.
Helicobacter pylori infection: Host immune response, implications on gene expression and microRNAs. World J Gastroenterol 2014; 20(6): 1424-1437 - URL: https://www.wjgnet.com/1007-9327/full/v20/i6/1424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i6.1424
Infection by Helicobacter pylori (H. pylori), a Gram-negative, microaerophilic, spiral-shaped bacteria that colonizes the gastric mucosa, is considered the most common bacterial infection worldwide. It is usually acquired during childhood and may persist in the gastric environment throughout life if not treated[1,2]. The persistent presence of H. pylori in the stomach can result in chronic gastritis and may remain silent for decades after infection, due to the synchronized balance between the pathogen and its host, or cause more severe diseases such as atrophic gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma or gastric adenocarcinoma[2,3]. Therefore, H. pylori infection is considered the strongest factor associated with this neoplasm, mainly due to the inflammatory process triggered in the gastric mucosa, increasing the risk of gastric cancer over six-fold compared to individuals without this infection[4,5]. Gastric cancer is considered the first one among the several cancer types associated with infection in the world, with almost 75% of cases being attributable to H. pylori infection[6]. As a consequence of this association, H. pylori was classified in 1994 by the International Agency for Research on Cancer as a type I carcinogen[7].
H. pylori infects over half of the world population, but there is variation in incidence among different geographic regions[8]. Eighty-five percent of H. pylori-infected individuals remain lifelong asymptomatic, while only 1% of these individuals develop gastric cancer[9] and 10% develop peptic ulcer[10].
Indeed, the clinical consequences of infection by H. pylori are determined by multiple factors, including genetic predisposition of the host, especially regarding certain cytokine, and receptor gene polymorphisms[11-15], gene regulation, environmental factors such as high dietary salt intake, and heterogeneity of H. pylori strains[2,16,17].
In most cases, H. pylori infection can persist lifelong in its host in the absence of eradicating antibiotics[1,18], because it is capable of adaptations to colonize the adverse environment of the stomach. H. pylori can survive in the gastric environment at a wide range of pHs, due to urease enzyme activity and the presence of flagella which facilitate the penetration into the mucus layer and reaching the gastric epithelium[19]. Urease hydrolyzes urea to ammonia and carbon dioxide, neutralizing the pH, which allows the bacterial survival and proliferation[19], circumventing host defenses such as the immune response[20].
In general, it is noted that colonization by H. pylori causes a strong systemic immune response, creating a chronically inflamed environment with reduced stomach acidity that favors the growth of other bacteria in the gastric environment, maintaining the inflammation and thereby reducing the level of vitamin C in the gastric juice. The inhibition of gastric acid secretion favors a change from antrum-predominant to corpus-predominant gastritis, initiating gastric atrophy and intestinal metaplasia, which characterize precancerous lesions[21].
Furthermore, the bacterial virulence factors cytotoxin-associated gene A antigen (CagA) and vacuolating cytotoxin (VacA) play a pivotal role in H. pylori-induced pathogenesis, and others, such as IceA (induced by contact with epithelium), blood group antigen-binding adhesion (BabA), sialic acid-binding adhesion (SabA), duodenal ulcer-promoting gene (DupA) and outer inflammatory protein (OipA), also allow a successful colonization of the mucosa[22,23]. These bacteria populations are highly heterogeneous with respect to virulence factors VacA and CagA[24], and several substantial pieces of evidence show that these genetic differences play an important role in the clinical outcome of the infection[17,25].
The cagA gene produces one of the most important virulence factors of H. pylori, being located in a segment of DNA called the cag pathogenicity island (cagPAI) that contains, besides the cagA gene, genes which give rise to the bacterial type IV secretion system (T4SS-type-IV secretion system)[26]. This system functions like a molecular syringe, injecting CagA, peptidoglycans and other factors into host epithelial cells[27]. After its entry into the cell, CagA can be phosphorylated by tyrosine kinases and interact with cellular proteins, acting in the signal transduction pathways to the nucleus, changes in the cytoskeleton, disruption of cell-cell junctions[28,29], stimulating the growth factor signaling, leading to changes in cell morphology and increased cell proliferation[30], as well as anti-apoptotic responses[29,31]. CagA is not found in all strains of Western H. pylori population[32]. Its occurrence is associated with more severe inflammation of the gastric mucosa[33,34], conferring a greater risk of developing stomach cancer[32,35,36].
The second most studied virulence factor is the VacA, encoded by vacA gene that induces the formation of vacuoles in eukaryotic cells and stimulates apoptosis in epithelial cells[37]. Unlike cag-A, all H. pylori strains possess the vacA gene, although only about 50% of them express the VacA protein. The regions with the highest diversity are located at the 5’ terminus signal (allele types s1a, s1b, s1c and s2), the mid-region (allele types m1 and m2) and the intermediate region (allele types i1 and i2)[38]. This combination of sequence diversity in vacA, considering that each gene contains a single allele (signal, mid-region and intermediate region allele), causes variations in cytotoxic activity[39], the s1m1 strain being highly toxigenic[40]. Humans infected with H. pylori-VacA+ strains are more prone to gastritis than those infected with strains that do not express this protein[41]. VacA may interfere with phagocytosis and antigen presentation[42,43], reducing the activation of Jurkat cells, thereby inhibiting the activation of NFAT, an important transcription factor that is necessary for the expression of genes involved in the expansion of T cells activated by bacterial antigens[44], thereby ensuring the evasion of H. pylori from the adaptive immune response.
The BabA and SabA adhesins are encoded by the babA and sabA genes that encodes an outer membrane protein, BabA, which binds to the type B blood group antigen in gastric cells[1], while sabA binds to the sialyl-Lewis x/a antigens[45]. The adhesion of bacteria to the gastric epithelium allowing the release of the CagA and VacA factors into the host cells is mediated by BabA, which facilitates colonization, induces mucosal inflammation and can influence the severity of the disease[46,47]. H. pylori strains which carry babA, vacAs1 and cagA together are associated with duodenal ulcer and present a higher risk of gastric cancer[48]. The inflammatory response may be increased due to sabA-mediated adhesion, by facilitating the utilization of nutrients exudated from damaged host cells. Thus, as the inflammatory response increases, the sabA expression may be switched off, allowing the contact between the bacteria and the inflamed epithelium to be broken, thus maintaining prolonged infection[45]. However, there is no clinical or epidemiological evidence associating sabA to gastric cancer. Another gene that encodes an outer membrane protein is oipA, located near cagPAI[49]. It is regulated by a slipped-strand repair mechanism based on the number of Cysteine-Threonine dinucleotide repeats in the 5’ regions of the gene[49]. The oipA gene has the ability to induce interleukin (IL)-8 from gastric epithelial cells, as cagA and its status have been linked to the discrimination of duodenal ulcer and gastritis[49,50].
The dupA gene, located in the plasticity region of the H. pylori genome, represents a marker of virulence with pathogenic potential[51]. This gene was reported to be associated with increased risk of duodenal ulcer[51], with lower gastric cancer incidence and lower acid output, including patients with peptic ulcer[52]. As opposed to these findings, there are studies of dupA status in which no association with any gastroduodenal disease was found[53].
The iceA gene, another virulence factor, has two variants, iceA1 and iceA2[54]. However, the function of iceA2 remains undefined[55], while the expression of iceA1 is increased in some populations by the contact of H. pylori with human gastric epithelial cells and is associated with peptic ulcer[56]. Nevertheless, the development of erosive gastritis has been related to strains carrying genes iceA1, cagA and vacAs1a/m1, while enanthematous gastritis is associated with vacAs2/m2 and iceA2 genotypes[57]. Moreover, the severity of gastritis is related with the coexistence of the iceA2 gene with cagA, vacAs1/m1 and babA2[58].
In this review, we first approached about the H. pylori infection and its virulence factors, topics widely addressed in other recent reviews[2,18,19]. Thus, we will focus mainly on modulation of host immune response triggered by H. pylori, and the advances in the fast developing field of gene expression profiles in gastric mucosa, which can change as a consequence of H. pylori infection, leading to the activation of transcription of genes involved in defense mechanisms, inflammatory and immunological responses, cell proliferation and apoptosis. Moreover, we highlighted the importance of the eradication of H. pylori, which plays an important role in the restoration of gastric mucosa inflammation and on gene expression levels. In light of the increasing involvement of microRNAs (miRNAs) in the regulation of posttranscriptional gene silencing, we addressed the action of different miRNAs in precancerous lesions, gastric cancer, and inflammatory processes induced by H. pylori, evidencing its participation in several steps of gastric carcinogenesis.
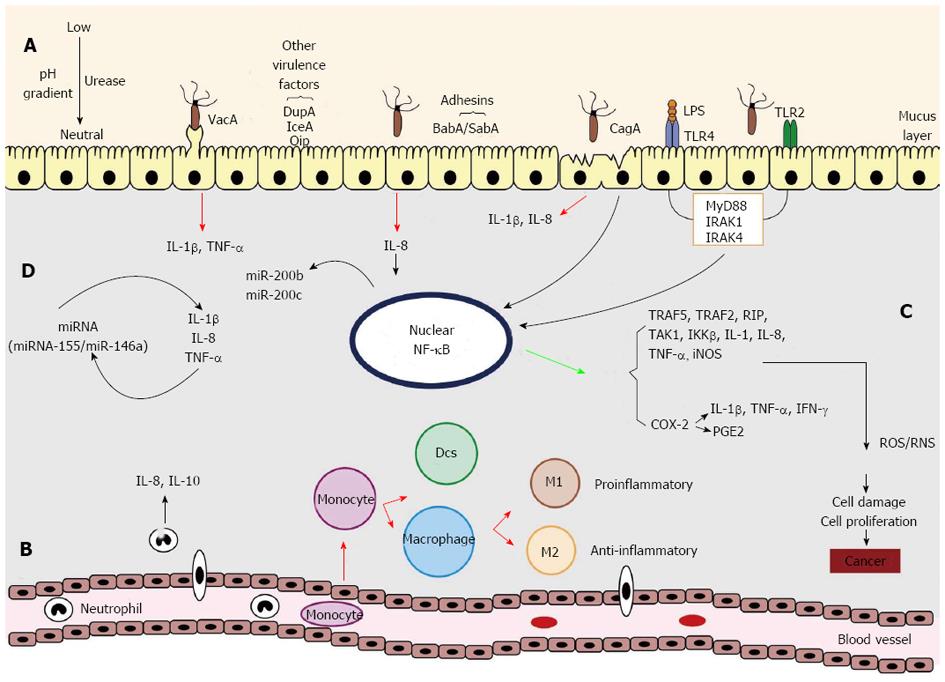
As soon as H. pylori bacteria colonize the stomach, the epithelial cells and their innate immune receptors, mainly the toll-like receptors (TLRs)[19], recognize the bacteria (Figure 1). This attachment process can be facilitated by the action of adhesins (SabA and BabA) expressed by bacteria, which favor the action of other virulence factors (CagA and VacA). Soon after, the host’s innate and adaptive immune systems are activated, leading to the recruitment of a wide variety of inflammatory cells and mediators, and the activation of transcription factor nuclear factor (NF)-κB and pro- and anti-inflammatory cytokines, cell proliferation and survival factors. The activation of the immune system in response to the presence of the bacteria increases the production of reactive oxygen and nitrogen species [reactive nitrogen species (RNS) and reactive oxygen species (ROS)] by increasing oxidative/genotoxic stress, which can cause cell and DNA damage, favoring the appearance of mutations that may facilitate the carcinogenic process. In addition, the expression of the immune response mediators can be regulated by miRNAs, and inflammatory mediators can change the miRNAs expression[59-61].
Members of the TLR family are essential components of the innate and adaptive immune response and comprise 10 types in humans, TLR1 to TLR10[62]. They recognize molecular structures of pathogenic microbe-associated molecular patterns (PAMPs), like lipopolysaccharides (LPS), lipoproteins, lipoteichoic acid, peptidoglycan, lipoarabinomannan and flagellin[63]. H. pylori LPS, as cell wall components, are recognized mainly by TLR4; however modifications of the LPS structure can alter this recognition and poorly stimulate the host immune response, enhancing the bacterial evasion and pathogenicity[64]. H. pylori is also recognized by TLR2 through other forms of LPS structurally different from those recognized by TLR4[65]. TLRs are dependent on the presence of MyD88 (myeloid differentiation primary-response gene 88) for efficient signal transduction. The MyD88 complex is associated with interleukin-1-receptor-associated kinase-1 (IRAK1) and IRAK4. IRAK1 is phosphorylated and then dissociated from MyD88. Subsequent dissociation of protein complexes occur by phosphorylation, and, as the last step, NF-κB is translocated into the nucleus, activating the expression of genes related to the inflammatory process[66] (Figure 1A).
CagA-positive strains contribute to the inflammatory response, since this virulence factor causes an increase in the production of certain cytokines such as IL-1β and IL-8[67,68] and activation of NF-κB, which can confer a proliferative phenotype to the bacteria, important in the process of carcinogenesis[69], promoting induction of growth factors and suppression of apoptosis[70]. Thus, CagA deregulates the cell signaling pathways and favors the arising of oncogenic cells, which is important in the pathogenesis of H. pylori[71]. The VacA factor induces a pro-inflammatory response[72] and multiple cellular activities that facilitate chronic colonization of the gastric mucosa by bacteria[68]. A recent study showed that overexpression of VacA led to the production of tumor necrosis factor (TNF)-α, IL-1β, nitric oxide, reactive oxygen species and the activation of NF-κB, which can be associated to pro-inflammatory cytokines and cell apoptosis[73]. VacA also can affect the immune system enabling H. pylori evade the adaptive immune response to establish persistent infection, since can interfere with phagocytosis and antigen presentation and also inhibit T cell proliferation[24].
H. pylori infection, after activation of NF-κB and cytokines production, causes chemotaxis of monocytes/macrophages and infiltration of polymorphonuclear leucocytes[74], recruitment of neutrophils and lymphocytes[75]) that also induces the production of IL-8 and IL-10 by neutrophils[76] (Figure 1B). In this pathway, monocytes secret interleukins such as IL-1β, IL-6, IL-10, and IL-12p40 (partially secreted as IL-23), dentritic cells (DCs) secret IL-1β, IL-6, IL-10, IL-12p40, IL-12p70, and IL-23, while M1 macrophages produce mainly IL-1β, IL-6, IL-10, IL-12p40 and IL-23. M2 macrophages synthesize IL-10 but produce less pro-inflammatory cytokines than M1 macrophages, which can control inflammation, leading to a chronic inflammatory response[77].
The activation of DCs and M1 macrophages is correlated with an increased capacity to induce T-cell proliferation like T helper cells and decreased phagocytosis[78,79], as well as Th17 that can promote chronic infection triggered by a chemotaxis system[80]. Even though H. pylori avoids phagocytosis and prevents the induction of an adaptive immune response, macrophages engulf the bacterium but cannot kill it, which facilitates the chronic infection[81]. This infection results in a predominantly T cell-mediated immunity rather than humoral immunity, with Th1 and Th17 responses, which increase the production of IL-1β, TNF-α and IL-8[82,83]. While Th17 cell differentiation is promoved by TNF-α and IL-6 from activated macrophages/dentritic cells, Th1 cell development is triggered by IL-12 and interferon (IFN)-γ[2]. In addition, the recruitment of antigen-specific regulatory T cells has also been reported, facilitating the permanent colonization of the stomach through direct cell-to-cell contact or by secreting cytokines [transforming growth factor (TGF)-β1 and IL-10] that modulate the immune response[84].
Moreover, NF-κB regulates the expression of several genes, for example TRAF5, TRAF2, RIP, TAK1 and IKKβ, some of which are associated with inflammation and cancer[85]. Its activation by LPS leads to the synthesis of IL-1, IL-8, IL-10 and TNF-α, and iNOS (Figure 1C). NF-κB also upregulates the expression of the pro-inflammatory cyclooxygenase (COX-2) enzyme, whose function is to induce cytokines such as TNF-α, interferon-γ and IL-1, inhibiting apoptosis, maintaining cell proliferation and stimulating angiogenesis in favor of carcinogenesis[20]. In H. pylori-associated gastric cancer there are reports showing increased expression of COX-2 and prostaglandin E2 activated by TLR2/TLR9 and NF-κB, and this induction is mediated by the activation of the epidermal growth factor receptor in gastric epithelial cells[86,87]. Added to inflammatory stress effects, influences on the cell cycle and cell polarity, H. pylori also activates multiple oncogenic mechanisms, such as the PI3K/AKT/GSK3β pathway that regulates many functions like cell growth, proliferation, differentiation and motility, and its aberrant activation is associated with various types of cancer, including stomach cancer[88,89]. The presence of these bacteria also affects the STAT3 protein pathway that regulates cell growth, differentiation and apoptosis, in which a high expression of STAT3 is associated with advanced stage and poor prognosis of gastric cancer[90]. All the high immune stimulation produced by these molecules results in the production of ROS and RNS by neutrophils attracted to the infection site, which can cause cell damage, leading to gene mutations and cell proliferation, favoring the emergence of gastric cancer[91].
Other important members of the class of immune regulators are miRNA[92]. Recent reports have highlighted the regulatory role of miRNAs in H. pylori infection and associated diseases (Figure 1D). For example, a strong inflammatory response characterized by the early production of pro-inflammatory TNF-α and IL-6 cytokines, followed by IL-10, IL-1β and IL-23 secretion as a consequence of miR-146a up-regulation and strong miR-155 induction, which raised the TNF-α production[93]. In contrast, IL-8, TNF-α and IL-1β could contribute to the induction of miR-146a during H. pylori infection[94]. Therefore, miRNAs modulate the H. pylori infection and are also affected by these bacteria, as, for example, the synthesis of the transcription factor NF-κB that can act as a transactivator of miR-200b and miR-200c[60]. This issue will be discussed in more detail in the last section of this review. Thus, all the pathways reported above show the need for new approaches in order to reach a better understanding of the influence of H. pylori on the host immune system, allowing the working out of preventive measures and efficient new strategies of H. pylori eradication.
In addition to a marked inflammatory response of the host, activation of signaling pathways and gastric mucosa injury, H. pylori infection can enhance cell proliferation and apoptosis of gastric epithelial cells[95]. Thus, to counteract H. pylori infection, the host activates gene transcription involved in his defense mechanisms, inflammatory and immunological response and control of cell kinetics[31,96,88]. Gene expression profiling analysis in gastric biopsies and cell lines in response to H. pylori infection might be one approach to better understand the role of important factors involved in the pathogenic mechanism of these bacteria.
In this respect, Hofman et al[97] (2007) evaluated the gene expression profile of the gastric mucosa of H. pylori-infected compared to noninfected patients and highlighted a distinct transcriptional pattern in biopsies of the antral and fundic regions, associated also with bacterial density and virulence factors such as cagA, vacA and babA2. The authors reported up-regulation in receptors and co-receptors involved in bacterial recognition such as TLR2, TLR4, LY96, ITGB2, VCAM1, MAPK8, RAC2, SLA, ADAM, MMP, IFITM1 and PAP, signal transduction, inflammation and immune response, proteolysis, apoptosis and cell proliferation in antral biopsies from infected patients in comparison with biopsies from noninfected individuals. It was also observed that several transcripts encoding chemokines and their receptors were up-regulated in response to H. pylori infection. More recently, microarray data of gene expression profiling in gastric antral mucosa from chronic superficial gastritis patients infected by H. pylori and uninfected subjects revealed 38 differentially expressed genes, including 23 up-regulated and 15 down-regulated genes related to protein metabolism, inflammatory and immunological reaction, signal transduction, gene transcription and trace element metabolism[98]. These data indicate that H. pylori infection could induce carcinogenesis by altering cellular gene expression processes, evade the host defense mechanism, increase inflammatory and immune responses, activate NF-κB and Wnt/β-catenin signaling pathways, and disturb the metal ion homeostasis. However, the functional significance of these selected genes needs to be further evaluated in other studies.
TLRs expression has been evaluated in H. pylori infection due to its relevant role in the recognition of pathogenic components such as bacterial LPS. In H. pylori-negative normal gastric mucosa, TLR5 mRNA is the most expressed, followed by TLR2 and TLR4, whereas in H. pylori-infected normal gastric mucosa, intestinal metaplasia, independently of H. pylori infection, and in the dysplasia/cancer sequence TLR2 and TLR4 are the most overexpressed[99]. Therefore, these findings suggest that progressive activation of these receptors, initially by H. pylori, but also by other PAMPs or damage-associated molecular patterns at later stages, may have an important role in gastric carcinogenesis and tumor progression[99]. However there is also indication of no quantitative differences in the TLR4 and TLR5 mRNA levels, regardless of the presence or absence of H. pylori, in both gastric epithelial cell biopsies and AGS cells, suggesting that the mRNA levels of these receptors may not be influenced by the infection process, or at least not at the time points selected for analysis[100].
H. pylori-CagA+ strains often trigger more potent inflammatory and immune responses, leading to a more severe disease, which may be mediated by nucleotide oligomerization domain 1 (NOD1) by recognizing the intracellular pathogen and initiating pro-inflammatory signaling cascades[101,102]. Gastric epithelial cells co-cultured with H. pylori-CagA+ strains show increased production of IFN-γ-inducible chemokines, IP-10 and MIG, in response to IFN-γ stimulation. In addition, gastric biopsies from infected and non-infected patients with gastritis or gastric cancer show increased mRNA expression levels of NOD1, CXCL8, IRF1 and CXCL10, when compared with normal tissue[103]. Likewise, up-regulation of pro-inflammatory molecules expression also occurs in gastric tumor tissues compared to matched non-tumor samples such as IRF1, NOD1 and CXCL8. Thus it is proposed that NOD1 and the IFN-γ signaling pathway regulate the expression levels of the tumor suppressor gene IRF1. That could, in some instances, potentiate oncogenic changes in the gastric mucosa as a consequence of infection with virulent H. pylori-CagA+ strains and exacerbate disease severity and progression during chronic H. pylori infection.
In addition, H. pylori-CagA+ strains also appear to be related with differential activation of two signaling proteins, STAT3 and ERK1/2 in gastritis patients[104]. The differential activation of these two signaling proteins may in part explain the increased predisposition to gastric cancer when infected with H. pylori-CagA+ strains compared to their CagA- counterparts, due to the activation of epithelial cell turnover, thus increasing the likelihood of gaining somatic mutations and subsequent cellular transformation. Recently, in AGS cells incubated with H. pylori-CagA+ strains 147A- and 147C was observed specific and significant alterations in gene expression profiles[105]. Up-regulated genes primarily encoded signal transduction (23.2%), transport (13.8%), transcription (12.6%), metabolic (11.3%), immune and inflammatory responses (6.9%), adhesion and migration (5.9%), and development proteins (5.0%), while down-regulated genes encoded metabolic (16.1%), transcription (14.6%), transport (14.6%), signal transduction (10.6%), translation (5.9%), cell cycle (5.1%), and apoptosis (3.0%). Among the differentially expressed genes compared to non-treated AGS cells, the EMT (epithelial-mesenchymal transition) gene was selected because it seems to facilitate the invasion of cancerous cells into both local and distant tissues. Thus, the H. pylori-CagA+ strain plays a significant role in epithelial-mesenchymal transition, so the prevention of H. pylori-CagA+ infection may be an effective approach in preventing the progression or metastasis of tumor cells that occurs via EMT-inducing genes.
Considering the role of H. pylori infection as a key event in triggering all these changes in gene expression of the infected gastric mucosa, and even the risk of malignant progression, the eradication of these bacteria has been recommended in various countries[106]. Once the gastric colonization by the pathogen is rarely eliminated spontaneously, H. pylori eradication is regarded as a first-line therapy to reverse the pre-neoplastic lesions and prevent malignant progression[107]. The standard triple treatment regimen of infection consists of two or three antibiotics (amoxicillin or clarithromycin) and a proton pump inhibitor, associated or not with bismuth salts, for 1 or 2 wk[108], reaching an eradication rate higher than 90%[109,110].
Although the eradication of H. pylori can result in partial regression of pre-neoplastic lesions, to this date few studies have evaluated the role of treatment for the restoration of gastric mucosa inflammation and normalization of gene expression levels. Tsai et al[107] (2006) employed microarray technology to investigate changes in gene expression profiles using samples from a double-blinded, placebo-controlled clinical trial, associated with H. pylori infection and eradication of the bacteria. One year after the bacteria eradication therapy, were identified 30 genes whose expression was significantly down-regulated, the majority of which were associated with immune response and inflammation (CXCL1, CXCL14, IGLC2, LOC400986, TNFSF10 and OAS1), while in the placebo group the expression of 55 genes differed significantly in the same period (32 up-regulated and 23 down-regulated). Among them, genes involved in cell-cell adhesion and lining, cell cycle differentiation, and lipid metabolism and transport were down-regulated over time in the treatment group but up-regulated in the placebo group. Taken together, these findings showed that H. pylori infection and its subsequent eradication resulted in alterations of gene expression associated with cell damage, inflammation, proliferation, apoptosis and intestinal differentiation, suggesting that H. pylori eradication may stop or reverse ongoing malignancy-related molecular processes in the stomach. In this respect, further studies are needed to evaluate the use of these genes as possible markers for gastric cancer risk.
The eradication therapy also appears to influence the expression of the transcription factor FOXP3 by CD4+CD25 regulatory T cells in the gastric and duodenal mucosa leading to reduced expression in response to treatment[111]. Moreover, was observed a decrease of IFN-γ and IL-10 gene expression in the antral mucosa after eradication of H. pylori. Thus, it is possible that in the infected mucosa the overall immune response may be shifted towards an anti-inflammatory response. This could indicate that a moderate regulatory mechanism is induced in the presence of the bacteria, keeping an immunologic balance where the inflammation is maintained at a controlled level by the suppressive regulatory T cells. This effect may explain why H. pylori infections become chronic.
The effect of H. pylori eradication therapy was also observed on receptors expression levels such as genes human beta defensin 2 (hBD2) and hBD3, which codify antimicrobial peptides on the mucosal surface and act in the innate immune responses to human pathogens[112]. Up-regulation of both hBD2 and hBD3 transcripts were observed in H. pylori-positive subjects that correlated with the degree of gastritis in corpus and antrum. However, after successful eradication therapy, while the mucosal hBD2 transcript levels returned to normal, the hBD3 protein expression level remained unchanged. In addition, while infiltrating granulocytes disappeared completely, higher lymphocytic infiltration still persisted compared to H. pylori-negative subjects[112]. Possibly H. pylori-positive patients were most likely infected in their early childhood and had carried the bacteria for decades, speculating whether the decreased expression of hBD3 after 3 mo of treatment should be attributed to long-lasting effects on the epithelial cells that had not been completely renewed or to the lymphocytic infiltration still present at the time of study.
In a broader perspective, despite the still limited studies on the role of H. pylori eradication in the normalization of gene expression levels in gastric mucosa, such studies showing genes with significant changes of expression over time may help reveal molecular markers involved in inflammatory processes and mechanisms of progression from precancerous lesions to malignancy.
miRNAs, non-coding ribonucleic acids with about 22 nucleotides[113], are involved in the process of posttranscriptional gene silencing through the pairing with mRNA target, promoting its degradation[114,115] or, mostly in animals, causing repression of mRNA translation[116,117]. Since the discovery of miRNAs, their key role in the regulation of gene expression[118,119] and their participation in various cellular and systemic functions, they have been associated with various pathologies, such as inflammation and cancer[120,121].
The expression of miRNAs is tissue-specific and they have different cellular functions, such as regulation of proliferation, apoptosis[122,123], differentiation[124,125] and carcinogenesis, and can be used as biomarkers for tumor origin[120,126]. With particular regard to the stomach, there are various studies reporting different miRNAs in normal mucosa[127], H. pylori-induced precancerous lesions and gastric cancer (Table 1)[60,123,128-140]. Studies on miRNA in precancerous gastric lesions are still scarce. For example, chronic gastritis experimentally induced by H. pylori showed the action of hsa-miR-155 in regulating the response of Th1 and Th17 cells to control infection and, in the meantime, induced precancerous pathologies associated with this bacterium by IFN-γ production[121,141]. In intestinal metaplasia was demonstrated that the CagA bacterial protein stimulates the expression of hsa-miR-584 and hsa-miR-1290, which results in down-regulation of the forkhead box A1 (Foxa1) gene, thus inducing transdifferentiation of gastric epithelial cells[140].
| miRNAs | Regulation | Targets and action | Ref. |
| let-7b | Down | TLR4, NF-κB, COX-2, Cyclin D1, IL1BInitiation of immune response | [128,129] |
| has-miR-17 | ND | ND | [130] |
| hsa-miR-21 | Up | RECK, TGFBR1, TGFBR2Promotes proliferation, migration and invasion, inhibits apoptosis | [123,130] |
| hsa-miR-25 | Up | ND | [130] |
| has-miR-93 | Up | ND | [130] |
| has-miR-103 | Down | TNFαND | [128] |
| hsa-miR-146a | Up | IRAK1, CARD10, COPS8, PTGS2Inhibits tumor-promotes cytokines and growth factors | [131,132] |
| hsa-miR-155 | Up | SMAD2Attenuation of the inflammatory response | [133] |
| hsa-miR-194 | Up | NDND | [130] |
| hsa-miR-196 | Up | ND | [130] |
| hsa-miR-200b | Up | ZEB1Promotes EMT | [60] |
| has-miR-200c | Up | ZEB1, IL6ND | [60,128] |
| has-miR-222 | Up | RECKPromotes cell proliferation | [134] |
| has-miR-223 | Up | IL6, IL1B, ND | [135,136] |
| hsa-miR-370 | Down | FoxM1Promotes proliferation | [137] |
| has-miR-371-5p | Down | LATS2Inhibits cell cycle progression | [138] |
| has-miR-372 | Down | LATS2Inhibits cell cycle progression | [138] |
| has-miR-373 | Down | LATS2Inhibits cell cycle progression | [138] |
| hsa-miR-375 | Down | IL8ND | [128] |
| has-miR-449b | Down | MET, GMNN, CCNE2, SIRT1, HDAC1Promotes proliferationInhibits senescence and apoptosis | [139] |
| has-miR-584 | Up | PPP2a, Foxa1Promotes EMT and stem cells differentiation | [140] |
| hsa-miR-1290 | Up | NKRF, Foxa1Promotes EMT and stem cells differentiation | [140] |
In H. pylori-associated gastric cancer, an increasing number of studies have described the occurrence of deregulation of miRNA expression and its involvement in the regulation of gene expression. H. pylori and CagA genotype inhibit has-miR-370 expression in both gastritis and gastric cancer, which led to overexpression of this target FoxM1. This increased expression was gradual from inflammation to cancer, resulting in cell proliferation for gastric carcinogenesis[137]. In gastric cancer cell line, non-malignant gastric cell line, as well as in human gastric mucosal tissue, H. pylori is able to increase expression of has-miR-222 promoting cell proliferation by gradually decrease the expression of their target RECK[134], so H. pylori infection can induce carcinogenesis through altering expression of some miRNAs. Also H. pylori-infected AGS cell line results in the repression of hsa-miR-371-5p, hsa-miR-372 and hsa-miR-373, which leads to the inhibition of cell cycle progression by up-regulation of their target LATS2 (serine-threonine kinase)[138]. hsa-miR-200b and hsa-miR-200c that have a common target, ZEB1, are transactivated by transcription factor NF-κB due to the presence of the cagA genotype, so that the gastric epithelial cells begin to undergo mesenchymal transition[60].
Considering the importance of the treatment and eradication of H. pylori to restore gastric tissue homeostasis, Matsushima et al[135] (2011) found 31 miRNAs differentially expressed in infected- noncancerous gastric mucosa compared to non-infected individuals. Of these miRNAs, only has-miR-223 showed increased expression in H. pylori-positive individuals. In a subgroup of four patients in which H. pylori was eradicated, was observed that 14 miRNAs that were down-regulated in the presence of the pathogen had their levels increased after four weeks of eradication therapy. However, in a patient in whom the therapy was not satisfactory, the levels of these miRNAS were unaltered[135]. However, eradication of the bacteria year after treatment did not change the expression of oncogenic miRNAs in metaplastic glands, but it was decreased in non-metaplastic glands, indicating that the treatment was effective in restoring the miRNAs expression only in the early stages of gastric transformation[130]. In addition, hsa-miR-21, hsa-miR-25, hsa-miR-93, hsa-miR-194 and hsa-miR-196 were overexpressed in gastric cancer in comparison to H. pylori-positive gastric ulcer or atrophic gastritis, and the eradication decreased the expression of these miRNAs only in atrophic gastritis[130]. These findings evidence that H. pylori is able to change the expression of miRNAs in noncancerous gastric mucosa, and this is one of the possible mechanisms for manipulating the host response.
H. pylori can remain in the stomach at high density levels and for a long time, indicating that the host immune response is not effective in eliminating the pathogen. This may be due to the deregulation caused by the bacteria in the expression pattern of miRNAs which target cytokines and other mediators of the immune response. The miRNA has-miR-21 is a possible regulator of H. pylori-induced inflammation, targeting the receptor of the TGFβ signaling pathway (TGFβR1 and TGFβR2)[142], and the mature form of this miRNA shows increased expression in both gastric cancer and H. pylori-infected gastric tissue[123]. hsa-miR-155 and hsa-miR-146a are also involved in the attenuation of the inflammatory response against H. pylori. In this process, the MyD88 complex and adaptor proteins (IRAK-1 and TRAF6) of the TLRs signaling cascade are targeted by these miRNAs, resulting in decreased NF-κB activation. In contrast, H. pylori also up-regulates hsa-miR-155 expression, which occurs in an NF-κB-dependent manner, resulting in decreased levels of pro-inflammatory mediators IL-8 and growth-related oncogene-α[131-133]. Moreover, H. pylori infection decreases the expression of let-7b, increasing the production of TLR4, NF-κB, COX-2 and Cyclin D1, thus contributing to the initiation of the immune response and the inflammation of the gastric mucosa[129]. Particularly, Isomoto et al[128] (2012) investigated the association of various miRNAs with cytokine expression in H. pylori-positive gastric mucosa and found a negative correlation among let-7b, hsa-miR-200c, hsa-miR-375 and hsa-miR-103 and interleukins IL-1β, IL-6, IL-8 and TNF-α, respectively. Other relationships between inflammatory mediators and miRNAs are described, as for example has-miR-370 and reduced expression of TGFβR2[143], has-miR-365 and negative regulation of IL-6[144], and has-miR-223 and the reduction of IL-6 and IL-1β[136].
Therefore, inflammatory process induced by H. pylori leading to precancerous gastric lesions and gastric cancer can alter the expression pattern of miRNAs in order to influence biological processes by changing the expression of mRNA targets. Eradication of the bacteria may be a strategy for restoring normal levels of these miRNAs in the gastric mucosa at early stages of malignant transformation, reducing the risk of gastric cancer.
After millennia of co-evolution of H. pylori bacteria with human hosts, complex mechanisms of interaction between pathogen and host developed, allowing its persistence and subversion of the immune system and successful colonization in the human stomach. Numerous studies about colonization and adhesion of bacteria in gastric epithelial cells, diversity of virulence factors, activation of signaling pathways, evasion and subversion of the immune system and, more recently, about changes in the gene expression profile of infected mucosa and participation of miRNAs have contributed to a better understanding of the host-pathogen relation. Taken together, these data may help to clarify pivotal biological and molecular mechanisms of infection pathogenesis and to identify clinically significant biomarkers, with the possibility of disclosing novel therapeutic targets for treatment strategies, especially in patients who developed resistance mechanisms. Taking into account that H. pylori infection is a relevant risk factor for the development of gastric cancer, strategies aiming for a better understanding of the mechanisms involved in its pathogenesis and effective eradication therapies are critical for the prevention of this type of malignancy.
The authors thank PROPE/UNESP and FUNDUNESP (Process No. 0302/019/13-PROPe/CDC) by support of english revision.
P- Reviewers: Samulski RJ, Shimoyama S, Zhong L S- Editor: Cui XM L- Editor: A E- Editor: Wu HL
| 1. | Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 2. | Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol. 2011;17:1383-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther. 1999;13 Suppl 1:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 5. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 6. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1719] [Article Influence: 132.2] [Reference Citation Analysis (1)] |
| 7. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 8. | Van Doorn LJ, Figueiredo C, Mégraud F, Pena S, Midolo P, Queiroz DM, Carneiro F, Vanderborght B, Pegado MD, Sanna R. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Sachs G, Scott DR. Helicobacter pylori: Eradication or Preservation. F1000 Med Rep. 2012;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Li ZW, Wu Y, Sun Y, Liu LY, Tian MM, Feng GS, You WC, Li JY. Inflammatory cytokine gene polymorphisms increase the risk of atrophic gastritis and intestinal metaplasia. World J Gastroenterol. 2010;16:1788-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Crusius JB, Canzian F, Capellá G, Peña AS, Pera G, Sala N, Agudo A, Rico F, Del Giudice G, Palli D. Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann Oncol. 2008;19:1894-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Oliveira JG, Duarte MC, Silva AE. IL-1ra anti-inflammatory cytokine polymorphism is associated with risk of gastric cancer and chronic gastritis in a Brazilian population, but the TNF-β pro-inflammatory cytokine is not. Mol Biol Rep. 2012;39:7617-7625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | de Oliveira JG, Rossi AF, Nizato DM, Miyasaki K, Silva AE. Profiles of gene polymorphisms in cytokines and Toll-like receptors with higher risk for gastric cancer. Dig Dis Sci. 2013;58:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | de Oliveira JG, Silva AE. Polymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian population. World J Gastroenterol. 2012;18:1235-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis. 2011;32:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Kim KK, Kim HB. Protein interaction network related to Helicobacter pylori infection response. World J Gastroenterol. 2009;15:4518-4528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol. 2006;9:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 274] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Bauer B, Meyer TF. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcer. 2011;2011:1-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1393] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 27. | Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, Naumann M, Meyer TF. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Higashi H, Nakaya A, Tsutsumi R, Yokoyama K, Fujii Y, Ishikawa S, Higuchi M, Takahashi A, Kurashima Y, Teishikata Y. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205-17216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Li N, Han L, Chen J, Lin X, Chen H, She F. Proliferative and apoptotic effects of gastric epithelial cells induced by coccoid Helicobacter pylori. J Basic Microbiol. 2013;53:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Maeda S, Yoshida H, Mitsuno Y, Hirata Y, Ogura K, Shiratori Y, Omata M. Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Mol Pathol. 2002;55:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Truong BX, Mai VT, Tanaka H, Ly le T, Thong TM, Hai HH, Van Long D, Furumatsu K, Yoshida M, Kutsumi H. Diverse characteristics of the CagA gene of Helicobacter pylori strains collected from patients from southern vietnam with gastric cancer and peptic ulcer. J Clin Microbiol. 2009;47:4021-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Peek RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760-770. [PubMed] |
| 34. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 35. | Yang JJ, Cho LY, Ma SH, Ko KP, Shin A, Choi BY, Han DS, Song KS, Kim YS, Chang SH. Oncogenic CagA promotes gastric cancer risk via activating ERK signaling pathways: a nested case-control study. PLoS One. 2011;6:e21155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Karami N, Talebkhan Y, Saberi S, Esmaeili M, Oghalaie A, Abdirad A, Mostafavi E, Hosseini ME, Mohagheghi MA, Mohammadi M. Seroreactivity to Helicobacter pylori antigens as a risk indicator of gastric cancer. Asian Pac J Cancer Prev. 2013;14:1813-1817. [PubMed] |
| 37. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 459] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 38. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 880] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 40. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 41. | Peek RM. Helicobacter pylori strain-specific modulation of gastric mucosal cellular turnover: implications for carcinogenesis. J Gastroenterol. 2002;37 Suppl 13:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Allen LA, Schlesinger LS, Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J Exp Med. 2000;191:115-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 2003;5:25-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 45. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 46. | Prinz C, Schöniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, Classen M, Rösch T, Schepp W, Gerhard M. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61:1903-1909. [PubMed] |
| 47. | Rad R, Gerhard M, Lang R, Schöniger M, Rösch T, Schepp W, Becker I, Wagner H, Prinz C. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J Immunol. 2002;168:3033-3041. [PubMed] |
| 48. | Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778-12783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533-7538. [PubMed] |
| 50. | Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Imagawa S, Ito M, Yoshihara M, Eguchi H, Tanaka S, Chayama K. Helicobacter pylori dupA and gastric acid secretion are negatively associated with gastric cancer development. J Med Microbiol. 2010;59:1484-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Nguyen LT, Uchida T, Tsukamoto Y, Kuroda A, Okimoto T, Kodama M, Murakami K, Fujioka T, Moriyama M. Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin Microbiol Infect. 2010;16:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Peek RM, Thompson SA, Donahue JP, Tham KT, Atherton JC, Blaser MJ, Miller GG. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998;110:531-544. [PubMed] |
| 55. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 56. | Sgouros SN, Bergele C. Clinical outcome of patients with Helicobacter pylori infection: the bug, the host, or the environment? Postgrad Med J. 2006;82:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Ramis IB, Fonseca TL, de Moraes EP, Fernandes MS, Mendoza-Sassi R, Rodrigues O, Juliano CR, Scaini CJ, da Silva PE. Molecular Basis of pathogenicity in Helicobacter pylori clinical isolates. J Clin Microbiol. 2010;48:3776-3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Garcia GT, Aranda KR, Gonçalves ME, Cardoso SR, Iriya K, Silva NP, Scaletsky IC. High prevalence of clarithromycin resistance and cagA, vacA, iceA2, and babA2 genotypes of Helicobacter pylori in Brazilian children. J Clin Microbiol. 2010;48:4266-4268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Chen L, Yang Q, Kong WQ, Liu T, Liu M, Li X, Tang H. MicroRNA-181b targets cAMP responsive element binding protein 1 in gastric adenocarcinomas. IUBMB Life. 2012;64:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Baud J, Varon C, Chabas S, Chambonnier L, Darfeuille F, Staedel C. Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS One. 2013;8:e60315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Liu X, Ru J, Zhang J, Zhu LH, Liu M, Li X, Tang H. miR-23a targets interferon regulatory factor 1 and modulates cellular proliferation and paclitaxel-induced apoptosis in gastric adenocarcinoma cells. PLoS One. 2013;8:e64707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Kang JY, Lee JO. Structural biology of the Toll-like receptor family. Annu Rev Biochem. 2011;80:917-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 63. | Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335-376. [PubMed] |
| 64. | Matsuura M. Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity. Front Immunol. 2013;4:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 65. | Yokota S, Ohnishi T, Muroi M, Tanamoto K, Fujii N, Amano K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol Med Microbiol. 2007;51:140-148. [PubMed] |
| 66. | Kumar Pachathundikandi S, Brandt S, Madassery J, Backert S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS One. 2011;6:e19614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Bartchewsky W, Martini MR, Masiero M, Squassoni AC, Alvarez MC, Ladeira MS, Salvatore D, Trevisan M, Pedrazzoli J, Ribeiro ML. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol. 2009;44:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Isomoto H, Moss J, Hirayama T. Pleiotropic actions of Helicobacter pylori vacuolating cytotoxin, VacA. Tohoku J Exp Med. 2010;220:3-14. [PubMed] |
| 69. | Argent RH, Thomas RJ, Letley DP, Rittig MG, Hardie KR, Atherton JC. Functional association between the Helicobacter pylori virulence factors VacA and CagA. J Med Microbiol. 2008;57:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1961] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 71. | Ding SZ, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol. 2010;6:851-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci USA. 2004;101:7727-7732. [PubMed] |
| 73. | Luo JJ, Li CY, Liu S, Yu W, Tang SY, Cai HL, Zhang Y. Overexpression of Helicobacter pylori VacA N-terminal fragment induces proinflammatory cytokine expression and apoptosis in human monocytic cell line through activation of NF-κB. Can J Microbiol. 2013;59:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Telford JL, Covacci A, Rappuoli R, Chiara P. Immunobiology of Helicobacter pylori infection. Curr Opin Immunol. 1997;9:498-503. [PubMed] |
| 76. | Alvarez-Arellano L, Camorlinga-Ponce M, Maldonado-Bernal C, Torres J. Activation of human neutrophils with Helicobacter pylori and the role of Toll-like receptors 2 and 4 in the response. FEMS Immunol Med Microbiol. 2007;51:473-479. [PubMed] |
| 77. | Fehlings M, Drobbe L, Moos V, Renner Viveros P, Hagen J, Beigier-Bompadre M, Pang E, Belogolova E, Churin Y, Schneider T. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect Immun. 2012;80:2724-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 773] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 79. | Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288-308. [PubMed] |
| 80. | Rolig AS, Cech C, Ahler E, Carter JE, Ottemann KM. The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota. Infect Immun. 2013;81:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Weiss G, Forster S, Irving A, Tate M, Ferrero RL, Hertzog P, Frøkiær H, Kaparakis-Liaskos M. Helicobacter pylori VacA suppresses Lactobacillus acidophilus-induced interferon beta signaling in macrophages via alterations in the endocytic pathway. MBio. 2013;4:e00609-e00612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964-5971. [PubMed] |
| 83. | Galgani M, Busiello I, Censini S, Zappacosta S, Racioppi L, Zarrilli R. Helicobacter pylori induces apoptosis of human monocytes but not monocyte-derived dendritic cells: role of the cag pathogenicity island. Infect Immun. 2004;72:4480-4485. [PubMed] |
| 84. | Bornschein J, Kandulski A, Selgrad M, Malfertheiner P. From gastric inflammation to gastric cancer. Dig Dis. 2010;28:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Au PY, Yeh WC. Physiological roles and mechanisms of signaling by TRAF2 and TRAF5. Adv Exp Med Biol. 2007;597:32-47. [PubMed] |
| 86. | Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465-1477. [PubMed] |
| 87. | Sierra JC, Hobbs S, Chaturvedi R, Yan F, Wilson KT, Peek RM, Polk DB. Induction of COX-2 expression by Helicobacter pylori is mediated by activation of epidermal growth factor receptor in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;305:G196-G203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Nagy TA, Frey MR, Yan F, Israel DA, Polk DB, Peek RM. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis. 2009;199:641-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation. Cell Microbiol. 2009;11:70-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Jang J, Lee S, Jung Y, Song K, Fukumoto M, Gould VE, Lee I. Malgun (clear) cell change in Helicobacter pylori gastritis reflects epithelial genomic damage and repair. Am J Pathol. 2003;162:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 889] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 93. | Hocès de la Guardia A, Staedel C, Kaafarany I, Clément A, Roubaud Baudron C, Mégraud F, Lehours P. Inflammatory cytokine and microRNA responses of primary human dendritic cells cultured with Helicobacter pylori strains. Front Microbiol. 2013;4:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 95. | Yokota S, Okabayashi T, Rehli M, Fujii N, Amano K. Helicobacter pylori lipopolysaccharides upregulate toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infect Immun. 2010;78:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 96. | Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem. 2004;279:44513-44521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Hofman VJ, Moreilhon C, Brest PD, Lassalle S, Le Brigand K, Sicard D, Raymond J, Lamarque D, Hébuterne XA, Mari B. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori. Mod Pathol. 2007;20:974-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Yang ZM, Chen WW, Wang YF. Gene expression profiling in gastric mucosa from Helicobacter pylori-infected and uninfected patients undergoing chronic superficial gastritis. PLoS One. 2012;7:e33030. [PubMed] |
| 99. | Pimentel-Nunes P, Gonçalves N, Boal-Carvalho I, Afonso L, Lopes P, Roncon-Albuquerque R, Henrique R, Moreira-Dias L, Leite-Moreira AF, Dinis-Ribeiro M. Helicobacter pylori induces increased expression of Toll-like receptors and decreased Toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesis. Helicobacter. 2013;18:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | Garza-González E, Bocanegra-García V, Bosques-Padilla FJ, Flores-Gutiérrez JP, Moreno F, Perez-Perez GI. mRNA levels of TLR4 and TLR5 are independent of H pylori. World J Gastroenterol. 2008;14:5306-5310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 102. | Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 103. | Allison CC, Ferrand J, McLeod L, Hassan M, Kaparakis-Liaskos M, Grubman A, Bhathal PS, Dev A, Sievert W, Jenkins BJ. Nucleotide oligomerization domain 1 enhances IFN-γ signaling in gastric epithelial cells during Helicobacter pylori infection and exacerbates disease severity. J Immunol. 2013;190:3706-3715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Jackson CB, Judd LM, Menheniott TR, Kronborg I, Dow C, Yeomans ND, Boussioutas A, Robb L, Giraud AS. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Sohn SH, Lee YC. The genome-wide expression profile of gastric epithelial cells infected by naturally occurring cagA isogenic strains of Helicobacter pylori. Environ Toxicol Pharmacol. 2011;32:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz Coelho LG, Fock M, Fedail S, Cohen H. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 107. | Tsai CJ, Herrera-Goepfert R, Tibshirani RJ, Yang S, Mohar A, Guarner J, Parsonnet J. Changes of gene expression in gastric preneoplasia following Helicobacter pylori eradication therapy. Cancer Epidemiol Biomarkers Prev. 2006;15:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 109. | Yoon JH, Baik GH, Sohn KM, Kim DY, Kim YS, Suk KT, Kim JB, Kim DJ, Kim JB, Shin WG. Trends in the eradication rates of Helicobacter pylori infection for eleven years. World J Gastroenterol. 2012;18:6628-6634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Kim SY, Lee SW, Hyun JJ, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Choi JH. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple “concomitant” therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013;47:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Kindlund B, Sjöling A, Hansson M, Edebo A, Hansson LE, Sjövall H, Svennerholm AM, Lundin BS. FOXP3-expressing CD4(+) T-cell numbers increase in areas of duodenal gastric metaplasia and are associated to CD4(+) T-cell aggregates in the duodenum of Helicobacter pylori-infected duodenal ulcer patients. Helicobacter. 2009;14:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Bauer B, Wex T, Kuester D, Meyer T, Malfertheiner P. Differential expression of human beta defensin 2 and 3 in gastric mucosa of Helicobacter pylori-infected individuals. Helicobacter. 2013;18:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M. A uniform system for microRNA annotation. RNA. 2003;9:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1332] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 114. | Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2287] [Cited by in RCA: 2292] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 115. | Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877-6888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1033] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 116. | Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2720] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 117. | Beilharz TH, Humphreys DT, Clancy JL, Thermann R, Martin DI, Hentze MW, Preiss T. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS One. 2009;4:e6783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 118. | Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8672] [Cited by in RCA: 8877] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 119. | Zhou P, Xu W, Peng X, Luo Z, Xing Q, Chen X, Hou C, Liang W, Zhou J, Wu X. Large-scale screens of miRNA-mRNA interactions unveiled that the 3’UTR of a gene is targeted by multiple miRNAs. PLoS One. 2013;8:e68204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 120. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7370] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 121. | Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 122. | Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 1572] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 123. | Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 124. | Kawasaki H, Taira K. Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature. 2003;423:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 125. | Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, Davis K, Weisel RD, Li RK. Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol. 2014;66:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 126. | Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425-436. [PubMed] [DOI] [Full Text] |
| 127. | Ribeiro-dos-Santos Â, Khayat AS, Silva A, Alencar DO, Lobato J, Luz L, Pinheiro DG, Varuzza L, Assumpção M, Assumpção P. Ultra-deep sequencing reveals the microRNA expression pattern of the human stomach. PLoS One. 2010;5:e13205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Isomoto H, Matsushima K, Inoue N, Hayashi T, Nakayama T, Kunizaki M, Hidaka S, Nakayama M, Hisatsune J, Nakashima M. Interweaving microRNAs and proinflammatory cytokines in gastric mucosa with reference to H. pylori infection. J Clin Immunol. 2012;32:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One. 2013;8:e56709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 130. | Shiotani A, Uedo N, Iishi H, Murao T, Kanzaki T, Kimura Y, Kamada T, Kusunoki H, Inoue K, Haruma K. H. pylori eradication did not improve dysregulation of specific oncogenic miRNAs in intestinal metaplastic glands. J Gastroenterol. 2012;47:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Liu Z, Xiao B, Tang B, Li B, Li N, Zhu E, Guo G, Gu J, Zhuang Y, Liu X. Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect. 2010;12:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 132. | Liu Z, Wang D, Hu Y, Zhou G, Zhu C, Yu Q, Chi Y, Cao Y, Jia C, Zou Q. MicroRNA-146a negatively regulates PTGS2 expression induced by Helicobacter pylori in human gastric epithelial cells. J Gastroenterol. 2013;48:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 133. | Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 134. | Li N, Tang B, Zhu ED, Li BS, Zhuang Y, Yu S, Lu DS, Zou QM, Xiao B, Mao XH. Increased miR-222 in H. pylori-associated gastric cancer correlated with tumor progression by promoting cancer cell proliferation and targeting RECK. FEBS Lett. 2012;586:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 135. | Matsushima K, Isomoto H, Inoue N, Nakayama T, Hayashi T, Nakayama M, Nakao K, Hirayama T, Kohno S. MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int J Cancer. 2011;128:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 136. | Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS One. 2012;7:e42971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 137. | Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W. FoxM1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 138. | Belair C, Baud J, Chabas S, Sharma CM, Vogel J, Staedel C, Darfeuille F. Helicobacter pylori interferes with an embryonic stem cell micro RNA cluster to block cell cycle progression. Silence. 2011;2:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 139. | Bou Kheir T, Futoma-Kazmierczak E, Jacobsen A, Krogh A, Bardram L, Hother C, Grønbæk K, Federspiel B, Lund AH, Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer. 2011;10:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 140. | Zhu Y, Jiang Q, Lou X, Ji X, Wen Z, Wu J, Tao H, Jiang T, He W, Wang C. MicroRNAs up-regulated by CagA of Helicobacter pylori induce intestinal metaplasia of gastric epithelial cells. PLoS One. 2012;7:e35147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 141. | Sayi A, Kohler E, Hitzler I, Arnold I, Schwendener R, Rehrauer H, Müller A. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182:7085-7101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 142. | Olivieri F, Spazzafumo L, Santini G, Lazzarini R, Albertini MC, Rippo MR, Galeazzi R, Abbatecola AM, Marcheselli F, Monti D. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech Ageing Dev. 2012;133:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 143. | Lo SS, Hung PS, Chen JH, Tu HF, Fang WL, Chen CY, Chen WT, Gong NR, Wu CW. Overexpression of miR-370 and downregulation of its novel target TGFβ-RII contribute to the progression of gastric carcinoma. Oncogene. 2012;31:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D, Luo R, Zhong Y, Chen HC, Fang LR. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-kappaB. J Biol Chem. 2011;286:21401-21412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |