Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1305
Revised: October 16, 2013
Accepted: November 1, 2013
Published online: February 7, 2014
Processing time: 163 Days and 22.5 Hours
AIM: To evaluate the expression of Bcl-xL, Bak, and Bax proteins in correlation with particular clinico-histopathological parameters, including tumor invasion front, in patients with colorectal cancer.
METHODS: The expression of these proteins was evaluated with the use of the immunohistochemical method in 50 primary tumors.
RESULTS: According to observations, a low expression of Bax and Bak proteins is related to the localization of the tumor in the rectum (P < 0.05 and P < 0.05 respectively), which may explain an increased incidence of colorectal cancer in this area. A positive expression of Bax protein also correlates with the presence of cancer cell infiltration to lymph and blood vessels (P < 0.05), which may suggest the participation of this protein in the early stages of colorectal cancer progression. Moreover, a positive expression of Bcl-xL protein correlated with a positive expression of Bak protein. This may suggest a greater participation of Bcl-xL protein in the inhibition of the proapoptotic Bak protein, but not the Bax protein.
CONCLUSION: Bax protein is probably very significant in the cancerogenesis mechanism in the large intestine.
Core tip: We determined the expressions of Bax, Bak, and Bcl-xL proteins in colorectal cancer tissue. We reported that low expression of Bax and Bak proteins is related to the localization of the tumor in the rectum, which may explain an increased incidence of colorectal cancer in this area. We also presented the participation of Bax protein in the early stages of colorectal cancer progression. Moreover, our results suggest a greater participation of Bcl-xL protein in the inhibition of the proapoptotic Bak protein, but not the Bax protein. Therefore, Bax protein is probably very significant in the carcinogenesis mechanism in the large intestine.
- Citation: Pryczynicz A, Gryko M, Niewiarowska K, Cepowicz D, Ustymowicz M, Kemona A, Guzińska-Ustymowicz K. Bax protein may influence the invasion of colorectal cancer. World J Gastroenterol 2014; 20(5): 1305-1310
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1305
In Poland, colorectal cancer is the second most common cause of malignant cancer death in men (after bronchial and lung cancers), as well as the third most common in women (after breast and lung cancers). Although the incidence rate in Poland is relatively low, colorectal cancer is becoming increasingly common. The worldwide approximate percentage of 5-year-survival is about 45%, whereas in Poland it is only 25%. Therefore, both prophylactics and improvement in diagnostic tests and cancer therapy are crucial.
Apoptosis is controlled by various cellular signals from within or without the cell, and thus apoptosis may have two paths (internal and external). The intracellular apoptosis path is regulated by the family of Bcl-2 proteins which control cytochrome c release from the mitochondria. The family of Bcl-2 proteins can be divided into two groups: apoptosis inducing (proapoptotic Bax, Bak, Bid, Bad, and Bok) and apoptosis impeding (antiapoptotic Bcl-2, Bcl-xL, and Bcl-w). The mechanism of apoptosis induction depends on the quantitative ratio of the proapoptotic proteins to the antiapoptotic proteins. The major impeding proteins are Bcl-2 and Bcl-xL. They connect with Bax and Bak domains, divide them, and prevent their oligomerization. This way they block the mitochondrial apoptosis path. Their overexpression strongly impedes apoptosis due to cytotoxic damage by elimination of free radicals, prevention of mitochondrial canal formation, and the release of c cytochrome[1-3].
Apoptosis plays a significant role in the prevention of cancer development in the body. As the cell cannot undergo apoptosis due to mutation or impediment by biochemical factors, it continues to divide and thus transforms into a tumor. At this point, the most important factor is Bcl-2 oncogene, as its mutations lead to tumor development. A number of studies have shown a gradual increase in the expression of this antiapoptotic protein in the adenoma-adenocarcinoma sequence, while others have suggested that Bcl-2 expression increases only at the early stages of carcinogenesis and then decreases at the later stages. Apart from Bcl-2, the overexpression of the antiapoptotic Bcl-xL also leads to the protection of cancer cells from death. Bax protein, which is a promoter of cell death, is inactivated in cancer cells. According to studies, as much as 50% of colorectal cancer cases have the BAX gene mutation, which is related to the decrease in the apoptotic index in cancer cells. Moreover, the relationship of Bcl-xL and Bax proteins, which is decreased in the normal epithelium of the intestinal mucous membrane, is a crucial factor in the regulation of apoptosis. The expression of Bcl-xL in adenocarcinoma increases while Bax and Bak decrease, which is related to the cancer cells’ ability to escape from death[4-6].
Therefore, the aim of this work was to evaluate the expression of Bcl-xL, Bak, and Bax proteins in correlation with particular clinico-histopathological parameters including tumor invasion front.
The study group consisted of 50 patients with colorectal carcinoma, operated on in the Surgical Department of the J. Śniadecki Hospital in Bialystok. Colorectal carcinoma specimens and 17 control samples of normal colorectal mucosa were examined. Sections, 4 µm-thick, were cut from paraffin blocks and stained with hematoxylin and eosin (H and E). The routine histopathological assessment of the sections referred to the histological type, malignancy grade (G), clinico-pathological pTN status, regional lymph node involvement, presence of distant metastases, lymphocytic infiltration, vascular invasion, and tumor budding[7]. Inflammatory lymphocytic infiltration was graded as: 0-lacking; 1-weak, diffused; 2-moderate; 3-strong, lymphocyte nests, according to the Jass classification[8]. Lymphatic and venous invasions were examined and assessed together as vascular invasion as in the Guzińska-Ustymowicz[9] article. Tumor budding was defined as the presence of isolated single undifferentiated cancer cells, or as clusters of five or more cancer cells forming microtubular cancerous glands scattered in the stroma at the invasive margin of colorectal cancer[10]. The study protocol was approved by the local Bioethics Committee.
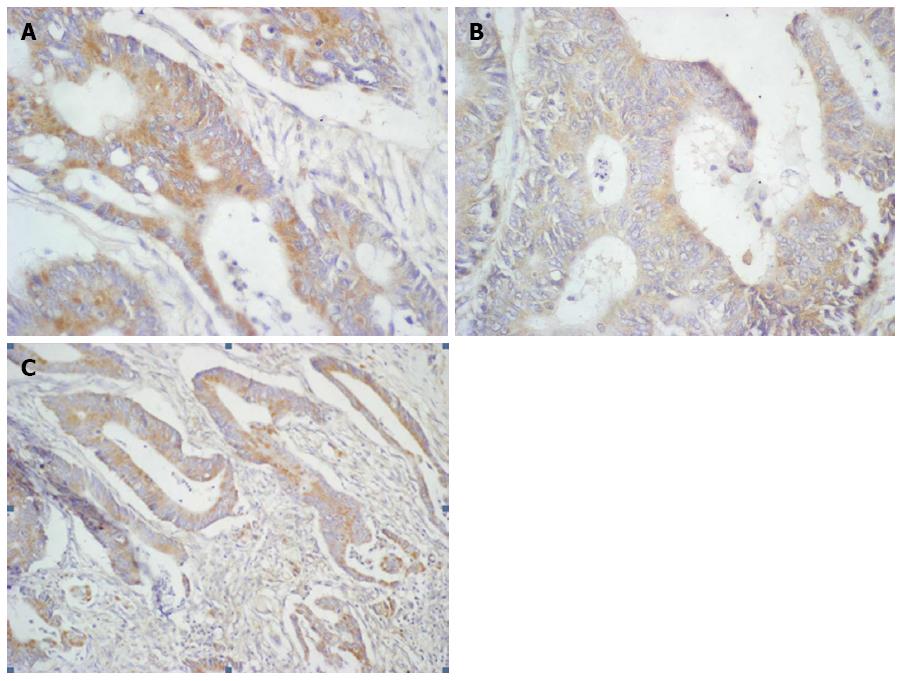
Formalin-fixed and paraffin-embedded tissue specimens were cut on a microtome into 4 μm sections. The sections were deparaffinized in xylenes and hydrated in alcohols. To visualize the antigen, the sections were heated in a microwave oven for 15 min in a citrate buffer (pH = 6.0). They were incubated with 3% hydrogen peroxide solution in order to block endogenous peroxidase. Next, incubation was performed with antibodies against Bcl-xL (goat polyclonal anti-human antibody, Sc-7122, Santa Cruz Biotechnology-incubation 1 h, dilution 1:300), Bak (goat polyclonal anti-human antibody, Sc-1035, Santa Cruz Biotechnology-incubation 1 h 30 min, dilution 1:150), and Bax (goat polyclonal anti-human antibody, Sc-526-G, Santa Cruz Biotechnology - incubation 1 h 30 min, dilution 1:100). The reaction was carried out using the EnVision FLEX two-step visualization system (DAKO, Poland). A color reaction for peroxidase was developed with chromogene DAB (DAKO, Poland). Bcl-xL, Bak, and Bax expression were determined by two independent pathologists using the semiquantitative method and assessed as strong (reaction visible in ≥ 25% of tumor cells) or weak (lack of reaction or the reaction present in < 25% of cancer cells). Positive reactions were calculated in at least 500 cancer cells in each tissue specimen under a light microscope (× 400) (Figure 1).
Statistical analysis was conducted using Student’s t test and the Fisher exact test. A P value of < 0.05 was considered statistically significant. Missing data were removed in pairs.
Positive expression was observed as follows: Bcl-xL protein: 64% of the patients; Bax protein: 42% of the patients; and Bak protein: 40% of the patients. Statistical analysis showed no statistically significant dependencies between the expression of these proteins and the following parameters: age, gender, histological type of neoplasm, pT stage, presence of metastases to regional lymph nodes, presence of distant metastases, infiltration from inflammatory cells, and tumor budding. According to observations, low expression of Bax and Bak proteins is related to the location of the tumor in the rectum (P < 0.05 and P < 0.05 respectively). Moreover, positive Bax expression correlates with the presence of cancer cell infiltration to lymph and blood vessels (P < 0.05) (Tables 1 and 2). According to the statistical analysis of mutual correlations, Bak expression correlated with Bcl-xL expression (P < 0.01) (Table 3). In 19 patients with positive Bak expression, a positive Bcl-xL was also observed in 94.74% (18/19 patients). No similar dependency was observed in the case of Bax protein.
| Variables | Bax expression | P value | Bak expression | P value | |||
| Weak | Strong | Weak | Strong | ||||
| Age | ≤ 60 | 5 (50) | 5 (50) | 0.566 | 5 (50) | 5 (50) | 0.470 |
| > 60 | 24 (60) | 16 (40) | 25 (62.5) | 15 (37.5) | |||
| Gender | Male | 14 (60,9) | 9 (39.1) | 0.704 | 15 (65.2) | 8 (34.8) | 0.487 |
| Female | 15 (55.6) | 12 (44.4) | 15 (55.6) | 12 (44.4) | |||
| Localization | Colon | 12 (42.9) | 16 (57.1) | < 0.050 | 13 (46.4) | 15 (53.6) | < 0.050 |
| Rectum | 17 (77.3) | 5 (22.7) | 17 (77.3) | 5 (22.7) | |||
| Adenocarcinoma type | Non-mucinous | 28 (62.2) | 17 (37.8) | 0.069 | 27 (60) | 18 (40) | 1.000 |
| Mucinous | 1 (20) | 4 (80) | 3 (60) | 2 (40) | |||
| pT stage | 2 | 3 (100) | 0 (0) | 0.128 | 3 (100) | 0 (0) | 0.144 |
| 3 | 26 (55.3) | 21 (44.7) | 27 (57. 5) | 20 (42.5) | |||
| Lymph node metastasis | Absent | 17 (60.7) | 11 (39.3) | 0.660 | 18 (64.3) | 10 (35.7) | 0.485 |
| Present | 12 (54.5) | 10 (45.5) | 12 (54.5) | 10 (45.5) | |||
| Distant metastasis | Absent | 27 (60) | 18 (40) | 0.390 | 26 (57.8) | 19 (42.2) | 0.335 |
| Present | 2 (40) | 3 (60) | 4 (80) | 1 (20) | |||
| Lymphocytic infiltration | Absent | 2 (40) | 3 (60) | 0.064 | 4 (80) | 1 (20) | 0.562 |
| Weak | 14 (56) | 11 (44) | 14 (56) | 11 (44) | |||
| Moderate | 13 (76.5) | 4 (23.5) | 11 (64.7) | 6 (35.3) | |||
| Strong | 0 (0) | 3 (100) | 1 (33.3) | 2 (66.7) | |||
| Vascular invasion | Absent | 17 (73.9) | 6 (26.1) | < 0.050 | 15 (65.2) | 8 (34.8) | 0.487 |
| Present | 12 (44.4) | 15 (55.6) | 15 (55.6) | 12 (44.4) | |||
| Tumor budding | Absent | 7 (63.6) | 4 (36.4) | 0.668 | 8 (72.7) | 3 (27.3) | 0.329 |
| Present | 22 (56.4) | 17 (43.6) | 22 (56.4) | 17 (43.6) | |||
| Variables | Bcl-xL expression | P value | ||
| Weak | Strong | |||
| Age | ≤ 60 | 3 (33.3) | 6 (66.7) | 0.853 |
| > 60 | 15 (36.6) | 26 (63.4) | ||
| Gender | Male | 9 (37.5) | 15 (62.5) | 0.831 |
| Female | 9 (34.6) | 17 (65.4) | ||
| Localization | Colon | 8 (28.6) | 20 (71.4) | 0.216 |
| Rectum | 10 (45.5) | 12 (54.5) | ||
| Adenocarcinoma type | Non-mucinous | 16 (35.6) | 29 (64.4) | 0.844 |
| Mucinous | 2 (40) | 3 (60) | ||
| pT stage | 2 | 2 (66.7) | 1 (33.3) | 0.253 |
| 3 | 16 (34) | 31 (66) | ||
| Lymph node metastasis | Absent | 8 (29.6) | 19 (70.4) | 0.309 |
| Present | 10 (43.5) | 13 (56.5) | ||
| Distant metastasis | Absent | 14 (31.8) | 30 (68.2) | 0.095 |
| Present | 4 (66.7) | 2 (33.3) | ||
| Lymphocytic infiltration | Absent | 2 (40) | 3 (60) | 0.423 |
| Weak | 8 (32) | 17 (68) | ||
| Moderate | 8 (47.1) | 9 (52.9) | ||
| Strong | 0 (0) | 3 (100) | ||
| Vascular invasion | Absent | 10 (43.5) | 13 (56.5) | 0.309 |
| Present | 8 (29.6) | 19 (70.4) | ||
| Tumor budding | Absent | 5 (45.4) | 6 (54.5) | 0.459 |
| Present | 13 (33.3) | 26 (66.7) | ||
| Bcl-xL expression | P value | Bak expression | P value | |||
| Weak | Strong | Weak | Strong | |||
| Bak weak | 15 (51.72) | 14 (48.28) | < 0.010 | 19 (65.52) | 10 (34.48) | 0.349 |
| strong | 1 (5.26) | 18 (94.74) | 11 (52.38) | 10 (47.62) | ||
| Bax weak | 11 (39.29) | 17 (60.71) | 0.300 | |||
| strong | 5 (25) | 15 (75) | ||||
It has been shown that the abnormalities in the expression of proteins which regulate programmed cell death, namely proapoptotic proteins (among others Bak, Bax, and Bid) and antiapoptotic proteins (among others Bcl-2 and Bcl-xL), lead to the formation and development of cancer.
According to the literature, the expression of the proapoptotic Bak protein in a normal mucous membrane of the large intestine is observed in the cells which are localized on the crypt surface. Bak expression in adenocarcinoma of the large intestine affects a deeper area of the crypts. As regards the pattern of malignant cancer of the large intestine, Bak expression is lower compared to the normal mucous membrane and it is also dispersed irregularly. In a normal mucous membrane of the large intestine, the expression of Bax protein is absent in the crypt base and increases towards the villa. Cancer cells in a malignant primary tumor of the large intestine show medium to strong Bax expression. As regards metastases to the liver, a strong decrease in Bax expression is observed. Moreover, Bax expression is lower compared to the normal intestinal mucous membrane[11-14]. The expression of the antiapoptotic Bcl-xL protein in most cancer cells is strong, while no expression of this protein is observed in most of the glandular and well-differentiated parts of colorectal cancer. The increase in Bcl-xL protein level is also related to the more advanced tumors T3 and T4, as well as the tumors which give metastases to lymph nodes and distant organs. According to this study, Bcl-xL protein plays a significant role in apoptosis impediment, which is related to a longer survival of neoplastic cells and cancer progression[15-18].
According to scientific documentation, a positive expression of the antiapoptotic Bcl-xL protein is observed in a higher number of patients (64%) compared to the proapoptotic Bax and Bak proteins (42% and 40% patients, respectively). Bcl-xL protein can form heterodimers with Bak and Bax proteins, and thus prevent their oligomerization and impede apoptosis. Therefore, a strong overexpression of the antiapoptotic proteins is significant in the carcinogenesis of colorectal cancer. According to this study, a positive Bcl-xL expression correlated with a positive Bak expression, however such dependency was not observed in the case of Bax protein. This may suggest a greater participation of Bcl-xL protein in the impediment of the proapoptotic Bak protein, but not Bax. This study may confirm the results obtained by other authors who have observed a decrease in Bak expression and an increase in Bax expression in neoplastic tissue[11-14]. Thus, Bax protein probably plays a very significant role in the carcinogenesis mechanism in the large intestine.
According to this study, a statistically-significant dependency between a positive expression of the proapoptotics Bax and Bak and tumor location was observed. Particularly, a negative expression of these proteins was related with the tumor location in the rectum (77.3% and 77.3% of the patients, respectively). In cases of patients with the tumor located in other parts of the large intestine, the expression of Bax and Bak proteins was positive in 57.1% and 53.6% of patients, respectively. This may indicate a greater incidence of colorectal cancer located in the rectal area. However, no such dependencies have been reported in the literature. According to this study, no relationship between Bcl-xL expression and tumor location was observed.
Studies on the relationship between apoptosis-regulating proteins and cancer histological type showed no dependencies. However, Jansson et al[13] observed that a decrease in Bax expression is related to poorly differentiated cancer and the mucogenic type of colorectal cancer. A similar dependency has also been observed by other authors[11,19]. According to Zhang et al[20], an increase in Bcl-xL expression correlated with a poorly-differentiated cancer type, which is contrary to other apoptosis proteins. Most likely, the proteins which regulate cell death are also involved in the differentiation of cancer cells, yet this process is poorly understood and requires further studies.
Statistical analysis of this work also showed a correlation between Bax positive expression and the presence of cancer cell infiltration to lymph and blood vessels. Bax protein seems to participate in colorectal cancer cell spread, which may indicate this protein’s participation in the early stages of colorectal cancer progression. However, Ogura et al[21] observed that a positive Bax expression is related with a smaller cancer cell infiltration to lymph vessels. The most probable cause of these contradictive results is due to the study group, or difference in the methods of immunohistochemical staining and the estimation of this protein’s expression.
Our study showed no dependency between the expression of the apoptosis regulating proteins, local neoplasm advancement, and the presence of colorectal metastases to regional lymph nodes and distant organs. However, apart from the results similar to this study, such dependencies have also been reported in the literature. The increase in Bcl-xL expression is associated with the presence of metastases to regional lymph nodes and the C + D stage of colorectal cancer in Duke’s classification. Thus, it is suggested that Bcl-xL protein plays a significant role in the development of colorectal cancer and its metastases[20]. A decrease in the expression of Bax proapoptotic protein was related mainly with an increase in cancer stage by Duke’s classification. Moreover, the percentage of positive cells in the metastasis to the liver decreased with the occurrence of primary tumor metastases to regional lymph nodes, as well as with an increase in its size[11,19]. A decreased Bax expression is related with the loss of mitochondrial apoptosis activation. This mechanism allows for easy cancer cell development. In this case, a decrease in Bax expression is connected with a poor prognosis for the patient. Moreover, scientists noticed that Bax-negative patients with colorectal cancer show a shorter survival compared to Bax-positive patients[21]. According to other scientists, a positive expression of this protein is related to a poor prognosis in patients with colorectal metastases. The discrepancies resulted mainly from different study groups. As has been mentioned, these proteins play a more or less significant role depending on the colorectal cancer stage. Therefore, changes in their expression give different prognoses in different study groups.
No dependencies between the expression of Bcl-xL, Bax, and Bak and the presence of buds in the tumor invasion front were observed. The presence of tumor buds, single or small groups of invasive cancer cells in the tumor front which infiltrate the desmoplastic stroma, is an indicator of malignancy potential, including metastatic abilities. Despite reports suggesting the existence of a relationship between the expression of the antiapoptotic Bcl-2 protein and tumor budding presence, Bcl-xL, Bax, and Bak proteins seem not to be involved in the process of their formation[22].
Colorectal cancer is still the most common cancer in terms of incidence and cancer motility. Processes responsible for the carcinogenesis of cancers are still investigated. One of these processes is apoptosis, essential for the removal of abnormal and damaged cells. Therefore the authors wanted to present the development of resistance to apoptosis in colorectal cancer cells in correlation with clinicopathological parameters.
It is known that the ratio of proapoptotic to antiapoptotic proteins in cancer cells is disrupted. The main causes of this disorder are mutations in the Bcl-2 and Bax genes. However, it is unknown whether any of these proteins play a more important role in the carcinogenesis of colorectal cancer.
The authors showed that low expression of Bax and Bak proteins is related with the localization of the tumor in the rectum, which may explain an increased incidence of colorectal cancer in this area. They also presented the participation of Bax protein in colorectal cancer progression by vascular invasion. This is also the first study which presents a greater participation of the Bcl-xL protein in the inhibition of the proapoptotic Bak protein, but not the Bax protein. Therefore Bax protein is probably very significant in the carcinogenesis mechanism in the large intestine.
By demonstrating Bax proteins as an important protein involved in the regulation of apoptosis in colorectal cancer cells, this fact can be used by experts as a target in cancer therapy.
The authors evaluated the expression of Bcl-xL, Bak, and Bax proteins in correlation with particular clinico-histopathological parameters, including tumor invasion front, in patients with colorectal cancer, and demonstrated that a low expression of Bax and Bak proteins is related with the localization of the tumor in the rectum. They also demonstrated that a positive expression of Bax protein correlates with the presence of cancer cell infiltration to lymph and blood vessels, which may suggest the participation of this protein in the early stages of colorectal cancer progression. This manuscript is very interesting and provides novel evidence.
P- Reviewer: Nakajima A S- Editor: Cui XM L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 522] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 2. | Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567-1574. [PubMed] |
| 3. | Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 2377] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 4. | Glinsky GV. Apoptosis in metastatic cancer cells. Crit Rev Oncol Hematol. 1997;25:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Holdenrieder S, Stieber P. Apoptotic markers in cancer. Clin Biochem. 2004;37:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Available from: http://cancerstaging.org/Pages/default.aspx. |
| 8. | Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Guzińska-Ustymowicz K. Aggressive and non-aggressive tumor budding in colorectal cancers. New York: Nova Science Publishers, Inc 2006; 105-116. |
| 10. | Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539-543. [PubMed] |
| 11. | Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, Lorenz M, Dörken B, Daniel PT. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol. 1999;17:1364-1374. [PubMed] |
| 12. | Partik G, Kahl-Rainer P, Sedivy R, Ellinger A, Bursch W, Marian B. Apoptosis in human colorectal tumours: ultrastructure and quantitative studies on tissue localization and association with bak expression. Virchows Arch. 1998;432:415-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Jansson A, Sun XF. Bax expression decreases significantly from primary tumor to metastasis in colorectal cancer. J Clin Oncol. 2002;20:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Alper M, Cukur S, Belenli O, Suna M. Evaluation of the immunohistochemical stain patterns of survivin, Bak and Bag-1 in colorectal cancers and comparison with polyps situated in the colon. Hepatogastroenterology. 2008;55:1269-1273. [PubMed] |
| 15. | Badvie S, Hanna-Morris A, Andreyev HJ, Cohen P, Saini S, Allen-Mersh TG. A “field change” of inhibited apoptosis occurs in colorectal mucosa adjacent to colorectal adenocarcinoma. J Clin Pathol. 2006;59:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Maurer CA, Friess H, Bühler SS, Wahl BR, Graber H, Zimmermann A, Büchler MW. Apoptosis inhibiting factor Bcl-xL might be the crucial member of the Bcl-2 gene family in colorectal cancer. Dig Dis Sci. 1998;43:2641-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Koornstra JJ, de Jong S, Hollema H, de Vries EG, Kleibeuker JH. Changes in apoptosis during the development of colorectal cancer: a systematic review of the literature. Crit Rev Oncol Hematol. 2003;45:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422-2427. [PubMed] |
| 19. | Tsamandas AC, Kardamakis D, Petsas T, Zolota V, Vassiliou V, Matatsoris T, Kalofonos H, Vagianos CE, Scopa CD. Bcl-2, bax and p53 expression in rectal adenocarcinoma. Correlation with classic pathologic prognostic factors and patients’ outcome. In Vivo. 2007;21:113-118. [PubMed] |
| 20. | Zhang YL, Pang LQ, Wu Y, Wang XY, Wang CQ, Fan Y. Significance of Bcl-xL in human colon carcinoma. World J Gastroenterol. 2008;14:3069-3073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ogura E, Senzaki H, Yamamoto D, Yoshida R, Takada H, Hioki K, Tsubura A. Prognostic significance of Bcl-2, Bcl-xL/S, Bax and Bak expressions in colorectal carcinomas. Oncol Rep. 1999;6:365-369. [PubMed] |
| 22. | Karamitopoulou E, Lugli A, Panayiotides I, Karakitsos P, Peros G, Rallis G, Patsouris ES, Terracciano L, Zlobec I. Systematic assessment of protein phenotypes characterizing high-grade tumour budding in mismatch repair-proficient colorectal cancer. Histopathology. 2010;57:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |