Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1325
Revised: October 18, 2013
Accepted: November 12, 2013
Published online: February 7, 2014
Processing time: 160 Days and 5.6 Hours
AIM: To develop a new hemostatic device for endoscopic surgery that can control the bleeding without completely occluding the bleeding vessel.
METHODS: A hemostatic clip and its applier that can stanch bleeding while maintaining blood flow through the clipped vessel was introduced, and the performance of the proposed clip was evaluated using in vitro and in vivo experiments.
RESULTS: During in vitro experiments, no leakage was found after clipping at cuts made in artificial vessels, and flow was maintained through the clipped artificial vessels. In experiments on rats, all the implanted clips occluded the target vessels successfully, and no bleeding or tissue damage was observed at the operative site after the rats were euthanized on postoperative day 7. In experiments on pigs, bleeding stopped immediately after partial clipping of a damaged vessel, and some amount of blood flow was consistently maintained through the clipped vessel after hemostasis.
CONCLUSION: We believe that the proposed hemostatic clip and clip applier can enhance patient safety during laparoscopic surgery.
Core tip: This paper present a preclinical experimental study aimed at demonstrating the utility of a new clip designed to preserve blood flow while clipping a bleeding vessel. It can be a reasonable solution for partial large vessel injuries or avulsion injuries.
- Citation: Nam KW, Lee SB, Kim IY, Kim KG, Park SJ. A new hemostatic clip for endoscopic surgery that can maintain blood flow after clipping. World J Gastroenterol 2014; 20(5): 1325-1331
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1325.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1325
Most laparoscopic operations are performed using the limited 2-dimensional visual information about the operative site that is supplied by an endoscope; as a result, accidents due to the narrow field of vision and low depth perception frequently occur. One of the most common accidents is blood vessel injury caused by the misuse of cutting tools such as scissors, electrocautery, or harmonic scalpel[1]. Various hemostatic tactics can be applied to accidental vascular injuries that occur during laparoscopic surgery. These include the use of energy-based electrosurgical devices[2,3], insertion of hemostatic tubes or catheters[4,5], injection of hemostatic solution[6], use of hemostatic clips alone[7,8] or with epinephrine injection[9,10], injection of Histoacryl[11], or the use of topical hemostatic agents such as gelatins, collagens, thrombin and fibrin sealants, and synthetic glues[12-15].
However, energy-based vessel-sealing devices can cause thermal injuries that can result in neural damage and necrosis[16] or tissue distortions[17]. Additionally, when the damaged vessel cannot safely be completely occluded, it is difficult with conventional hemostatic clips to achieve hemostasis while maintaining blood flow through the damaged vessel after clipping[18].
In this article, we propose a new hemostatic clip and dedicated clip applier that can stanch bleeding from a damaged vessel during laparoscopic surgery without completely occluding the vessel and report on our in vitro and in vivo evaluation of their performance.
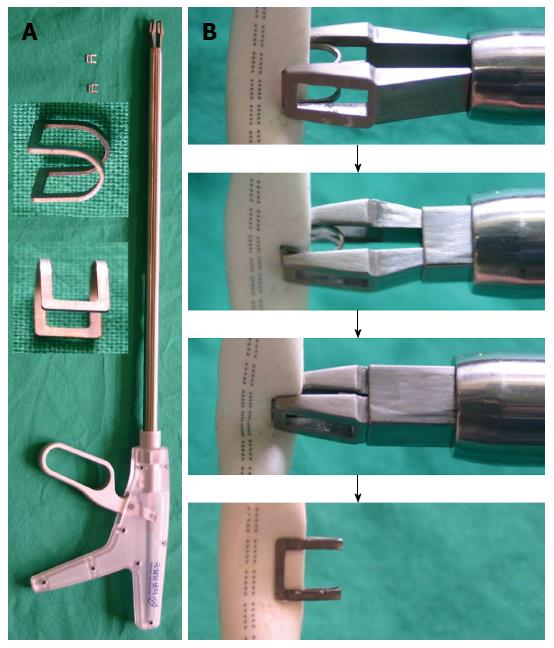
The proposed hemostatic clip and clip applier are shown in Figure 1A. The clip is 6 mm wide, 6 mm long, and 5 mm high, and the total length of the clip applier is 480 mm with a working length of 320 mm. When accidental vascular injury and bleeding occur during laparoscopic surgery, the clip is to be used as follows: (1) temporarily stop the main procedure; (2) grasp the bleeding vessel using laparoscopic forceps; (3) locate the clip applier so that the defect in the vessel is completely surrounded by the clip; (4) manipulate the handle of the clip applier to hold the hemostatic clip in place until the bleeding is stopped; and (5) resume the main procedure (Figure 1B).
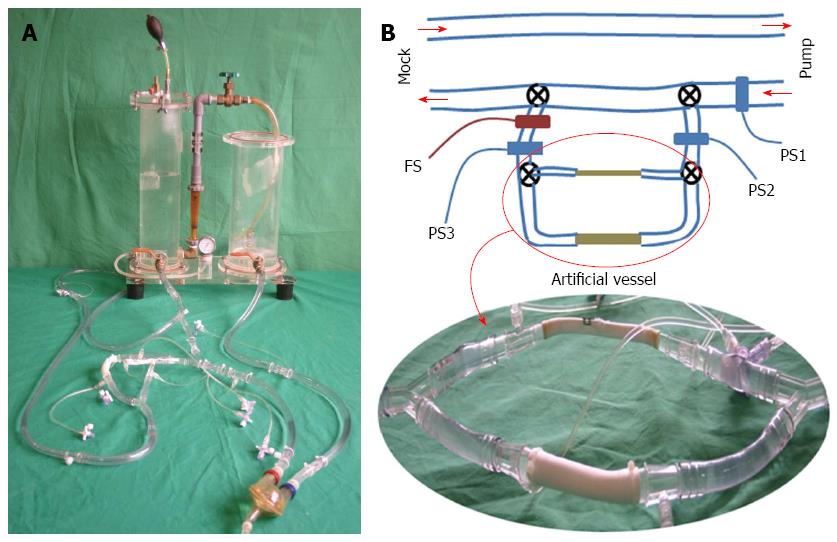
To evaluate the hemostatic performance of the clip in situations where it is important to maintain blood flow through the bleeding vessel, a closed-flow circuit consisting of a pneumatic pulsatile-flow pump and a Donovan-type mock circulatory system was assembled to simulate the main circulatory system of the human body, as shown in Figure 2A. The mock circulatory system and the sac of the pulsatile-flow pump were connected with 2 Tygon tubes with an internal diameter of 1/2 inch, to simulate an aorta and a vena cava. Then, the aortic tube was bifurcated to establish 2 flow paths for 1/2-inch and 1/4-inch artificial vessels, as shown in Figure 2B. Three hydraulic pressure sensors (PS9030VY™; Sensortechnics GmbH, Puchheim, Germany) and an ultrasonic flow meter (TS410; Transonic Systems Inc., Ithaca, NY, United States) were attached to the closed-flow circuit to observe the variations of hydraulic pressure and flow in accordance with the degree of vessel occlusion.
During the experiments, the average afterload pressure of the mock circulatory system was adjusted to 100 mmHg and the pumping rate of the pulsatile pump was fixed to 50 beats per minute to limit the maximal hydraulic pressure within the artificial vessel to ≤ 200 mmHg when the artificial vessel was completely occluded (average outflow of the hydraulic pump = 3.0 L/min). During experiments on the 1/4-inch artificial vessel, the tubes before and after the 1/2-inch artificial vessel were completely occluded using 2 clamping scissors and similarly, the tubes before and after the 1/4-inch artificial vessel were completely occluded during experiments on the 1/2-inch artificial vessel.
To assess the stability and clinical feasibility of the clip, short-term animal experiments were performed using 4 rats (all males; average age = 3 mo) for full-occlusion tests and 2 pigs (all females; average age = 3 mo, average weight = 40 kg) for partial-occlusion tests. The rat experiments were performed at the Korean National Cancer Center, and the pig experiments were performed at the Yonsei University (Sinchon Severance Hospital). The experimental protocols were approved by the ethics committees for animal study of both medical institutions, and the studies were performed in strict compliance with the Korean Ministry of Health and Welfare Guidelines for the Care and Use of Research Animals.
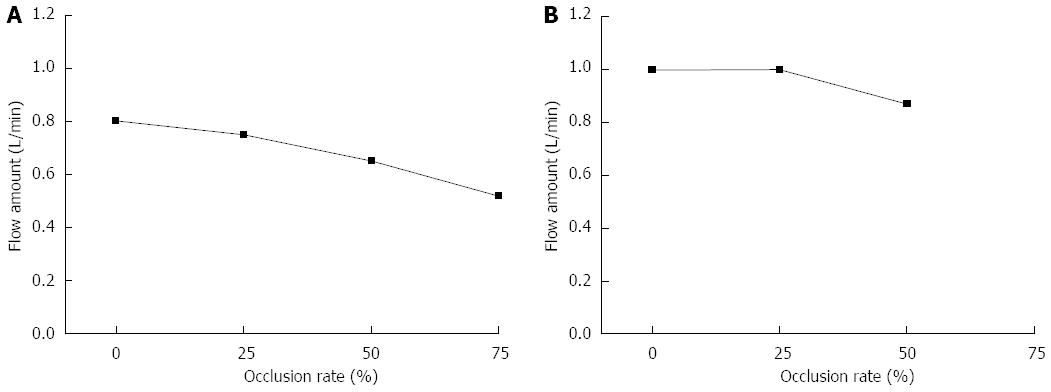
Table 1 shows the measurements of the 3 pressure sensors during in vitro experiments lasting 3 h. The 1/4-inch artificial vessel was occluded 0%, 25%, 50%, and 75% using the proposed hemostatic clip and the 1/2-inch artificial vessel was occluded 0%, 25%, and 50%. Figure 3 shows the measurements of flow variation through the clipped artificial vessels. In all cases, no leakage at the cut area was found after clipping, and flow through the clipped artificial vessel was maintained after clipping the cut area. The average decreases in flow rates due to the clipping were 6.25% at 25% occlusion, 18.75% at 50% occlusion, and 35% at 75% occlusion for the 1/4-inch artificial vessel and 0% at 25% occlusion and 13% at 50% occlusion for the 1/2-inch artificial vessel.
| D | OR | PS1 (mmHg) | PS2 (mmHg) | PS3 (mmHg) |
| 1/4 | 0% | 149.57 ± 0.73 | 138.06 ± 0.59 | 129.83 ± 0.64 |
| 25% | 132.06 ± 0.80 | 123.15 ± 0.54 | 117.71 ± 0.82 | |
| 50% | 149.85 ± 1.27 | 139.95 ± 1.20 | 138.05 ± 1.32 | |
| 75% | 149.09 ± 0.56 | 135.18 ± 0.50 | 136.16 ± 0.57 | |
| 1/2 | 0% | 160.38 ± 1.22 | 152.42 ± 1.70 | 143.89 ± 2.45 |
| 25% | 124.20 ± 2.26 | 124.20 ± 2.26 | 125.62 ± 3.41 | |
| 50% | 137.30 ± 1.46 | 130.25 ± 1.17 | 122.44 ± 1.40 |
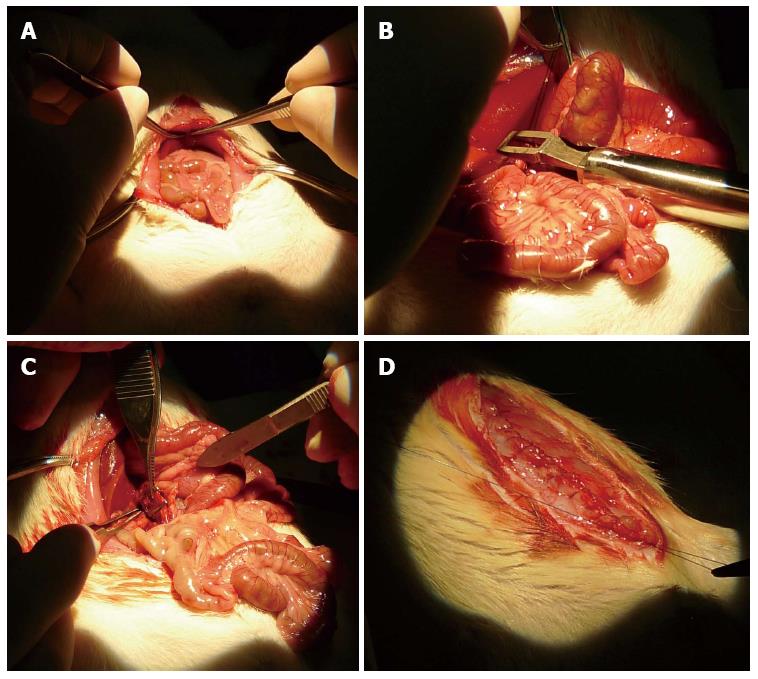
Figure 4 demonstrates the procedures of the full-occlusion test. During the operation, each rat was laid on the operating table in a supine position; in 2 rats, the clip was implanted on the right iliac vein, in 1, on the inferior vena cava, and in 1, on the splenic artery and vein. The target vessels were completely occluded by the clip. The rats fully recovered from anesthesia within a few hours and were given food and water for 1 wk without any additional medical treatments. After the operation, 1 of the 4 clip-implanted rats died on postoperative day 3 and the remaining 3 lived for 1 wk. All of the surviving rats were euthanized on postoperative day 7 by anesthetic overdose. During autopsy, all of the implanted clips were seen to successfully occlude the target vessels and no bleeding or tissue damage was observed at the operative site.
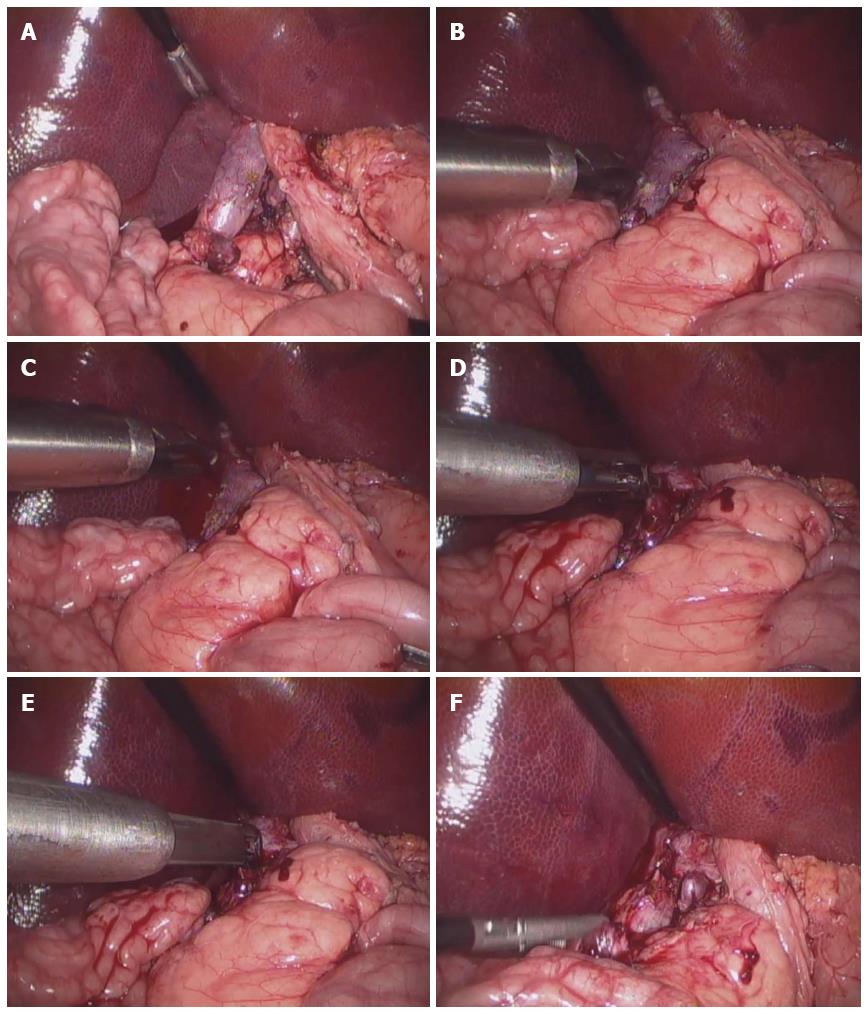
Figure 5 demonstrates the procedures of the partial-occlusion test. Partial-occlusion tests were performed as laparoscopic surgeries. The target vessel (infra-hepatic inferior vena cava) was separated from the surrounding tissues, and then bleeding was intentionally induced on the surface of the target vessel using electrocautery. After the intentional injury, the armed clip applier was inserted through a trocar, and the bleeding site was occluded by the clip. During the experiment, the bleeding stopped immediately after clip implantation.
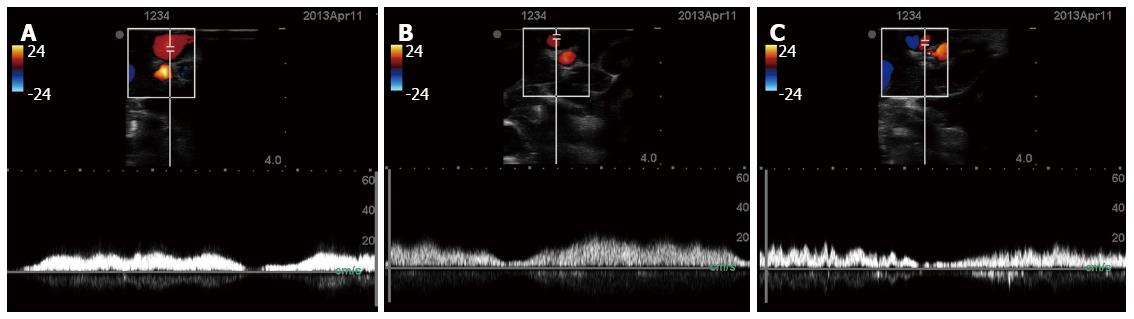
To assess blood flow through the clipped vessel, the flow through the partially-clipped vessel was monitored using ultrasound (M-Turbo; Fujifilm Sonosite Inc., Tokyo, Japan). Because the ultrasonic probe utilized was not designed for laparoscopic use, the abdomen was opened and the probe was applied on the surface of the clipped vessel. Figure 6 shows the measurements of blood flow through the partially-clipped vessel. As is shown in the figure, some amount of blood flow was consistently maintained after clipping. After the measurements were taken, the pigs were euthanized by anesthetic overdose.
The clip and clip applier proposed in this article have a similar operating mechanism to the conventional over-the-scope clip[19,20] or the deep pistol-shaped clip applier[21]. The over-the-scope clip is primarily used for closing a perforation or fistula during laparoscopic surgery, but the proposed clip aims to immediately stanch bleeding from an injured blood vessels while maintaining blood flow through the clipped vessel. Additionally, the deep pistol-shaped clip applier aims to seal the bleeding of the venous channel at the intercavernous sinus during dural incision, but the proposed clip can cover a wider range of vascular injuries and therefore can minimize the number of clips needed to control bleeding from an injured vessel. Furthermore, unlike conventional hemostatic clips[22,23] that completely occlude blood vessels, the proposed clip can maintain a certain amount of blood flow through a clipped vessel.
Currently, the proposed clip is manufactured using biocompatible titanium; however, this presents the problem of postoperative incompatibility with magnetic resonance imaging[24]. To prevent this, the clip needs to be manufactured using non-metallic materials or non-magnetic metals (such as nitinol[25]) that have sufficient strength, durability, and biocompatibility. Additionally, applying the metallic clip to the bleeding vessel has a potential risk of tearing if the handle of the clip applier is tightened excessively by the surgeon, which might exacerbate the bleeding condition. This risk can be reduced by further improving the design of the clip applier to restrict the power transfer to the distal site of the clip applier, and surgeons undergoing additional preoperative training. Furthermore, sometimes, the clip may be applied on an unwanted area by mistake and should be removed immediately to reapply at the desired site. However, since currently developed clip and clip applier do not have such clip-removal ability, we are in the process of implementing such ability by redesigning the structure of the clip and clip applier in the future. By adding such ability, it may even be possible to remove the clip after the bleeding site is sealed, using additional hemostatic agents and preventing clinical complications from the clip remaining in the body. Furthermore, if it is possible to manufacture the clip using bio-degradable materials such as absorbable hemostatic agents[26], the clip will degrade naturally after the bleeding is controlled.
Currently, the tip of the clip applier is straight, which makes it difficult to clip the bleeding vessel accurately in the perpendicular direction. To improve clinical feasibility, it will be necessary to redesign the clip applier to have 1 or 2 degrees of freedom at the distal end to be able to clip the bleeding vessel at various angles.
In this study, the sealing performance of the proposed clip was evaluated by in vitro and short-term animal experiments. Further studies are needed to investigate additional clinical concerns about the proposed hemostatic clip including intra-operative blood loss, surgical time, clip retention rate, and physiological and pathological effects on the vessel and surrounding tissues after long-term application of the clip[27-29] to verify its clinical feasibility.
In conclusion, we believe that the proposed hemostatic clip and clip applier have the potential to enhance patient safety during laparoscopic surgery.
Most laparoscopic operations are performed depending on the limited 2-D visual information and as a result, accidents such as blood vessel injury occur frequently. Currently, it is extremely difficult with conventional hemostatic clips to achieve hemostasis while maintaining blood flow through the clipped vessel.
In the area of bleeding vessel hemostasis, the research hot spot is how to seal the bleeding site easily and quickly during operation.
Unlike conventional hemostatic clips, the hemostatic clip proposed in this paper can maintain a certain amount of blood flow through a clipped vessel.
The proposed hemostatic clip can cover a wider range of vascular injuries during laparoscopic surgeries.
Hemostatic clip refers to the mechanical clip that seals or occludes the bleeding site during operation.
The authors tried to do their best to establish the new sealed system to control intraoperative bleeding during the laparoscopic surgery. This new hemostatic clips and its applicator seemed to be function well, based on the in vitro and in vivo studies.
P- Reviewers: ArezzoA, Golffier C, Wang PH S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Ma S
| 1. | Mechchat A, Bagan P. Management of major vascular complications of laparoscopic surgery. J Visc Surg. 2010;147:e145-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Dionigi G, Boni L, Rausei S, Frattini F, Ferrari CC, Mangano A, Leotta A, Franchin M. The safety of energy-based devices in open thyroidectomy: a prospective, randomised study comparing the LigaSure™ (LF1212) and the Harmonic® FOCUS. Langenbecks Arch Surg. 2012;397:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Burdette TE, Kerrigan CL, Homa K. Harmonic scalpel versus electrocautery in breast reduction surgery: a randomized controlled trial. Plast Reconstr Surg. 2011;128:243e-249e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Yoshida H, Mamada Y, Taniai N, Yoshioka M, Hirakata A, Kawano Y, Mizuguchi Y, Shimizu T, Ueda J, Uchida E. Treatment modalities for bleeding esophagogastric varices. J Nippon Med Sch. 2012;79:19-30. [PubMed] |
| 5. | Morita S, Shibata M, Nakagawa Y, Yamamoto I, Inokuchi S. Successful hemostasis of intractable nasal bleeding with a Sengstaken-Blakemore tube. Otolaryngol Head Neck Surg. 2006;134:1053-1054. [PubMed] |
| 6. | Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Diener MK, Seiler CM, von Frankenberg M, Rendel K, Schüle S, Maschuw K, Riedl S, Rückert JC, Eckmann C, Scharlau U. Vascular clips versus ligatures in thyroid surgery--results of a multicenter randomized controlled trial (CLIVIT Trial). Langenbecks Arch Surg. 2012;397:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ljubicic N, Budimir I, Biscanin A, Nikolic M, Supanc V, Hrabar D, Pavic T. Endoclips vs large or small-volume epinephrine in peptic ulcer recurrent bleeding. World J Gastroenterol. 2012;18:2219-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Hu ML, Wu KL, Chiu KW, Chiu YC, Chou YP, Tai WC, Hu TH, Chiou SS, Chuah SK. Predictors of rebleeding after initial hemostasis with epinephrine injection in high-risk ulcers. World J Gastroenterol. 2010;16:5490-5495. [PubMed] |
| 10. | Lecleire S, Antonietti M, Iwanicki-Caron I, Duclos A, Ramirez S, Ben-Soussan E, Hervé S, Ducrotté P. Endoscopic band ligation could decrease recurrent bleeding in Mallory-Weiss syndrome as compared to haemostasis by hemoclips plus epinephrine. Aliment Pharmacol Ther. 2009;30:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Monsanto P, Almeida N, Rosa A, Maçôas F, Lérias C, Portela F, Amaro P, Ferreira M, Gouveia H, Sofia C. Endoscopic treatment of bleeding gastric varices with histoacryl (N-butyl-2-cyanoacrylate): a South European single center experience. Indian J Gastroenterol. 2013;32:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Saif R, Jacob M, Robinson S, Amer A, Kei-Hui D, Sen G, Manas D, White S. Use of fibrin-based sealants and gelatin-matrix hemostats in laparoscopic liver surgery. Surg Laparosc Endosc Percutan Tech. 2011;21:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sileshi B, Achneck H, Ma L, Lawson JH. Application of energy-based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18:197-204. [PubMed] |
| 14. | Sileshi B, Achneck HE, Lawson JH. Management of surgical hemostasis: topical agents. Vascular. 2008;16 Suppl 1:S22-S28. [PubMed] |
| 15. | Samudrala S. Topical hemostatic agents in surgery: a surgeon’s perspective. AORN J. 2008;88:S2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Eberli D, Hefermehl LJ, Müller A, Sulser T, Knönagel H. Thermal spread of vessel-sealing devices evaluated in a clinically relevant in vitro model. Urol Int. 2011;86:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kakarala K, Faquin WC, Deschler DG. A comparison of histopathologic margin assessment after steel scalpel, monopolar electrosurgery, and ultrasonic scalpel glossectomy in a rat model. Laryngoscope. 2010;120 Suppl 4:S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Ping H, Xing NZ, Zhang JH, Yan Y, Kang N, Niu YN. Application of the Hem-o-lok ligation system in laparoscopic nephrectomy. Surg Endosc. 2010;24:1494-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Albert JG, Friedrich-Rust M, Woeste G, Strey C, Bechstein WO, Zeuzem S, Sarrazin C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest Endosc. 2011;74:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Hong Y, Chen S, Guo S, Yu J, Wu Q, Zhang J. A new technique for management of intercavernous sinus bleeding with titanium clips in transsphenoidal surgery. Neurol India. 2010;58:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Gallotta V, Fanfani F, Rossitto C, Vizzielli G, Testa A, Scambia G, Fagotti A. A randomized study comparing the use of the Ligaclip with bipolar energy to prevent lymphocele during laparoscopic pelvic lymphadenectomy for gynecologic cancer. Am J Obstet Gynecol. 2010;203:483.e1-483.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Fibla JJ, Molins L, Mier JM, Vidal G. Effectiveness of sympathetic block by clipping in the treatment of hyperhidrosis and facial blushing. Interact Cardiovasc Thorac Surg. 2009;9:970-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Raju GS. Magnetic resonance imaging incompatibility of clips is an issue. Gastrointest Endosc. 2010;72:905-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Mohrs OK, Petersen SE, Nowak B, Kauczor HU, Voigtlaender T. MRI after implantation of a novel femoral closure device following intra-arterial catheterisation: implications for subsequent contrast-enhanced MR angiography. Eur Radiol. 2010;20:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Gabay M. Absorbable hemostatic agents. Am J Health Syst Pharm. 2006;63:1244-1253. [PubMed] |
| 27. | Risselada M, Ellison GW, Bacon NJ, Polyak MM, van Gilder J, Kirkby K, Kim SE. Comparison of 5 surgical techniques for partial liver lobectomy in the dog for intraoperative blood loss and surgical time. Vet Surg. 2010;39:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Jensen DM, Machicado GA. Hemoclipping of chronic canine ulcers: a randomized, prospective study of initial deployment success, clip retention rates, and ulcer healing. Gastrointest Endosc. 2009;70:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kume K, Yamasaki M, Yoshikawa I. Sepsis caused by endoscopic clipping for colonic diverticular bleeding: a rare complication. World J Gastroenterol. 2009;15:3817-3818. [PubMed] |