Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1289
Revised: September 28, 2013
Accepted: November 12, 2013
Published online: February 7, 2014
Processing time: 185 Days and 4.8 Hours
AIM: To investigate the factors other than fibrosis stage correlating with acoustic radiation force impulse (ARFI) elastograpy in chronic hepatitis C.
METHODS: ARFI elastograpy was performed in 108 consecutive patients with chronic hepatitis C who underwent a liver biopsy. The proportion of fibrosis area in the biopsy specimens was measured by computer-assisted morphometric image analysis.
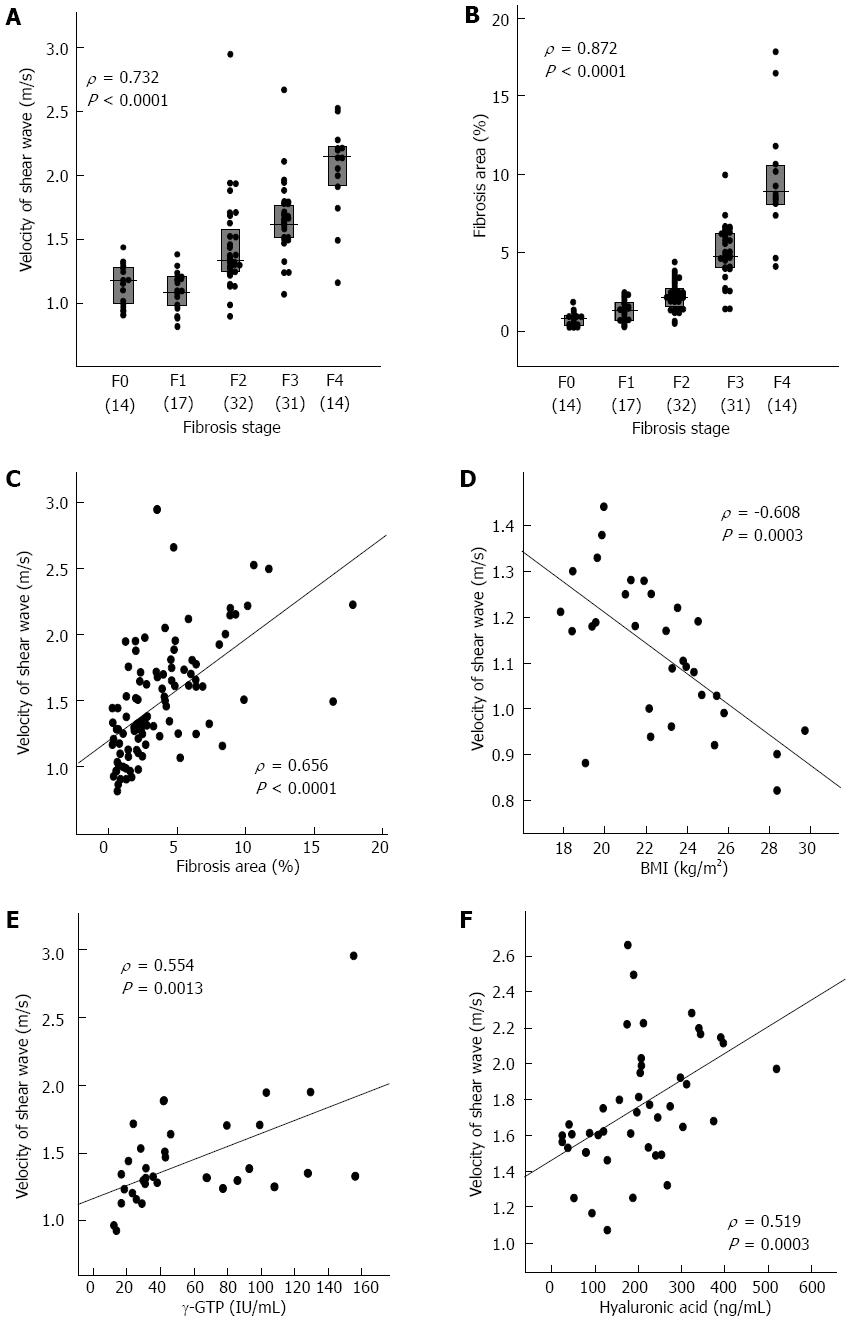
RESULTS: ARFI correlated significantly with fibrosis stage (β = 0.1865, P < 0.0001) and hyaluronic acid levels (β = 0.0008, P = 0.0039) in all patients by multiple regression analysis. Fibrosis area correlated significantly with ARFI by Spearman’s rank correlation test but not by multiple regression analysis. ARFI correlated significantly with body mass index (BMI) (β = -0.0334, P = 0.0001) in F 0 or F 1, with γ-glutamyltranspeptidase levels (β = 0.0048, P = 0.0012) in F 2, and with fibrosis stage (β = 0.2921, P = 0.0044) and hyaluronic acid levels (β = 0.0012, P = 0.0025) in F 3 or F 4. The ARFI cutoff value was 1.28 m/s for F≥ 2, 1.44 m/s for F≥ 3, and 1.73 m/s for F 4.
CONCLUSION: ARFI correlated with fibrosis stage and hyaluronic acid but not with inflammation. ARFI was affected by BMI, γ-glutamyltranspeptidase, and hyaluronic acid in each fibrosis stage.
Core tip: The assessment of liver fibrosis stage is important to estimate prognosis and to identify the patients requiring antiviral treatment in chronic hepatitis C. Liver biopsy is a gold standard for assessing fibrosis, but is invasive. Thus methods for noninvasively assessing fibrosis have been developed. Liver stiffness measurement (LSM) by Fibroscan and acoustic radiation force impulse correlate with fibrosis stage. However, LSM may be affected by factors other than fibrosis, such as edema, steatosis, and inflammation.
- Citation: Nishikawa T, Hashimoto S, Kawabe N, Harata M, Nitta Y, Murao M, Nakano T, Mizuno Y, Shimazaki H, Kan T, Nakaoka K, Takagawa Y, Ohki M, Ichino N, Osakabe K, Yoshioka K. Factors correlating with acoustic radiation force impulse elastography in chronic hepatitis C. World J Gastroenterol 2014; 20(5): 1289-1297
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1289.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1289
The assessment of liver fibrosis stage is important to estimate prognosis and to identify the patients requiring antiviral treatment in chronic hepatitis C.
Methods for noninvasively assessing liver fibrosis have been developed. Liver stiffness measurement (LSM) by transient elastography (TE) with Fibroscan[1-3] and velocity of shear wave (Vs) measured by acoustic radiation force impulse (ARFI)[4-6] correlate with liver fibrosis stage in various liver diseases. However, LSM is affected by factors other than liver fibrosis, such as edema, steatosis, inflammation and necrosis. In particular, inflammation affects LSM; acute or chronic inflammation can result in a high LSM, indicating the presence of falsely higher fibrosis stage than the actual fibrosis stage by both TE[7-9] and ARFI[10-12]. However, Rizzo et al[13] reported that ARFI is not correlated with alanine aminotransferase (ALT) levels[13].
Liver fibrosis is usually semi-quantitatively assessed by the numerical systems of Scheuer[14], the Metavir group[15] or Ishak et al[16]. Direct measurements of the amount of fibrosis in a biopsy specimen by computer-assisted morphometric image analysis has been reported, in which morphometric collagen content is measured quantitatively; it has been shown to correlate well with liver biopsy assessment numerical systems scores[17-19]. Isgro et al[20] reported that fibrosis area has a better relationship with TE than Ishak stage[20], whereas our previous study demonstrated a better correlation of TE with fibrosis stage than with fibrosis area in patients with chronic hepatitis C[21].
In the present study, factors other than fibrosis stage that affect ARFI were investigated in patients with chronic hepatitis C. The proportion of fibrosis area was quantitatively measured by image analysis software in liver biopsy specimens and the correlation with ARFI was assessed.
This study was performed in strict accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Fujita Health University ethics committee. All study participants provided written informed consent.
A total of 108 consecutive patients with chronic hepatitis C virus infection who underwent a liver biopsy before treatment with interferon at Fujita Health University Hospital from October 2009 to October 2012 were included (Table 1). Liver biopsy was performed using a 14G disposable true-cut needle under ultrasonographic guidance. Sections were stained with hematoxylin-eosin and azan stain. Liver specimens of at least 1.5 cm length with more than 8 portal tracts were assessed. Liver biopsy specimens were assessed by two hepatologists (Yoshioka K and Nakaoka K) blinded to the clinical data according to Metavir score[15]. Fibrosis was staged as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis. Activity was graded as follows: A0, none; A1, mild; A2, moderate; and A3, severe activity. Steatosis was graded according to the nonalcoholic fatty liver disease activity score as follows: grade 0; < 5% of hepatocytes involved, grade 1, 5%-33%; grade 2, >33%-66%; and grade 3, > 66%[22]. When fibrosis stage, activity grade, or steatosis grade evaluated by the two hepatologists differed, the higher fibrosis stage, activity grade, or steatosis grade was adopted.
| All patients (n = 108) | F0 (n = 14) | P values of Mann-Whitney U test between F0 and F1 | F1 (n = 17) | P values of Mann-Whitney U test between F0-1 and F2 | F2 (n = 32) | P values of Mann-Whitney U test between F2 and F3 | F3 (n = 31) | P values of Mann-Whitney U test between F3 and F4 | F4 (n = 14) | |
| Age (yr)1 | 59.5 (49.0-66.0) | 48.0 (41.0-60.0) | NS | 51.0 (41.8-65.5) | NS | 61.5 (51.5-66.5) | NS | 61.0 (52.0-67.0) | NS | 60.5 (54.0-66.0) |
| Gender (female/male)2 | 52/56 | 8/6 | NS | 8/9 | NS | 15/17 | NS | 13/18 | NS | 8/6 |
| BMI | 22.5 (20.5-24.6) | 22.0 (20.0-23.2) | NS | 23.5 (19.8-25.4) | NS | 23.0 (20.7-24.4) | NS | 23.2 (21.1-26.1) | NS | 21.9 (19.0-23.4) |
| Fibrosis stage (F0/F1/F2/F3/F4) | 14/17/32/31/14 | - | - | - | - | - | ||||
| Inflammatory grade (A0/A1/A2/A3) | 12/32/53/11 | 9/5/0/0 | 0.0261 | 2/15/0/0 | 0.0001 | 1/9/22/0 | 0.0060 | 0/2/22/7 | NS | 0/1/9/4 |
| Steatosis grade (S0/S1/S/2/S3) | 42/42/14/10 | 8/5/0/1 | NS | 7/9/0/1 | NS | 10/11/6/5 | NS | 9/14/5/3 | NS | 8/3/3/0 |
| AST (IU/L)1 | 44.0 (31.5-82.0) | 28.5 (24.0-38.0) | NS | 36.0 (23.0-41.3) | 0.0033 | 48.5 (36.5-101.5) | NS | 48.0 (42.5-85.3) | NS | 65.5 (37.0-88.0) |
| ALT (IU/L)1 | 55.0 (35.0-91.5) | 37.5 (22.0-59.0) | NS | 39.0 (24.8-52.0) | 0.0095 | 65.0 (41.0-153.0) | NS | 70.0 (41.3-109.0) | NS | 64.0 (36.0-91.0) |
| γ-GTP (IU/L)1 | 33.0 (23.5-75.0) | 23.0 (14.0-27.0) | 0.0802 | 28.0 (19.5-71.3) | NS | 39.5 (24.5-89.5) | NS | 41.0 (28.0-96.8) | 0.0329 | 30.5 (27.0-38.0) |
| Platelet count (× 104/μL)1 | 14.3 (11.3-17.6) | 14.6 (11.7-20.2) | NS | 18.2 (16.6-21.2) | 0.0107 | 16.1 (14.0-17.4) | 0.0080 | 12.2 (11.3-14.3) | 0.0078 | 10.1 (7.1-11.6) |
| Prothrombin time (INR)1 | 1.00 (0.96-1.06) | 0.95 (0.90-0.99) | NS | 0.96 (0.93-1.02) | NS | 1.00 (0.95-1.03) | 0.0144 | 1.03 (1.00-1.08) | 0.0229 | 1.10 (1.03-1.12) |
| Albumin (g/dL)1 | 4.2 (4.0-4.5) | 4.4 (4.1-4.6) | NS | 4.4 (4.2-4.5) | NS | 4.3 (4.0-4.5) | 0.0524 | 4.1 (3.8-4.3) | NS | 4.0 (3.8-4.2) |
| Total cholesterol (mg/dL)1 | 170 (150-188) | 193 (177-207) | 0.0619 | 169 (155-193) | NS | 172 (156-189) | 0.0615 | 159 (141-177) | NS | 160 (144-183) |
| γ-globulin (g/dL)1 | 1.51 (1.33-1.79) | 1.28 (1.14-1.40) | 0.0262 | 1.40 (1.28-1.70) | NS | 1.44 (1.34-1.64) | 0.0067 | 1.63 (1.51-2.11) | NS | 1.66 (1.43-1.97) |
| Hyaluronic acid (ng/mL)1 | 89 (49-206) | 39 (30-64) | NS | 49 (26-77) | 0.0041 | 89 (66-185) | 0.0601 | 184 (82-245) | 0.0291 | 232 (191-338) |
| HCV genotype (1/2) | 81/26 | 10/4 | NS | 12/5 | NS | 22/9 | NS | 5/26 | NS | 11/3 |
| HCV RNA (logIU/mL)1 | 6.6 (5.8-7.0) | 6.5 (6.0-6.9) | NS | 6.6 (5.4-7.0) | NS | 6.6 (5.8-7.1) | NS | 6.7 (5.9-7.1) | NS | 6.6 (6.3-6.8) |
| Fibrosis area (%)1 | 2.63% (1.35-4.95) | 0.85% (0.41-1.04) | 0.0111 | 1.37% (0.73-1.85) | 0.0022 | 2.20% (1.62-2.74) | < 0.0001 | 4.83% (4.03-6.24) | < 0.0001 | 8.87% (8.04-10.52) |
| Velocity of shear wave (m/s)1 | 1.38 (1.19-1.71) | 1.2 (1.0-1.3) | NS | 1.1 (1.0 -1.2) | 0.0010 | 1.3 (1.2-1.6) | 0.0014 | 1.6 (1.5-1.8) | 0.0008 | 2.1 (1.9-2.2) |
Vs measurement by ARFI was performed with a Siemens ACUSON S2000 (Mochida Siemens Medical Systems Co., Ltd., Tokyo, Japan) within 1 wk of liver biopsy[4]. A region in liver to be examined for elastic properties is targeted with a region-of-interest (ROI) cursor while performing B-mode imaging. Tissue at the ROI is mechanically excited using acoustic push pulses to generate localized tissue displacements. The displacements result in propagation of shear-wave away from the region of excitation which is tracked using ultrasonic correlation-based methods. The maximal displacement is estimated for many ultrasound tracking beams laterally adjacent to the single push-beam. By measuring the time to peak displacement at each lateral location, the shear wave propagation velocity can be reconstructed. The examination was performed on the right lobe of the liver. A measurement depth of 2-3 cm below the liver capsule was chosen. Ten successful acquisitions at different locations were performed on each patient, and the results are expressed in meters/second (m/s), and the median value was calculated. The shear wave propagation velocity is considered to be proportional to the square root of tissue elasticity.
The procedures were performed by two investigators (Nishikawa T and Hashimoto S) who were blind to clinical, serological and histological data. The correlation in Vs measurement between two operators was good (r = 0.934).
The proportion of fibrosis area in the biopsy specimens was measured by computer-assisted morphometric image analysis. Liver biopsy specimens were stained with azan stain. Microscopic images of the entire biopsy specimen were obtained with a digital microscope (BZ-9000, Keyence, Tokyo, Japan). Fibrosis area, which was stained blue with azan, was marked and measured with Image Pro Plus 4.0 imaging software (Nippon Roper Co., Ltd., Tokyo, Japan).
Patients were categorized according to fibrosis stage. The groups were compared with the χ2 test and Mann-Whitney U test. Factors correlated with ARFI were estimated by Spearman’s rank correlation test. Factors independently correlated with ARFI were assessed by multiple regression analysis. The diagnostic performance of ARFI and fibrosis area was determined in terms of sensitivity, specificity, positive and negative predictive value, positive likelihood ratio, diagnostic accuracy, and area under the receiver operating characteristics (ROC) curve. Optimal cutoff values between fibrosis categories were determined at maximum sum of sensitivity and specificity. Data were analyzed using StatFlex version 5.0 for Windows (StatFlex, Osaka, Japan). A two-sided P value of < 0.05 indicated statistical significance.
The liver biopsies of the 108 patients were assessed by the Metavir system. Fibrosis stage was F0 in 14 patients, F1 in 17, F2 in 32, F3 in 31 and F4 in 14 (Table 1).
ARFI was significantly correlated with fibrosis stage (ρ = 0.732, P < 0.0001) (Figure 1A). ARFI values differed significantly between stages F1 and F2 (P = 0.0010), between F2 and F3 (P = 0.0014), and between F3 and F4 (P = 0.0008) (Table 1).
The ARFI cutoff values for different fibrosis stages determined by ROC analysis were 1.28 m/s for F≥ 1, 1.28 m/s for F≥ 2, 1.44 m/s for F≥ 3, and 1.73 m/s for F4 (Table 2).
| F≥1 | F≥2 | F≥3 | F4 | |
| Cutoff value (m/s) | 1.28 | 1.28 | 1.44 | 1.73 |
| Positive predictive value | 97.0% | 94.0% | 78.4% | 48.0% |
| Negative predictive value | 29.3% | 65.9% | 91.2% | 97.6% |
| Sensitivity | 69.1% | 81.8% | 88.9% | 85.7% |
| Specificity | 85.7% | 87.1% | 82.5% | 86.2% |
| Positive likelihood ratio | 4.8 | 6.3 | 5.1 | 6.2 |
| Diagnostic accuracy | 71.3% | 83.3% | 85.2% | 86.1% |
| AUROC | 0.810 | 0.909 | 0.869 | 0.885 |
| Standard error of AUROC | 0.046 | 0.027 | 0.036 | 0.058 |
The proportion of fibrosis area was significantly correlated with fibrosis stage as assessed by the Metavir system (ρ = 0.872, P < 0.0001) (Figure 1B). The fibrosis area values differed significantly between stages F0 and F1 (P = 0.0111), F1 and F2 (P = 0.0022), F2 and F3 (P < 0.0001), and between F3 and F4 (P < 0.0001) (Table 1).
The fibrosis area cutoff values for the different fibrosis stages determined by ROC analysis were 1.17% for F≥ 1, 1.80% for F≥ 2, 3.71% for F≥ 3, and 7.32% for F4 (Table 3).
| F≥1 | F≥2 | F≥3 | F4 | |
| Cutoff value | 1.17% | 1.8% | 3.71% | 7.32% |
| Positive predictive value | 97.7% | 94.3% | 93.0% | 92.3% |
| Negative predictive value | 60.0% | 71.1% | 92.3% | 97.9% |
| Sensitivity | 91.5% | 85.7% | 88.9% | 85.7% |
| Specificity | 85.7% | 87.1% | 95.2% | 98.9% |
| Positive likelihood ratio | 6.4 | 6.6 | 18.7 | 80.6 |
| Diagnostic accuracy | 90.7% | 86.1% | 92.6% | 97.20% |
| AUROC | 0.935 | 0.927 | 0.963 | 0.962 |
| Standard error of AUROC | 0.025 | 0.024 | 0.018 | 0.023 |
ARFI was significantly correlated with fibrosis stage (P < 0.0001) (Figure 1A), inflammatory grade (P < 0.0001), aspartate aminotransferase (AST) levels (P < 0.0001), ALT levels (P = 0.0008), γ-glutamyltranspeptidase (γ-GTP) levels (P < 0.0001), platelet count (P < 0.0001), prothrombin time (INR) (P = 0.0003), albumin levels (P = 0.0002), total cholesterol levels (P = 0.0004), γ-globulin levels (P = 0.0087), hyaluronic acid levels (P < 0.0001), and fibrosis area (P < 0.0001) (Figure 1) by Spearman’s rank correlation test (Table 4). ARFI tended to be higher in genotype 1 [median, 1.49 (interquartile range, 1.22-1.75) m/s] than in genotype 2 [1.30 (1.17-1.46)] (P = 0.0728). The multiple regression analysis selected fibrosis stage (β = 0.1865, P < 0.0001) and hyaluronic acid levels (β = 0.0008, P = 0.0039) as factors that independently correlated with ARFI, whereas inflammatory grade, AST, ALT and fibrosis area were not selected (Table 4).
| All patients (n = 108) | Patients with F0 or F1 (n = 31) | Patients with F2 (n = 32) | Patients with F3 or F4 (n = 45) | |||||||||||||
| Spearman's rank correlation test | Multiple regression analysis | Spearman's rank correlation test | Multiple regression analysis | Spearman's rank correlation test | Multiple regression analysis | Spearman's rank correlation test | Multiple regression analysis | |||||||||
| ρ | P value | β | P value | ρ | P value | β | P value | ρ | P value | β | P value | ρ | P value | β | P value | |
| Age (yr) | NS | NS | NS | NS | ||||||||||||
| Gender (female/male)1 | NS | NS | NS | NS | ||||||||||||
| BMI | NS | -0.608 | 0.0003 | -0.033 | 0.0001 | NS | NS | |||||||||
| Fibrosis stage | 0.732 | < 0.0001 | 0.187 | 0.0001 | NS | 0.505 | 0.0004 | 0.292 | 0.0044 | |||||||
| Inflammatory grade | 0.612 | < 0.0001 | NS | NS | NS | NS | ||||||||||
| Steatosis grade | NS | NS | NS | NS | ||||||||||||
| AST (IU/L) | 0.430 | < 0.0001 | NS | NS | NS | NS | ||||||||||
| ALT (IU/L) | 0.318 | 0.0008 | NS | 0.343 | 0.0593 | NS | NS | NS | ||||||||
| γ-GTP (IU/L) | 0.407 | < 0.0001 | NS | 0.340 | 0.0614 | NS | 0.544 | 0.0013 | 0.005 | 0.0012 | NS | |||||
| Platelet count (× 104/μL) | -0.441 | < 0.0001 | NS | NS | NS | -0.425 | 0.0036 | NS | ||||||||
| Prothrombin time (INR) | 0.344 | 0.0003 | NS | NS | NS | 0.390 | 0.0080 | NS | ||||||||
| Albumin (g/dL) | -0.347 | 0.0002 | NS | NS | NS | -0.459 | 0.0015 | NS | ||||||||
| Total cholesterol (mg/mL) | -0.337 | 0.0004 | NS | NS | NS | NS | ||||||||||
| γ-globulin (g/dL) | 0.252 | 0.0087 | NS | NS | -0.344 | 0.0581 | NS | NS | ||||||||
| Hyaluronic acid (ng/mL) | 0.576 | < 0.0001 | 8.00E-4 | 0.0039 | NS | NS | 0.519 | 0.0003 | 0.001 | 0.0025 | ||||||
| HCV genotype (1/2)1 | 0.0728 | NS | NS | NS | NS | |||||||||||
| HCV RNA (logIU/mL) | NS | NS | NS | NS | ||||||||||||
| Fibrosis area (%) | 0.656 | < 0.0001 | NS | NS | NS | 0.296 | 0.0481 | NS | ||||||||
| R | 0.707 | 0.645 | 0.546 | 0.634 | ||||||||||||
| Adjusted R | 0.490 | 0.396 | 0.275 | 0.373 | ||||||||||||
| F | 51.800 | 20.700 | 12.700 | 14.100 | ||||||||||||
| P value | < 0.0001 | 0.0001 | 0.0012 | < 0.0001 | ||||||||||||
To elucidate the factors affecting ARFI other than fibrosis stage, patients with stage F0 or F1, those with F2, and those with F3 or F4 were analyzed separately.
Body mass index (BMI) was significantly correlated with ARFI (P = 0.0003) (Figure 1D) and ALT levels (P = 0.0593) and γ-GTP levels (P = 0.0614) tended to be correlated with ARFI by Spearman’s rank correlation test in the 31 patients with stage F0 or F1 (Table 4). Only BMI was correlated with ARFI by multiple regression analysis (β = -0.0334, P = 0.0001).
γ-GTP levels were significantly correlated with ARFI (P = 0.0013) (Figure 1E) and γ-globulin levels (P = 0.0581) tended to be correlated with ARFI in the 32 patients with stage F2 by Spearman’s rank correlation test (Table 4). The multiple regression analysis only selected γ-GTP levels as a factor correlating with ARFI (β = 0.0048, P = 0.0012).
In the patients with stage F3 or F4, fibrosis stage (P = 0.0004), platelet count (P = 0.0036), prothrombin time (INR) (P = 0.0080), albumin levels (P = 0.0015), hyaluronic acid levels (P = 0.0003) (Figure 1F), and fibrosis area (P = 0.0481) were significantly correlated with ARFI by Spearman’s rank correlation test (Table 4). The multiple regression analysis selected fibrosis stage (β = 0.2921, P = 0.0044) and hyaluronic acid levels (β = 0.0012, P = 0.0025) as factors correlating with ARFI.
The assessment of fibrosis stage is important to estimate prognosis and to identify the patients requiring antiviral treatment in chronic hepatitis C. A lot of noninvasive methods to assess liver fibrosis stage other than liver biopsy are available, for example, ARFI, TE, real-time elastography[23], and algorithm of serum fibrosis markers such as FibroTest[24] and APRI[25]. They provide good performances in estimation of fibrosis stage, while there are problems such as influence of inflammation. In the present study, factors other than fibrosis stage that affect ARFI were investigated in patients with chronic hepatitis C.
The present study confirmed findings reported previously that ARFI correlates with fibrosis stage[10-13,26,27]. The ARFI cutoff values for different fibrosis stages were 1.28 m/s for F≥ 1, 1.28 m/s for F≥ 2, 1.44 m/s for F≥ 3 and 1.73 m/s for F4. This result suggests that distinguishing between F0 and F1 is impossible, as the cutoff value for F≥ 1 and that for F≥ 2 are the same. However, Sporea et al[26] reported that the cutoff value is 1.19 m/s for F≥ 1, 1.33 m/s for F≥ 2, 1.43 m/s for F≥ 3, and 1.55 m/s for F4[26]. Rizzo et al[13] reported that the cutoff value is 1.3 m/s for F≥ 2, 1.7 m/s for F≥ 3 and 2.0 m/s for F4[13]. Thus, discrepancies are apparent among the cutoff values reported in different studies. The discrepancies are probably attributed to the difference in the population studied. Further studies should be conducted to establish standard ARFI cutoff values for staging fibrosis.
In the present study, AST, ALT and inflammatory grade were correlated with ARFI in the univariate analysis that included all patients, but were not selected as factors independently correlating with ARFI in the multiple regression analysis. In addition, inflammatory factors did not correlate with ARFI when patients with different fibrosis stages were analyzed separately. These results suggest that inflammatory activity does not affect ARFI in patients with chronic hepatitis C. Rizzo et al[13] also reported that ARFI is not associated with ALT, BMI, Metavir grade, or liver steatosis, whereas TE is significantly correlated with ALT[13]. Bota et al[10] reported that discordance of at least two fibrosis stages between ARFI and histologic assessment were associated with female sex, interquartile range interval (IQR) ≥ 30%, high AST and high ALT in univariate analysis, while, in multivariate analysis, the female gender and IQR ≥ 30% (P = 0.004) were associated with the discordances. In contrast, Yoon et al[12] reported that the optimum ARFI cutoff values are 1.13 m/s for F≥ 2 and 1.98 m/s for F4, whereas these values decreased to 1.09 m/s for F≥ 2 and 1.81 m/s for F4 when patients with normal ALT levels were selected. Chen et al[11] reported that ALT, ActiTest A score, Metavir activity (A) grade, Metavir F stage, BMI, and platelet count are independently associated with ARFI and suggested that a 100 IU/L increase in serum ALT levels augmented ARFI by approximately 0.155 m/s. In the present study, only 25 patients had ALT levels of 100 IU/L or higher. The low ALT levels among the patients studied may be a reason why ALT was not correlated with ARFI.
A multiple linear regression analysis in our previous study on TE selected fibrosis area, ALT levels, γ-GTP levels, prothrombin time, and hyaluronic acid levels as factors correlating with TE[21]. Many studies on TE have reported that LSM is affected by ALT levels. Franquelli et al[28] reported that TE fibrosis staging is overestimated by necroinflammatory activity and steatosis. Coco et al[7] found that LSM is higher in patients with an elevated ALT than in those with either spontaneous biochemical remission or after antiviral therapy. Thus, it is probable that ALT or inflammatory activity affects TE. However, it is still unclear whether they also affect ARFI. Further studies are needed to clarify factors that affect ARFI other than fibrosis stage.
ARFI was significantly correlated with BMI in the 31 patients with stage F0 or F1; the higher the BMI, the lower the ARFI. However, ARFI was not associated with steatosis grade. Motosugi et al[29] reported that fat deposition in the liver does not affect ARFI. Thus, the negative correlation between BMI and ARFI could not be attributed to steatosis, which accompanies higher BMI[30]. Actually, BMI and steatosis grade were not correlated in patients with stage F0 or F1 in the present study (data not shown). The mechanism of the association between higher BMI and lower ARFI is unclear. Because a higher BMI is associated with lower ARFI, and may cause an underestimation of fibrosis staging, careful attention should be paid to BMI during ARFI staging of fibrosis in patients with stage F0 or F1 disease.
ARFI significantly correlated with γ-GTP levels in patients with F2 and with fibrosis stage and hyaluronic acid levels in patients with stage F3 or F4. γ-GTP[24,31] and hyaluronic acid[32,33] levels have been regarded as the most informative fibrosis markers. Thus, it is reasonable that γ-GTP and hyaluronic acid levels independently correlated with ARFI.
Isgro et al[20] showed that the collagen proportional area has a better relationship with TE and with hepatic venous pressure gradient compared with Ishak stage. In the present study, fibrosis area was correlated significantly with fibrosis stage, but only fibrosis stage and hyaluronic acid levels were selected as factors independently correlating with ARFI. Our previous study demonstrated a better correlation of TE with fibrosis stage than with fibrosis area in patients with chronic hepatitis C[21]. The Metavir stages represent categories of increasing fibrosis severity based on a combination of location and quantity of scarring as well as whether the fibrous tissue forms septa, bridges, or nodules. Fibrosis area represents only the quantity of fibrosis in liver tissues. Our results indicate that not only the quantity of fibrosis but also other histological factors such as patterns of fibrosis also affect ARFI.
The present study demonstrated that ARFI correlated with fibrosis stage but was not associated with inflammation. BMI negatively correlated with ARFI in the patients with stage F0 or F1. γ-GTP and hyaluronic acid levels were positively correlated in those with stage F2 and in those with F3 or F4, respectively. Thus, careful attention should be paid to BMI, γ-GTP levels, and hyaluronic acid levels when estimating fibrosis stage by ARFI. Fibrosis stage showed a better correlation with ARFI than fibrosis area, indicating that not only the quantity of fibrosis but also other factors such as patterns of fibrosis also affect ARFI. Since the number of the patients studied is small, further studies are needed to confirm the conclusion of the present study.
The authors thank Ms. Hiroko Sugiyama, Ms. Wakana Aoyama, Ms. Ai Shibata and Ms. Shiori Kishi of the Clinical Laboratory of Medicine, Fujita Health University Hospital, and Ms. Makiko Shimazaki and Ms. Akie Tsuda of the Department of Liver, Biliary Tract, and Pancreatic Diseases, Fujita Health University for assisting with the data collection and analysis. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Most studies reported that liver stiffness measurement by Fibroscan was affected by inflammation. There have been both of the reports which demonstrated the correlation of inflammation and acoustic radiation force impulse (ARFI) and those which denied their correlation. The present study confirmed findings reported previously that ARFI correlates with fibrosis stage, and demonstrated that aspartate aminotransferase, alanine aminotransferase and inflammatory grade did not independently correlate with ARFI in the multiple regression analysis. The present study also demonstrated the correlation of body mass index (BMI) and ARFI for the first time.
The new findings of this study are the correlation of BMI and ARFI, and the denial of the correlation between ARFI and inflammation.
The results showed that ARFI correlated significantly with liver fibrosis stage and hyaluronic acid in all patients. ARFI correlated significantly with BMI in fibrosis stage F0-1, with γ-glutamyltranspeptidase (GTP) in F2, and with fibrosis stage and hyaluronic acid in F3-4. In conclusion, ARFI correlated with fibrosis stage and hyaluronic acid but not with inflammation. ARFI was affected by BMI, γ-GTP, and hyaluronic acid in each fibrosis stage.
The authors reported the utilities of ARFI elastography for evaluation of hepatic fibrosis in patients with chronic hepatitis C. This paper looks very important and has a novelty in this study field.
P- Reviewers: Kanda T, Zapata R S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1935] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 2. | Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, Kurita S, Saito Y, Iwai H, Ishii H. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol Res. 2004;29:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 4. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 6. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol. 2008;14:6154-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [PubMed] |
| 10. | Bota S, Sporea I, Sirli R, Popescu A, Jurchis A. Factors which influence the accuracy of acoustic radiation force impulse (ARFI) elastography for the diagnosis of liver fibrosis in patients with chronic hepatitis C. Ultrasound Med Biol. 2013;39:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Effects of patient factors on noninvasive liver stiffness measurement using acoustic radiation force impulse elastography in patients with chronic hepatitis C. BMC Gastroenterol. 2012;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Yoon KT, Lim SM, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Cho M, Lee JW, Kim SU. Liver stiffness measurement using acoustic radiation force impulse (ARFI) elastography and effect of necroinflammation. Dig Dis Sci. 2012;57:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1198] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 15. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 16. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3783] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 17. | Friedenberg MA, Miller L, Chung CY, Fleszler F, Banson FL, Thomas R, Swartz KP, Friedenberg FK. Simplified method of hepatic fibrosis quantification: design of a new morphometric analysis application. Liver Int. 2005;25:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Lazzarini AL, Levine RA, Ploutz-Snyder RJ, Sanderson SO. Advances in digital quantification technique enhance discrimination between mild and advanced liver fibrosis in chronic hepatitis C. Liver Int. 2005;25:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Goodman ZD, Becker RL, Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology. 2007;45:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Isgro G, Calvaruso V, Andreana L, Luong TV, Garcovich M, Manousou P, Alibrandi A, Maimone S, Marelli L, Davies N. The relationship between transient elastography and histological collagen proportionate area for assessing fibrosis in chronic viral hepatitis. J Gastroenterol. 2013;48:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Nitta Y, Kawabe N, Hashimoto S, Harata M, Komura N, Kobayashi K, Arima Y, Shimazaki H, Nakano T, Murao M. Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol Res. 2009;39:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8234] [Article Influence: 411.7] [Reference Citation Analysis (5)] |
| 23. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1037] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 25. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] |
| 26. | Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112-4118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 27. | Takaki S, Kawakami Y, Miyaki D, Nakahara T, Naeshiro N, Murakami E, Tanaka M, Honda Y, Yokoyama S, Nagaoki Y. Non-invasive liver fibrosis score calculated by combination of virtual touch tissue quantification and serum liver functional tests in chronic hepatitis C patients. Hepatol Res. 2013;Apr 10; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Motosugi U, Ichikawa T, Niitsuma Y, Araki T. Acoustic radiation force impulse elastography of the liver: can fat deposition in the liver affect the measurement of liver stiffness? Jpn J Radiol. 2011;29:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Matos CA, Perez RM, Pacheco MS, Figueiredo-Mendes CG, Lopes-Neto E, Oliveira EB, Lanzoni VP, Silva AE, Ferraz ML. Steatosis in chronic hepatitis C: relationship to the virus and host risk factors. J Gastroenterol Hepatol. 2006;21:1236-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] |
| 32. | Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 33. | McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |