Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1332
Revised: October 10, 2013
Accepted: November 12, 2013
Published online: February 7, 2014
Processing time: 199 Days and 22.1 Hours
AIM: To develop and initially test a potential fecal protein biochip for the screening of colorectal cancer (CRC).
METHODS: Fecal protein from 20 colorectal cancer patients and 20 healthy controls were extracted from all of the fecal samples and screened for proteomic differences using a Biotin label-based protein array. Candidate proteins were then verified by ELISA. Finally, we will select out the significant protein and a seven-target multiplex fecal protein biochip was generated and tested for 20 fecal samples to determine the effectiveness of the biochip on identifying CRC. And the value of the protein biochip would be discussed.
RESULTS: After tested by protein biochip of the fecal protein from 20 colorectal cancer patients and 20 healthy controls and levels of calprotectin, M2-pyruvatekinase, angiopoietin-2, fibroblast growth factor-23 (FGF-23), proteins of the matrix metalloproteinase, thrombopoietin (TPO) and interleukin-13 (IL-13) were significantly different between CRC and healthy controls. The sensitivity of all the seven proteins combined was 0.7, specificity was 0.4, and area under the receiver operating characteristics was 0.729. The most promising combinations of test proteins were FGF-23, TPO, and IL-13, reaching a sensitivity of 0.7 and a specificity of 0.7. The combination of FGF-23 and TPO scored highest with sensitivity of 0.7 and specificity of 0.8. Its mean that the combination of FGF-23 and TPO has the highest value for the diagnosis of CRC in our study.
CONCLUSION: A protein biochip composed of proteins found to be elevated in the feces of colorectal cancer patients has great potential as a noninvasive diagnostic for colorectal cancer. The addition of new protein biomarkers and technologies, as they are discovered, is an excellent avenue of future research.
- Citation: Wang HP, Wang YY, Pan J, Cen R, Cai YK. Evaluation of specific fecal protein biochips for the diagnosis of colorectal cancer. World J Gastroenterol 2014; 20(5): 1332-1339
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1332
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide. About 1 million CRC cases occur each year, of which 500000 people will die[1]. There are multiple stages along the pathological progression of CRC. The progression occurs over 7-10 years from normal mucosa through adenoma to cancer[2]. Unfortunately, about 30%-50% of patients have metastases at the time of diagnosis; the 5-year survival rate is less than 10%[1]. In contrast, the 5-year survival of patients who are in Dukes A with surgical resection is more than 90%[3]. Therefore, it is imperative to detect CRC at an early stage in order to reduce mortality and morbidity.
There are many methods to detect CRC. Non-invasive methods include fecal occult blood test (FOBT), fecal immunochemical test (FIT), computer tomography (CT) colonoscopy, magnetic resonance imaging (MRI) colonoscopy, and barium enema. More invasive methods include flexible sigmoidoscopy and colonoscopy. FOBT, which is low-cost, simple, and painless, is the most widely used method. However, it has a high incidence of false positive and false negative results, as well as low specificity. The flexible sigmoidoscopy procedure can only detect cancer within the sigmoid colon and rectum[4]. Colonoscopy is considered the gold standard in the diagnosis of CRC[5], but its application is limited because of its high cost, invasiveness, patient discomfort during preparation, and complications, such as hemorrhage, intestinal perforation, and myocardial infarction.
As the normal biological function of colorectal epithelial cells becomes damaged, changes in oncogenes and tumor-suppressor genes result in mutations at the DNA, RNA, and protein levels[5]. Therefore, it may possible to develop a new method that detects these molecular changes in blood or feces; carcinoembryonic antigen (CEA) is an example of the earliest application of this strategy[6]. A detection method that uses fecal material has the advantages of being simple, flexible, and non-invasive, with no need for bowel preparation, and may reflect pathologies involving the whole gut. Molecules found in the feces include DNA, RNA, and protein. Detection of mutations in the genes encoding KRAS, APC, BRAF, and TP53 have been proposed recently, but the sensitivity is too low[5] and it is a high cost, complex operation. Proteins, in contrast, are more suitable for such a large-scale screen, because of their stability, high sensitivity, and operation simplicity[7]. Therefore, fecal detection is a very promising method for the detection of biomarkers in the feces of CRC patients.
In this study, we collected fecal specimens from CRC patients and healthy controls, and used protein biochip methods to measure several significant proteins chosen from the current literature. Finally, we verified the value of them as combined CRC biomarkers in the diagnosis of CRC by protein biochip.
A total of 20 CRC patients (10 men and 10 women) and 20 cases of healthy controls (14 men and 6 women) were collected. The average patient age was 67.5 years (range: 46-88 years). Within the CRC group, diagnoses were all confirmed as CRC by pathology from a colonoscopy biopsy. For both groups, patients were excluded if they had any one of the following: long-term history of irritable bowel disease (IBD); ulcerative colitis (UC); intestinal polyp; chronic constipation; diarrhea; hereditary intestinal polyposis syndrome and intestinal surgery; any diseases of the respiratory, urinary, cardiovascular, and nervous systems; or other diseases of the digestive system excluding the gastrointestinal tract. Patients were instructed to eat a normal protein diet for 1-2 wk prior to electronic colonoscopy. Information about the demographics of all test subjects is detailed in Table 1.
| Colorectal cancer (n = 20) | Healthy controls (n = 20) | |
| Gender | ||
| Male | 10 (50) | 14 (70) |
| Female | 10 (50) | 6 (30) |
| Age group (yr) | ||
| 46-55 | 5 (25) | 5 (25) |
| 56-65 | 3 (15) | 4 (20) |
| 66-75 | 6 (30) | 7 (35) |
| ≥ 75 | 6 (30) | 4 (20) |
| Location of tumor | ||
| Ascending colon | 6 (30) | - |
| Transverse colon | 1 (5) | - |
| Descending colon | 6 (30) | - |
| Rectum | 7 (35) | - |
| Tumor stage (TNM) | ||
| I | 2 (10) | - |
| II | 7 (35) | - |
| III | 9 (45) | - |
| IV | 2 (10) | - |
All stool specimens were collected 1-2 wk before surgery. Avoiding urine or water pollution, samples were collected, cut to 8.0 cm3 in size (2.0 cm × 2.0 cm × 2.0 cm), and immediately preserved in -80 °C for at least 30 min. For each sample, 0.1 g feces weighed precisely, added to 0.4 mL RIPA lysis buffer, vortexed for 1 min alternating with a 5-min ice bath until no visible feces granules remained, and then centrifuged. The supernatant fraction was collected and put on ice for about 60 min, centrifuged for 30 min at 10000 rpm at 4 °C, and then the supernatant fraction was collected again. The final supernatant was stored at -80 °C. The samples of CRC were labeled as C1-C20; the healthy controls were labeled as N1-N20.
The protein concentration was measured first by spectrophotometry using a NanoDrop ND-2000 (Thermo Fisher, United States), and then by bicinchoninic acid (BCA) using a standard protocol. The protein concentration was tested by colorimetric determination under 562 nm and compared to a standard curve of bovine serum albumin.
Fecal samples were analyzed by RayBio Biotin Label-based Human Antibody Array1 (507 Human Proteins), using Raybiotech kits. Samples were dialyzed, biotin labeled, dried, blocked, and then incubated in Antibody Array. Raw data were collected by fluorescence detection (GenePix 4000 b, Axon Instruments, United States), using the GenePix Pro 6.0 software (Axon Instruments, United States).
After screening by protein biochip and literature review, we chose seven proteins for enzyme linked immunosorbent assay (ELISA) analysis. For each protein, 10 μL of sample were tested by ELISA using the manufacture’s protocol (Labsystems Multiskan MS, Finland). A standard curve was drawn with the concentration of the standard as abscissa, the A value as ordinate.
This study was approved by the ethics committee of The 5th People’s Hospital of Shanghai, Fudan University. All the patients in the study signed consent forms before participating.
The statistical differences concentration of selected proteins between CRC and healthy controls were determined using the t test. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software.
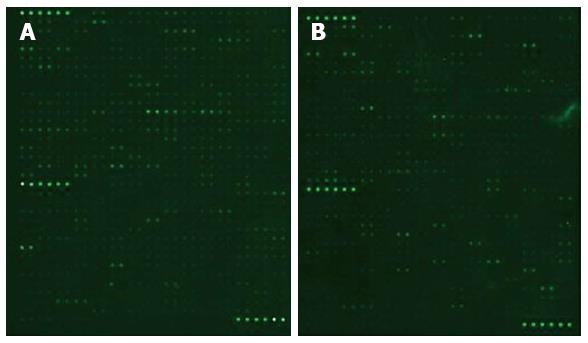
To determine the proteomic differences in fecal matter of CRC and healthy control samples, we screened fecal samples using a biotin label-based human antibody array 1. The results are shown as scanned images (Figure 1). The raw data were read using GenePix Pro 6.0 software, and the normalized results are shown in Table 2. Normalization was calculated by the size ratio of CRC group (C)/healthy controls (N). Initially, we found five proteins with maximum differences between the cohorts: angiogenin 2 (Ang-2), matrix metallopeptidase 10 (MMP-10), fibroblast growth factor-23 (FGF-23), thrombopoietin (TPO), and interleukin 13 (IL-13). We decided to use these five candidate proteins for further analysis.
| Name | C-Nor | N-Nor | C/N |
| Angiopoietin-2 | 263.60 | 6.89 | 38.27 |
| MMP-10 | 223.58 | 10.14 | 22.05 |
| FGF-23 | 345.69 | 22.60 | 15.3 |
| Thrombopoietin | 229.29 | 18.03 | 12.72 |
| IL-13 | 243.38 | 21.05 | 11.56 |
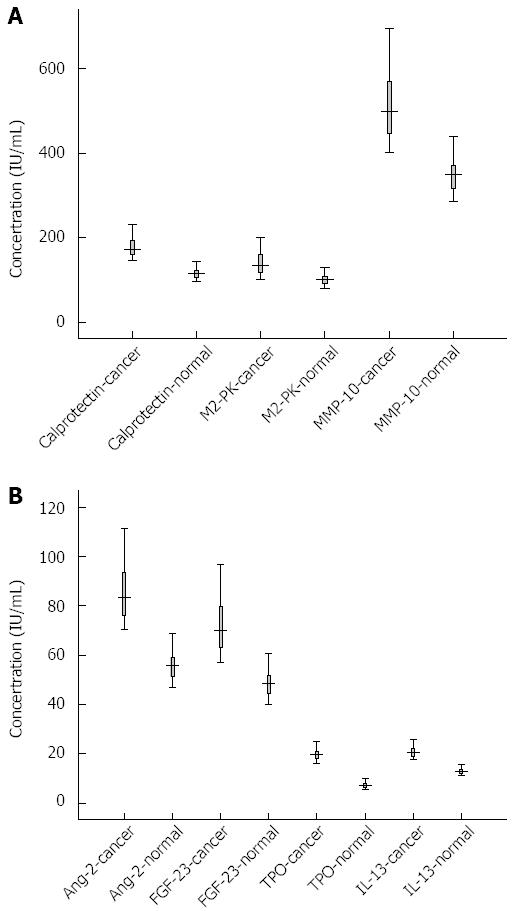
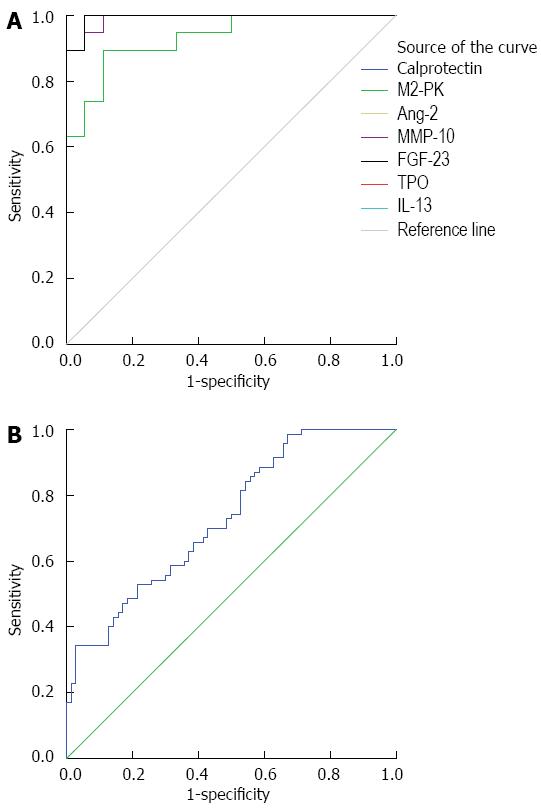
Through a literature search, we uncovered two additional candidate proteins for analysis: calprotectin and pyruvate kinase isoenzyme type M2 (M2-PK). We measured all seven proteins in the fecal samples using ELISA. During sample processing, we lost C5 from the CRC group and N9 and N17 from the control group. Thus, the final sample sizes were: CRC, n = 19; Healthy, n = 18. The concentrations of the seven proteins in CRC group and healthy controls are shown in Figure 2A and B. After receiver operating characteristic curve (ROC) analysis, the cut off and area under the curve (AUC), sensitivity and specificity were calculated, as shown in Figure 3A and Table 3. The levels of calprotectin, M2-PK, Ang-2, FGF-23, MMP-10, TPO, and IL-13 were significantly higher in CRC cases compared with the controls.
| Test result variable(s) | Area | SE | Asymptotic sig | Asymptotic 95%CI | |
| Lower bound | Upper bound | ||||
| Calprotectin | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 |
| M2-PK | 0.933 | 0.039 | 0.000 | 0.856 | 1.009 |
| Ang-2 | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 |
| MMP-10 | 0.991 | 0.010 | 0.000 | 0.972 | 1.011 |
| FGF-23 | 0.994 | 0.008 | 0.000 | 0.979 | 1.010 |
| TPO | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 |
| IL-13 | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 |
Calprotectin, sensitivity 1, specificity 1, average concentration of group C was 179.12 IU/mL, N was 116.08 IU/mL; M2-PK, sensitivity 0.895, specificity 0.889, average concentration of group C was 141.15 IU/mL, N was 100.19 IU/mL; Ang-2, sensitivity 1, specificity 1, average concentration of group C was 86.46 IU/mL, N was 55.85 IU/mL; MMP-10, sensitivity 0.895, specificity 1, average concentration of group C was 518.37 IU/mL, N was 348.61 IU/mL; FGF-23, sensitivity 1, specificity 0.944, average concentration of group C was 72.84 IU/mL, N was 48.55 IU/mL; TPO, sensitivity 1, specificity 1, average concentration of group C was 19.76 IU/mL, N was 7.39 IU/mL; IL-13, sensitivity 1, specificity 1, average concentration of group C was 21.01 IU/mL, N was 12.99 IU/mL. After T-test and P values are calculated by SPSS16.0, P < 0.05, Table 4.
| Name | SE | mean | 95%CI | Cutoff value | P value |
| Calprotectin-C | 5.725 | 179.12 | 167.09-191.14 | 144.39 | 0.00 |
| Calprotectin-N | 2.906 | 116.08 | 109.95-122.21 | ||
| M2-PK-C | 1.516 | 141.15 | 137.97-144.34 | 114.49 | 0.00 |
| M2-PK-N | 0.725 | 100.19 | 98.66-101.72 | ||
| Ang-2-C | 0.627 | 86.46 | 85.14-87.78 | 69.62 | 0.00 |
| Ang-2-N | 0.324 | 55.85 | 55.16-56.53 | ||
| MMP-10-C | 4.505 | 518.37 | 508.91-527.84 | 440.41 | 0.00 |
| MMP-10-N | 2.267 | 348.61 | 343.82-353.89 | ||
| FGF-23-C | 0.607 | 72.84 | 71.56-74.11 | 56.33 | 0.00 |
| FGF-23-N | 0.304 | 48.55 | 47.91-49.19 | ||
| TPO-C | 0.118 | 19.76 | 19.51-20.01 | 13.08 | 0.00 |
| TPO-N | 0.069 | 7.39 | 7.24-7.53 | ||
| IL-13-C | 0.122 | 21.01 | 20.76-21.27 | 16.76 | 0.00 |
| IL-13-N | 0.064 | 12.99 | 12.85-13.12 |
Finally, a protein biochip was made composed of the seven proteins. We selected 20 samples of 10 cases of CRC and 10 cases of healthy controls for retesting. The results are shown in Figure 3B. For the seven protein combined, the AUC = 0.729, the sensitivity was 0.7, and the specificity was 0.4. We also examined the sensitivity and specificity for select subsets of the seven proteins. For a subset containing FGF-23, IL-13, and TPO, the sensitivity was 0.7 and the specificity was 0.7. For a subset containing FGF-23 and TPO only, the sensitivity was 0.7 and the specificity was 0.8, making this the best combination for CRC screening. We also analyzed the correlation of the seven proteins with age of patients, tumor location (ascending colon, transverse colon, descending colon, or rectum), and tumor stage (Dukes stage or TNM stage) by t test; there were no statically significant correlations. Thus, the abundance of these seven proteins is independent of patient age, tumor location, and tumor stage.
There is an ongoing search for new methods and markers for the diagnosis of CRC. Many studies of attempted proteomics on CRC samples, but the results lack consistency for a number of possible reasons. First, there is always heterogeneity and variation in the experimental material, such as a varied patient population and different genetic and environmental factors, which can lead to different results. Second, the use of different research methods, such as 2D-polyacrylamide gel electrophoresis and mass spectrometry, hinders the ability to properly compare results. Another factor is the lack of detailed experimental methods from independent researchers, thus direct comparisons cannot be made.
In contrast, there are several advantages to using stool specimens and protein biochip technology. First of all, the use of feces is noninvasive, resulting in a good patient compliance rate. Second, comparing abundance of a small set of proteins has shown to have relatively high sensitivity and specificity. This approach overcomes the disadvantage of diagnosing cancers using a single molecular marker. Third, the method is simple, fast and convenient. Fourth, the amount of fecal sample does not limit the analysis[8,9]. Finally, it shows that the abundance of tumor-specific proteins in feces is significantly high compared with blood; this sample type and can reflect the full length of the colorectum[1].
There are several types of proteins found in feces, including products of digestion, and cell secretion, shedding from the lumen, and bacteria. Protein from food has no specificity in stool specimens; however, in this study patients were requested to limit their intake of high-protein foods before sample collection, especially animal protein. In feces, bacteria constitute nearly half of the total sample weight, excluding water. By centrifuging the samples, these proteins were cleared completely[1]. Proteins that come from lumen can be roughly classified into three categories: leakage, secretion, and exfoliation[10]. Due to dysfunction of the blood and lymphatic vessels of tumor, certain molecules leaked into the feces. However, this is not a consistent or continuous process, and it can also occur with tumor lesions, thus it lacks sensitivity and specificity. The most typical example in this case is hemoglobin[11]. Many other molecules are secreted by the epithelial cells of the colorectal lumen. Cancer cells can secrete unusual glycosylated proteins, such as mucin[12,13], CEA, and mini chromosome maintenance proteins (MCM2). These proteins can be detected by specific antibodies, but the intestinal bacteria secrete proteases and other glycosidic enzymes that can interfere with this process. Still other exfoliated molecules are released into the lumen during apoptosis of epithelial cells; they often come directly from the tumor itself. As a result, they have a high specificity for the presence of tumors. The renewal speed of tumor tissue is usually higher than that of normal tissue, therefore these molecules are even more likely to be found. Tumor sources of protein or peptide sequence are very stable in the feces, such as M2-PK, DAF, CEA, etc., and have high specificity[10].
In this study, calprotectin, M2-PK, Ang-2, MMP-10, FGF-23, TPO and IL-13 were identified and verified as statistically significant tumor markers. Every molecule was analyzed individually. The sensitivity and specificity of calprotectin, Ang-2, TPO, and IL-13 are all highest, with a value of 1. This result may have been because of the small sample size. Calprotectin, a 36.5 kDa zinc/calcium-binding protein, is made up of three polypeptide chains. calprotectin mainly comes from neutrophils[14]. CRC is tightly linked to acute inflammation reactions[15], therefore it was reasonable to test the levels of calprotectin as a marker of CRC[16]. Calprotectin is stable in feces, and small samples can be measured reliably. Tibble et al[16] found that the sensitivity and specificity of calprotectin are 79% and 72%, respectively. In the same study, concentration of FOBT was found to have no correlation with the tumor location or Dukes staging; the same results were found in this experiment.
M2-PK, an important enzyme for the metabolism of tumor cells, is a dimer of pyruvate kinase isoenzyme and target of many oncogenes. Hardt et al[17,18] and Ayling et al[19] reported that M2-PK can be detected in feces of CRC patients by ELISA. Hardt et al[18] found that M2-PK concentrations in feces were significantly higher in the 60 cases of CRC than the control group by ELISA; the sensitivity was 73%, specificity was 78%, and the concentration of M2-PK is closely related to TNM and Dukes staging. Because of the high sensitivity and specificity of M2-PK, it has high potential as a biomarker of CRC.
Ang-2, the Tie-2 receptor ligand of tyrosine specificity in endothelial cells, can disrupt cellular stability to induce angiogenesis[20]. Ahmad et al[21] detected the expression of Ang-1 and Ang-2 by RT-PCR and double staining fluorescence immunoassay in CRC cell lines, as well as a CRC resection specimen and normal mucosa. The results showed that Ang-2 was not only expressed in normal cells, it was also highly expressed in colon cancer cells. These results indicate that Ang-2 may be the promoting factor in tumor angiogenesis.
FGF-23, which is 32 kDa, it is secreted by bone cells or osteoblasts. Its target organ is the kidney, and its main function is to regulate phosphate and vitamin D metabolism. Leaf et al[22] found the levels of FGF-23 were higher than normal in one patient with colonic carcinoma, suggesting a correlation between high FGF-23 levels and CRC[22,23]. MMP-10 is a member of the zinc-dependent family of endopeptidases. It can decompose the extracellular matrix and break the blood-brain barrier. Because the reconstruction of the extracellular matrix is related to tumor metastasis and invasion, MMP-10 is considered to be related to these events. Asano et al[24] found the expression of MMP-10 to be increased in 112 cases of colorectal tumor tissues.
IL-13 is a cytokine which has anti-inflammatory and immunomodulatory effects. Its overexpression in cancer tissue is associated with tumor infiltration, apoptosis suppression, tumor metastasis, and lower mucin calcium[25]. Formentini et al[26] investigated the expression of IL-13R in epithelial cells of ulcerative colitis, colonic cancer and control patients. They found that IL-13R levels were significantly higher in colonic cancer samples than in the control and ulcerative colitis groups. TPO is a cytokine produced mainly by the liver, but also by the kidneys and other organs. In the process of tumorigenesis, blood coagulation dysfunction, platelet activation, increased number of platelet and thrombosis are common. Dymicka-Piekarska et al[27] found that TPO levels in a set of 38 cases of CRC was significantly higher than that of a control group of 35 individuals. Furthermore the concentration was higher in stage III tumors.
In this study, the concentration of all seven of these protein molecules was significantly higher in the CRC patients than the healthy controls, consistent with other research results. This result further validates the relationship between these seven proteins and CRC. Additionally, a protein biochip was used to combine measurements for all proteins simultaneously. With the seven protein molecules combined, sensitivity was 0.7 and specificity was 0.4. By combining FGF-23, IL-13, and TPO, the sensitivity was 0.7 and specificity was 0.7. If FGF-23 and TPO only were considered, the sensitivity was 0.7 and specificity was 0.8. Hence, FGF-23 and TPO is the best combination found in this study to accurately identify the presence of tumors from a fecal sample of the patient. We also analyzed the relationship between the seven protein levels and patient age, tumor location (ascending colon, transverse colon, descending colon, and rectum), and tumor staging (Dukes staging and TNM staging) by t test. Although the test was limited by sample size and a non-normal distribution, the results were not significant. In this study, the diagnosis value of a single molecular maker is comparable with other studies. However, due to the different sample size and research methods, the results also have slight differences. Studies using feces as a specimen source to discover protein biomarkers for CRC is scarce. Moreover, it is rarer to see this strategy combined with the protein biochip approach. Stefanie Bunger detected CEA, IL-8, vascular endothelial growth factor, S100A11, macrophage colony-stimulating factor, C3adesArg, CD26 in the blood of patient using a protein biochip approch, and the best combinations in this study were (1) CEA with IL-8; and (2) CEA with C-reactive protein; the sensitivity/specificity were 37%/83% and 35%/81%, respectively. This study provided a basis for the successful application of protein chips in the aid of CRC diagnosis. However, this study used blood samples as its specimen, but stool specimens may have additional advantages.
In our study, the sample size is small, so additional samples would afford increased confidence in our findings. The use of proteins as molecular markers may also have its shortcomings. First, proteins could be decomposed easily by proteases, leading to a false negative result. Second, there is no method to amplify protein as there is for DNA or RNA, thus limiting the application. Thirdly, changes in the phenotype of the tumor occur at the genomic level first, rather than the protein sequence[28]. Thus, protein molecules used as biomarkers need further in-depth study before they can be used widely for cancer screening.
In summary, we found seven proteins whose abundance is significantly higher in the feces of CRC compared with healthy controls. The strongest test combination is FGF-23 and TPO, with sensitivity of 0.7 and specificity of 0.8. These proteins did not correlate in any way to patient age, tumor stage, or location. We have shown that protein biochips have potential to be used as a noninvasive screening method for CRC. Additional protein biomarkers and optimized technology are areas for future research to develop this new diagnostic technique. With the discovery of new protein molecules and progress of diagnosis technology, the value of this method for CRC diagnosis will be subjected to further improvement and development.
We thank the Science and Technology Committee of the Shanghai Minhang Government. We also thank Shanghai Biochip Company for technical assistance.
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide. Fecal occult blood test and others have a high incidence of false positive and false negative results, as well as low specificity. Proteins, using fecal material have the advantages of being simple, flexible, and non-invasive, with no need for bowel preparation, and may reflect pathologies involving the whole gut, are more suitable for such a large-scale screen, because of their stability, high sensitivity, and operation simplicity. Therefore, fecal detection is a very promising method for the detection of biomarkers in the feces of CRC patients.
There are many researches in the finding out the biomarkers of CRC. DNA, RNA and protein are the most hotspots in this field. However, single molecular of which is deeply researched. And most of the single molecular biomarker in blood had a low sensitivity and specificity, especially there are less research of the fecal molecular biomarkers.
Fecal biomarker is a new method for cancer research. The aim of this study is not only to evaluation the value of specific fecal protein biochips, but also the authors wanted to find out new fecal molecular biomarkers for the diagnosis of CRC. It is significant for the diagnosis and treatment of CRC. The study is a research paper, and in the next period we will continue to focus on the specific fecal protein and the mechanism of them.
The study results suggest that the combination of fibroblast growth factor-23 (FGF-23) and thrombopoietin (TPO) by protein biochip method has a high value for the diagnosis of CRC and it may be used in clinical in the future.
There is an ongoing search for new methods and markers for the diagnosis of CRC. Stool specimens and protein biochip technology is noninvasive, simple, fast and convenient, resulting in a good patient compliance rate and overcomes the disadvantage of diagnosing cancers using a single molecular marker. Proteins that come from lumen can be roughly classified into three categories: leakage, secretion, and exfoliation.
This is a good retrospective study in which the authors analyzed the value of the protein biochip in the diagnosis of CRC. The results are valuable and suggest that the combination of FGF-23 and TPO by protein biochip method has a high value for the diagnosis of CRC. It is also need to be have a deep research in the future.
P- Reviewers: Chen JL, Stanojevic GZ S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Ang CS, Rothacker J, Patsiouras H, Burgess AW, Nice EC. Murine fecal proteomics: a model system for the detection of potential biomarkers for colorectal cancer. J Chromatogr A. 2010;1217:3330-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Kanthan R, Senger JL, Kanthan SC. Fecal molecular markers for colorectal cancer screening. Gastroenterol Res Pract. 2012;2012:184343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ang CS, Phung J, Nice EC. The discovery and validation of colorectal cancer biomarkers. Biomed Chromatogr. 2011;25:82-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Jenkinson F, Steele RJ. Colorectal cancer screening - methodology. Surgeon. 2010;8:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Bosch LJ, Carvalho B, Fijneman RJ, Jimenez CR, Pinedo HM, van Engeland M, Meijer GA. Molecular tests for colorectal cancer screening. Clin Colorectal Cancer. 2011;10:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Ouyang DL, Chen JJ, Getzenberg RH, Schoen RE. Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol. 2005;100:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Bonanno E, Rulli F, Galatà G, Pucci S, Sesti F, Farinon AM, Spagnoli LG. Stool test for colorectal cancer screening: what is going on? Surg Oncol. 2007;16 Suppl 1:S43-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Xu Z, Du W, Zhang P, Wang X, Ma X, Shi L, Song L. Development of a protein biochip to identify 6 monoclonal antibodies against subtypes of recombinant human interferons. Assay Drug Dev Technol. 2010;8:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Dupuy AM, Lehmann S, Cristol JP. Protein biochip systems for the clinical laboratory. Clin Chem Lab Med. 2005;43:1291-1302. [PubMed] |
| 10. | Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Ahlquist DA, McGill DB, Fleming JL, Schwartz S, Wieand HS, Rubin J, Moertel CG. Patterns of occult bleeding in asymptomatic colorectal cancer. Cancer. 1989;63:1826-1830. [PubMed] |
| 12. | Boland CR, Montgomery CK, Kim YS. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci USA. 1982;79:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Kim Y. Mucin glycoproteins in gastrointestinal malignancies and metastasis. Eur J Gastroenterol Hepatol. 1993;5:219-225. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tøn H, Brandsnes S, Holtlund J, Skuibina E, Schjønsby H, Johne B. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Tibble J, Sigthorsson G, Foster R, Sherwood R, Fagerhol M, Bjarnason I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Hardt PD, Ngoumou BK, Rupp J, Schnell-Kretschmer H, Kloer HU. Tumor M2-pyruvate kinase: a promising tumor marker in the diagnosis of gastro-intestinal cancer. Anticancer Res. 2000;20:4965-4968. [PubMed] |
| 18. | Hardt PD, Mazurek S, Toepler M, Schlierbach P, Bretzel RG, Eigenbrodt E, Kloer HU. Faecal tumour M2 pyruvate kinase: a new, sensitive screening tool for colorectal cancer. Br J Cancer. 2004;91:980-984. [PubMed] |
| 19. | Ayling RM. New faecal tests in gastroenterology. Ann Clin Biochem. 2012;49:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255-1259. [PubMed] |
| 21. | Ahmad SA, Liu W, Jung YD, Fan F, Reinmuth N, Bucana CD, Ellis LM. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138-1143. [PubMed] |
| 22. | Leaf DE, Pereira RC, Bazari H, Jüppner H. Oncogenic osteomalacia due to FGF23-expressing colon adenocarcinoma. J Clin Endocrinol Metab. 2013;98:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Kovesdy CP, Quarles LD. Fibroblast growth factor-23: what we know, what we don’t know, and what we need to know. Nephrol Dial Transplant. 2013;28:2228-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Asano T, Tada M, Cheng S, Takemoto N, Kuramae T, Abe M, Takahashi O, Miyamoto M, Hamada J, Moriuchi T. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. J Surg Res. 2008;146:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Mandal D, Levine AD. Elevated IL-13Ralpha2 in intestinal epithelial cells from ulcerative colitis or colorectal cancer initiates MAPK pathway. Inflamm Bowel Dis. 2010;16:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Formentini A, Braun P, Fricke H, Link KH, Henne-Bruns D, Kornmann M. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int J Colorectal Dis. 2012;27:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Dymicka-Piekarska V, Kemona H. Thrombopoietin and reticulated platelets as thrombopoietic markers in colorectal cancer. Thromb Res. 2008;122:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Miller S, Steele S. Novel molecular screening approaches in colorectal cancer. J Surg Oncol. 2012;105:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |