Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1361
Revised: October 29, 2013
Accepted: November 12, 2013
Published online: February 7, 2014
Processing time: 150 Days and 9.9 Hours
Nodular fasciitis is a benign proliferative lesion composed of fibroblast-like cells that affects various sites in the body. We describe a patient with nodular fasciitis in the mesentery, encountered during laparotomy for the treatment of ascending colon cancer. The nodular fasciitis in our patient resembled peritoneal dissemination of malignancy on macroscopic observation. Because the treatment options change with concomitant peritoneal dissemination of gastrointestinal tract malignancy, recognition of this rare condition and preparation for unexpected nodular lesions are crucial.
Core tip: Nodular fasciitis is a rare benign lesion composed of fibroblast-like cells that occurs at various sites in the body. In our patient, firm nodular lesions were incidentally identified in the mesentery during surgical treatment for colon cancer. Although the lesions were initially diagnosed as peritoneal carcinomatosis, the pathological examination revealed nodular fasciitis. This case highlights the need for all clinicians to know about this rare condition and to prepare for unexpected nodular lesions during surgical treatment of gastrointestinal tract malignancy.
- Citation: Shiga M, Okamoto K, Matsumoto M, Maeda H, Dabanaka K, Namikawa T, Uemura S, Munekage M, Kobayashi M, Hanazaki K. Nodular fasciitis in the mesentery, a differential diagnosis of peritoneal carcinomatosis. World J Gastroenterol 2014; 20(5): 1361-1364
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1361
Nodular fasciitis is a rare benign lesion comprising a proliferation of spindle, plump, or stellate fibroblast-like cells embedded in myxomatous stroma[1,2]. Nodular fasciitis involves subcutaneous fat, fascia, and less commonly, areas without true fascia[3,4]. Thus, nodular fasciitis can occur in any part of the body, although the upper extremities or trunk are the sites of predilection[1]. Nodular fasciitis is a reactive fibroblastic growth; however, the majority of patients lack an apparent (recognized) history of injury[1], and its etiology remains unclear.
The classical course of nodular fasciitis varies from stable size to rapid growth[1,2]. Surgical resection is often the treatment for growing nodules and those causing symptoms (such as tenderness and pain), while careful observation of asymptomatic nodules and those that are stable in size is recommended because of possible spontaneous regression[5]. Diagnosis of nodular fasciitis relies largely on the clinical features and careful morphological evaluation of the specimen, including hematoxylin and eosin (HE) staining[6]. Efforts are being made to establish the typical pattern of immunohistochemical staining of nodular fasciitis to help distinguish it from other soft tissue tumors.
Nodular fasciitis is well known to dermatologists and pathologists due to its preferential occurrence in subcutaneous tissue and importance in the differential diagnosis of soft-tissue tumors. Nodular fasciitis is rarely encountered in other fields of clinical practice. In this case report, we describe a patient with nodular fasciitis in the mesentery that mimicked peritoneal dissemination of colon cancer macroscopically.
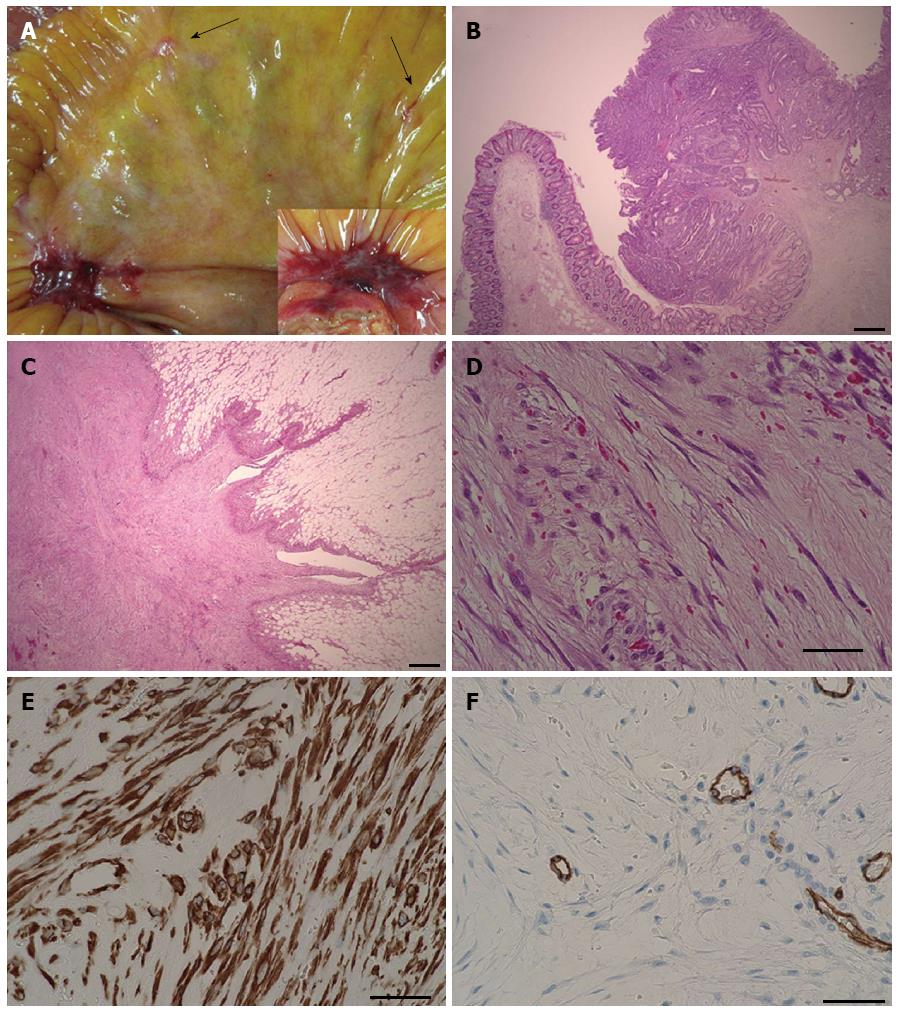
An 88-year-old male patient presented with intermittent gross hematochezia. He had a history of appendectomy for appendicitis about 40 years ago. Preoperative colonoscopy revealed a tumor mass with mild distortion of the intestinal lumen, suggesting tumor infiltration deeper than the submucosal layer. The biopsy confirmed tubular adenocarcinoma of the ascending colon. Computed tomography (CT) and 18F-fluorodeoxy glucose positron emission tomography showed no sign of metastatic lesion or peritoneal dissemination. The preoperative diagnosis was colon cancer, which was staged as IIA (T3, N0, M0) according to the Union for International Cancer Control (UICC) classification[7]. Thus, the patient underwent laparoscope-assisted ileocecal resection with regional lymphadenectomy. After resection of blood vessels and mobilization of the ascending colon, the lesion was removed from the abdominal cavity through a 7-cm incision. A flat and slightly reddish hard nodule about 2 cm in diameter was observed on the mesentery of the terminal ileum (Figure 1A), and a few smaller, whitish nodules were found around it. Peritoneal dissemination of colon cancer was suspected, although intraoperative pathological examination was not considered to change the surgical method because the primary site of the colon cancer had been removed completely. Two large nodules that were suspected of being dissemination of the colon cancer were resected for pathological diagnosis and symptom prophylaxis. Functional end-to-end anastomosis was performed between the terminal ileum and ascending colon.
The postoperative course was uneventful, and the pathological examination revealed sub-mucosal infiltration of colon cancer without lymph node metastasis (Figure 1B) (T1, N0, M0, stage I of the UICC classification[7]). The resected nodule of the mesentery was composed of spindle-like cells in myxomatous stroma without proliferation of adenocarcinoma cells (Figure 1C and D). The nodule was located adjacent to the true muscle layer and there was no encapsulation. The cells of the nodule were positive for vimentin, α-smooth muscle actin (α-SMA), calponin, and negative for podoplanin (D2-40), thrombomodulin, cluster of differentiation molecule 34 (CD34), β-catenin, anaplase lymphoma kinase, S100 protein, cluster of differentiation molecule 56 (CD56), and stem cell factor receptor (c-kit) according to immunohistochemistry staining. The nodule was largely negative for AE1/AE3 and carletinin. A diagnosis of nodular fasciitis was made based on observation of the specimen with HE staining and the immunohistochemical results.
This case of nodular fasciitis in the mesentery mimicked peritoneal dissemination of colon cancer, and to the best of our knowledge, is the first such case report. A diagnosis of nodular fasciitis should thus be considered when unexpected nodular lesions suggesting peritoneal dissemination of gastroenterological malignancy are encountered, and such lesions should be treated carefully.
The pathological diagnosis of nodular fasciitis is difficult because of the rarity of the condition, the resemblance of its pathological features to other soft tissue tumors, and the lack of typical diagnostic symptoms or pattern of immunohistochemical staining. In this case, the fibroblast-like cells were arranged haphazardly in the myxomatous stroma, giving a “tissue-culture appearance”[8]. Although the fibroblast-like cells varied in terms of nucleus size and amount of cytoplasm, they differed from the cells found in sarcomas, which typically have higher cellularity, bizarre nuclei, and less intercellular material; therefore, the nodule was considered a reactive proliferative lesion rather than a true tumor. The nodule was positive for vimentin according to immunohistochemical staining, suggesting that the tumor was derived from soft tissue. The majority of nodular fasciitis cases are negative for cytokeratin, with few cytokeratin-positive cases reported[9]. A few spindle cells of nodular fasciitis in our patient were positive for cytokeratin and calretinin, indicating a possible relationship with the mesothelium. Positive staining of α-SMA and calponin suggested myofibroblastic differentiation and less possibility of a sarcoma[6]. The nodule was negative for CD34, CD56, S100 protein, and β-catenin, reducing the possibility of another differential diagnosis (Table 1). A previous report of seven patients demonstrated that approximately half of leiomyosarcoma lesions were negative for cluster of differentiation molecule 10 (CD10) staining[10], while all nodular fasciitis cases were positive for CD10[6]. In our patient, none of the cells expressed CD10, suggesting the need to further evaluate the utility of this marker. Comprehensive judgment based on examination of multiple related markers and close morphological observation is indispensable for the diagnosis of nodular fasciitis.
| Nodular fasciitis | Implication | |
| α-SMA | + | Myofibroblastic differentiation |
| Vimentin | + | Negative in epithelial tumors |
| Calponin | + | Often negative in myofibroblastic sarcomas |
| CD10 | + | Negative in 50% of leiomyosarcomas |
| DGP9.5 | + | Negative in dermatofibrosarcoma protuberans |
| AE1/AE3 | - | Positive in epithelial tumors |
| CD56 | - | Positive in leiomyomas, nerve sheath tumors, leiomyosarcomas |
| S100 protein | - | Positive in shwannomas |
| Desmin | - | Positive in various sarcomas |
| CD34 | - | Positive in solitary fibrous tumors |
| β-catenin | - | Positive in desmoid fibromatosis |
As many as 10% of patients with colorectal cancer develop peritoneal carcinomatosis during the course of the disease, and half of these are diagnosed at the initial evaluation[11]. While about 20% of T4 tumors are accompanied by peritoneal dissemination, less than 1% of T2 tumors have synchronous peritoneal dissemination. This suggests that less infiltrating tumors have a small, but definite, possibility of peritoneal dissemination, probably occurring via a hematological or lymphatic route. While preoperative CT or other imaging modalities may help assess primary tumors, detecting small peritoneal nodules is still difficult, and laparotomy remains an important method for identifying peritoneal dissemination. Ideally, the intraoperative pathological examination for unexpected peritoneal nodules should be performed to inform the choice of surgical methods. When intraoperative pathological diagnosis is not available, judgment regarding surgical options (ranging from observation and biopsy to aggressive surgical resection) must be based on multiple factors including the patient’s condition, tumor spread, and the surgeon’s skill. When resection seems inappropriate, biopsy of the nodule for subsequent pathological diagnosis should be performed without exception.
In conclusion, nodular fasciitis in the mesentery can present as unexpected nodules of the peritoneum that resemble peritoneal dissemination of gastrointestinal tract malignancy. It is important to recognize this rare condition because the existence of peritoneal dissemination influences the choice of treatment for gastrointestinal tract malignancy.
The patient had no symptoms related to nodular fasciitis.
Asymptomatic firm nodular lesions were incidentally identified in the mesentery during surgical treatment for colon cancer.
Because the peritoneal nodular fasciitis resembled peritoneal carcinomatosis on macroscopic observation, pathological examination was inevitable.
The lesions were not identified on the preoperative computed tomography (CT) or 18F-fluorodeoxy glucose positron emission tomography CT.
Hematoxylin and eosin staining revealed benign proliferation of spindle-like cells in myxomatous stroma, and immunohistochemistry staining suggested the diagnosis of nodular fasciitis.
The nodular fasciitis was surgically resected.
Nodular fasciitis could occur at any site in the body. However, the case of nodular fasciitis in the abdominal cavity is quite rare.
The nodular fasciitis in our patient closely resembled peritoneal dissemination of colon cancer. Because the treatment options could change with concomitant peritoneal dissemination of gastrointestinal tract malignancy, recognition of this rare condition and preparation for unexpected nodular lesions are crucial.
Although nodular fasciitis shows a reactive fibroblastic growth, the majority of patients, including the present patient, lack an apparent history of injury and etiology of nodular fasciitis remains unclear. The further accumulation of clinical experience is necessary to solve this clinical question.
P- Reviewers: Chen YJ, Pan WS, Kim YJ, Rodrigo L S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Price EB, Silliphant WM, Shuman R. Nodular fasciitis: a clinicopathologic analysis of 65 cases. Am J Clin Pathol. 1961;35:122-136. [PubMed] |
| 2. | Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 147] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Allen PW. Nodular fasciitis. Pathology. 1972;4:9-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 116] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Terai M, Oka M, Kunisada M, Kawakami F, Nishigori C. Intradermal nodular fasciitis. Eur J Dermatol. 2012;22:285-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Yanagisawa A, Okada H. Nodular fasciitis with degeneration and regression. J Craniofac Surg. 2008;19:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Morgen EK, Carter P, Weinreb I, Al-Habeeb A, Ghazarian DM. Immunohistochemistry in nodular fasciitis of the head and neck. Pathology. 2013;45:432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Leslie HS, Mary KG, Christian W. TNM classification of Malignant Tumors. 7th ed. New York: Wiley-Liss 2009; 100-105. |
| 8. | Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Weidner N. The Difficult Diagnosis in Surgical pathology. Philadelphia: Saunders 1996; 732-737. |
| 10. | Deniz K, Çoban G, Okten T. Anti-CD10 (56C6) expression in soft tissue sarcomas. Pathol Res Pract. 2012;208:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kerscher AG, Chua TC, Gasser M, Maeder U, Kunzmann V, Isbert C, Germer CT, Pelz JO. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer. 2013;108:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |