Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1228
Revised: December 21, 2013
Accepted: January 3, 2014
Published online: February 7, 2014
Processing time: 132 Days and 22.5 Hours
Only a very few systematic studies have investigated the frequency of neurologic disorders in patients with Crohn’s disease (CD) and ulcerative colitis (UC), which are the two main types of inflammatory bowel disease (IBD). Results have been inconsistent and variable, owing to differences in case-finding methods and evaluated outcomes in different studies. The most frequent neurologic manifestations reported in CD and UC populations are cerebrovascular disease (with either arterial or venous events), demyelinating central nervous system disease, and peripheral neuropathy (whether axonal or demyelinating); however, the literature describes numerous nervous system disorders as being associated with IBD. The pathogenesis of nervous system tissue involvement in IBD has yet to be elucidated, although it seems to be related to immune mechanisms or prothrombotic states. The recently-introduced tumor necrosis factor (TNF) inhibitors have proven successful in controlling moderate to severe IBD activity. However, severe neurologic disorders associated with TNF inhibitors have been reported, which therefore raises concerns regarding the effect of anti-TNF-α antibodies on the nervous system. Although neurological involvement associated with IBD is rarely reported, gastroenterologists should be aware of the neurologic manifestations of IBD in order to provide early treatment, which is crucial for preventing major neurologic morbidity.
Core tip: The neurological manifestations in inflammatory bowel disease (IBD) patients are often unrecognized or underestimated. A detailed revision of the literature about the neurological manifestations in ulcerative colitis and Crohn’s disease patients is relevant to the IBD community, especially in the biologics era. Gastroenterologists should be aware of the neurologic manifestations of IBD in order to provide prevention and early treatment, which is crucial for preventing major neurologic morbidity.
- Citation: Morís G. Inflammatory bowel disease: An increased risk factor for neurologic complications. World J Gastroenterol 2014; 20(5): 1228-1237
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1228
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main types of idiopathic inflammatory bowel disease (IBD), and they are clearly distinct pathophysiological entities. UC, the most common form of IBD worldwide, is a disease of the colonic mucosa only; it is less prone to complications and can be cured with colectomy. In contrast, CD is a transmural disease of the gastrointestinal mucosa which can affect the entire gastrointestinal tract from the mouth to the anu[1,2].
CD and UC should be considered systemic diseases since they are associated with clinical manifestations involving organs outside the alimentary tract. Extraintestinal manifestations (EIMs) involving several organs, and most EIMs occurring in the joints, skin, mouth, eyes and coagulation system, either precede the onset of intestinal manifestations or appear and evolve in parallel with them. They also respond to treatment for the underlying bowel disease. However, many EIMs tend to follow a course independent from that of bowel disease activity. These EIMs are observed in some 20%-40% of patients with IBD, with CD patients being more susceptible to EIMs than patients with UC[3-8].
Neurologic involvement associated with IBD is frequently underreported. Nevertheless, it is important to quantify the morbidity burden of clinically significant neurologic complications in IBD because early recognition and treatment of neurologic diseases are crucial for preventing major morbidity[9,10]. The available literature consists of case reports and small series and only a few of them have reviewed large groups of IBD patients to identify neurologic symptoms. Moreover, most of the recent reviews dealing with UC and CD include only a brief mention of nervous system involvement in IBD[2-5].
The pathogenesis of neurogenic disorders associated with IBD has not been established and it may involve diverse causes. Most of them have an immune basis, but other reported causes include prothrombotic states, nutrient deficiency (vitamin B12, folate, copper, thiamine, vitamin E) because of malabsorption, and iatrogenic complications of medical and surgical management of IBD. In addition, in recent years, use of tumor necrosis factor (TNF) inhibitors has emerged as a successful treatment for refractory IBD, although anti-TNF-α antibodies appear to predispose some patients to developing diverse peripheral and central nervous system (CNS) involvement. An approach to neurologic symptoms in patients diagnosed with IBD has recently been reported elsewhere[11].
Neurologic involvement in IBD as a subgroup of the EIMs may precede the appearance of digestive symptoms or develop after diagnosis of IBD. In addition, neurological symptoms may exacerbate during flare-ups of IBD or evolve independently from intestinal manifestations without responding to treatment provided for the underlying bowel disease[12,13].
This review will examine current knowledge about nervous system involvement in CD and UC and the neurologic manifestations secondary to the use of biological agents and other approaches to IBD management.
Few systematic studies have investigated the frequency of neurological disorders in patients with IBD. Additionally, results from these studies have been inconsistent, which is mainly due to discrepancies in case-finding methods. In most of the studies reported, magnetic resonance imaging was not part of the standard workup. Moreover, some studies have included neurological symptoms of iatrogenic origin or symptoms caused by malabsorption-related disorders secondary to vitamin deficiencies[14,15]. Most of these studies were retrospective and register-based, and only a few included a control group[16]. Finally, the latest reports on neurologic complications in IBD disease have focused on peripheral nervous system (PNS) involvement, and they lack data on clinical manifestations involving other nervous system components[14,17-20]. Table 1 presents clinical studies addressing the frequency of neurogenic disorders in CD and UC. In a large retrospective register-based study including 638 patients with UC or CD, Lossos et al[21] found neurological involvement in 3% of the cases. In contrast, 33.2% of patients in a CD population experienced neurological or neuropsychiatric complications, although this proportion decreased to 19.3% when considering only those cases with a direct relationship[15]. Finally, in another study, 67% of patients with CD and 53% of patients with UC had neurologic disorders, although authors did not report whether IBD and neurologic involvement were coincidental[19]. Table 2 lists neurological disorders reported in the literature as being associated with IBD.
| Author | Year | Country | Study design | Total patients | Type of IBD | Patients with NC | Age (range, yr) | Sex | Other EIM | Associated with IBD exacerbation | Incidence of NC |
| Lossos et al[21] | 1995 | Israel | Retrospective, computerized search | 638 | 377 CD261 UC | 10 CD patients9 UC patients | 11-71 | 11 M8 F | 50% | 2 patients (10%) | 19 patients (3%) |
| Elsehety et al[15] | 1997 | United States | Retrospective | 253 | CD | Probable: 49 patients Possible: 35 patients Total: 84 patients | NR | NR | NR | 5 patients (6%) | Probable: 19.3% Possible: 13.9%Total: 33.2% |
| Oliveira et al[14] | 2008 | Brazil | Prospectively, clinic-based, PN especially studied | 82 | 31 CD51 UC | 16 CD patients23 UC patients | 43 CD151 UC | 15 M24 F | NR | NR | PN:51.6% CD45.1% UC Headache:54.8% CD57% UC |
| Sassi et al[23] | 2009 | Tunis | Prospective, prevalence study PN | 102 | 88 CD14 UC | 6 UC patients23 CD patients | 22-64 | 7 F2 M | 33% | 29% | 8.80% |
| Benavente et al[12] | 2011 | Spain | Retrospective, hospitalized patients | 84 | NR | 13 UC patients12 CD patients | 17-74 | 12 M13 F | 4% | 10 patients (40%) | 30% |
| Shen et al[20] | 2012 | United States | Questionnaire-based, study of PN symptoms only | 173 | 102 CD71 UC | 67 patients | NR | NR | NR | NR | 38.70% |
| Figueroa et al[18] | 2013 | United States | Retrospective, observational population based-cohort | 772 | 342 CD430 UC | 9 patients/12 events6 UC patients3 CD patients | 21-83 | 5 F4 M | NR | 8 patients | 72 patients/100000 person-years |
| Babali et al[17] | 2013 | Greece | Prospective study of subclinical PN in asymptomatic patients | 453 | 30 CD15 UC | 0 patients | NR | NR | NR | NR | No patients had subclinical neuropathy |
| Cerebrovascular disease |
| Cerebral infarction |
| Transient brain ischemia |
| Cerebral venous thrombosis |
| Demyelinating disease |
| Multiple sclerosis |
| Asymptomatic focal white-matter lesions |
| Myelopathy |
| Optic neuritis |
| Inflammatory pseudotumor |
| Epilepsy |
| Seizures |
| Psychosis |
| Chorea |
| Major depression |
| Autonomic nervous system dysfunction |
| Vasculitis of the central nervous system |
| Restless legs syndrome |
| Sleep disruption |
| Headache |
| Cranial neuropathies |
| Melkersson-Rosenthal syndrome |
| Sensorineural hearing loss |
| Ischemic optic neuropathy |
| Bell’s palsy |
| Neuromuscular diseases |
| Myasthenia gravis |
| Myopathy |
| Dermatomyositis |
| Polymyositis |
| Vacuolar myopathy |
| Peripheral neuropathy |
| Sensory large-fiber polyneuropathy |
| Small-fiber polyneuropathy |
| Acute and chronic immune-mediated neuropathies |
| Monophasic immune radiculoplexus neuropathy |
| Chronic distal sensorimotor polyneuropathy |
| Mononeuritis multiplex |
Peripheral neuropathy (PN) is known to be related to IBD and it is one of the most frequently reported neurologic complications. Various studies have found PNS complications, rather than CNS involvement, to be predominant[14]. Initially, medical treatments for the gastrointestinal disease or vitamin deficiencies caused by malabsorption were thought to cause PN[21]. Ultimately, PN is a common adverse event associated with use of TNF inhibitors[22]. If these causes of neuropathy are excluded, however, the reported frequency of PN in IBD will vary greatly among published studies, with estimates ranging from 0% to 39% due to selection bias, use of different definitions of the disease, or population characteristics[14,15,18,20,21,23].
Two recent studies have indicated the low prevalence of PN in IBD. In the first study, carried out in Greece, 97 patients with any form of IBD were studied to identify any cases of PN. Fifty-two patients were excluded for different reasons, including presence of comorbidities associated with PN and presence of other neurologic symptoms. Nerve conduction studies in the remaining 45 asymptomatic patients yielded normal results except for one patient with a history of acute motor sensory polyneuropathy complicating UC and another patient with incidental carpal tunnel syndrome[17]. A second study in Olmsted County, Minnesota, determined the neuropathy incidence rate in a population-based cohort of patients newly diagnosed with IBD over a 64-year period. The overall incidence rate was 72 cases of neuropathy per 100000 IBD person-years with a cumulative incidence rate after 30 years of 2.4%. Moreover, the study found late occurrences of neuropathy in the course of IBD, mainly during IBD inactivity[18].
Regarding the type of neuropathy reported, studies describe demyelinating or axonal involvement of peripheral nerves in IBD, and both neuropathies may be acute or chronic. Several cases of immune-mediated neuropathies in IBD patients have also been reported[24-26]. In a retrospective review of patients with PN and IBD, more than two-thirds of the cases had axonal neuropathy. Neuropathies were predominantly sensory (either small-fiber or large-fiber) rather than sensorimotor for axonal neuropathies; only one-third of patients developed demyelinating forms of neuropathy[19]. Conversely, the clinical spectrum reported by Figueroa et al[18] consisted of monophasic immune radiculoplexus neuropathy and chronic distal sensorimotor polyneuropathy. The underlying pathophysiology in cases of neuropathy in IBD patients remains obscure. T-cells are clearly involved in the pathogenesis of demyelinating neuropathies. At the same time, the relationship between axonal damage and immune system disturbances remains unclear, but clinical improvement in patients treated with immunomodulatory agents suggests that there is a link[19].
Thromboembolic complications are two to four times more likely in patients with IBD than in healthy individuals; they occur at any age in both sexes and active IBD may increase the relative risk of such complications by as much as 15-fold[27]. The incidence of thromboembolism in IBD ranges between 1% and 7.7% in clinical studies, with postmortem studies reporting rates of 40%[28]. Deep vein thrombosis and pulmonary embolism are the most common entities, but cerebrovascular disorders also occur and they are probably underestimated[29]. In a meta-analysis published in 2013, CD and UC were found to be associated with an increase in the risk of ischemic or hemorrhagic stroke or transient ischemic attack (OR = 1.28, 95%CI: 1.17-1.41), especially among women and young patients[30]. Strokes may affect either arterial or venous territories, but it is unclear whether venous or arterial strokes are more frequent[31,32].
The literature describes cerebral infarction and transient brain ischemia due to small- and large-artery disease involving the anterior and posterior circulation. Carotid arterial thrombosis, retinal branch artery occlusion, carotid thromboembolism, cardiac embolism, and paradoxical embolism are other possible pathogeneses[33-36].
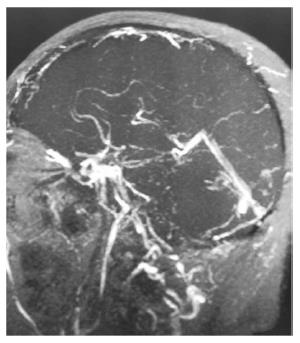
IBD accounts for 1%-6% of the total causes of cerebral venous thrombosis (CVT), and CVT develops without there being a temporal relationship with the course of IBD (Figure 1). There are no appreciable differences between IBD-related CVT and non-IBD related CVT in terms of clinical or radiological characteristics, prognosis, or treatment[37-39].
The reason for the increased rate of thromboembolic events in patients with IBD remains uncertain, but is most likely related to the interaction between acquired and genetic risk factors. Researchers have also reported interaction between cytokine mediators of chronic inflammation and the coagulation cascade[40].
Some genetic factors listed as possible promoters of the thrombotic manifestations of IBD include factor V Leiden and factor II, methylenetetrahydrofolate reductase gene mutation, plasminogen activator inhibitor type 1 gene mutation, and factor XIII[28]. However, no studies have convincingly demonstrated that IBD patients have a greater burden of prothrombotic genetic or non-genetic risk factors, such as factor V Leiden mutations, hyperhomocysteinemia, antiphospholipid antibodies, or thrombophilia, than the general population[41-43]. Moreover, genetic risk factors are generally not found more often in IBD patients than in the general population. However, when such factors are present, patients with IBD are more likely than healthy controls to suffer thromboembolic complications[28].
In addition to platelet and endothelial activation, some of the most often-cited impaired coagulation states are high levels of factor V, factor VIII, fibrinogen, and von Willebrand factor. In addition, low activity and low concentration of XIII factor subunit A have been detected. This decreased activity inversely correlates with intestinal inflammatory activity and fibrinogen levels[44]. In addition, IBD patients display decreased levels of protein C, protein S, antithrombin III, and tissue factor pathway inhibitor (all of which function as anticoagulants), and decreased levels of tissue plasminogen activator with increased plasminogen activator inhibitor-1; the above are all key players in the fibrinolysis pathway[36]. Finally, other mechanisms that may explain cerebrovascular disorders in IBD include vasculitis and consumption coagulopathy leading to hemorrhagic events[21]. Based on these data, recent studies have suggested that IBD may be an independent risk factor for thromboembolic disorders[27,36,45].
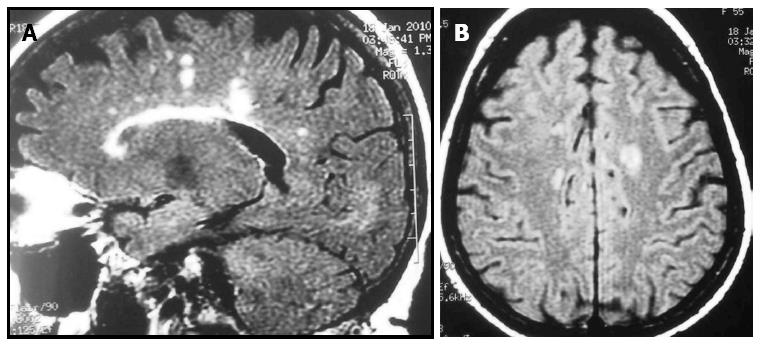
The relationship between multiple sclerosis (MS) and IBD was discovered by Rang et al[46] in the early 1980s when they detected high MS incidence and prevalence while studying patients who had undergone a colectomy for IBD (Figure 2). Retrospective cross-sectional studies have reported an increased incidence of demyelinating disease[16]; however, incidence of MS was reported to be higher in patients with UC than with CD[47]. In addition, one study found a high prevalence of asymptomatic focal white-matter brain lesions in IBD patients[48] but these data not have been confirmed by more recent studies[49]. Moreover, the incidence of white matter hyperintensities on T2-weighted images was found to be higher in CD patients (72%) than in age-matched controls (34%); however, this population was not completely asymptomatic and 20% of the CD patients were on TNF inhibitors.
Several studies have documented additional autoimmune diseases, including IBD, in patients with MS and their relatives; however, results were disparate due to differences in study design[50,51]. In a Danish study, Nielsen et al[52] found that MS patients were at a higher risk for developing UC and other autoimmune diseases, but not CD. This hypothesis is supported by a recent systematic review and meta-analysis. Here, the authors reported a significant relationship between MS and IBD (OR = 1.56, 95%CI: 1.28-1.90), although this association was not found in relatives of MS patients[53].
There may be a significant relationship between MS and IBD. This possibility is supported by the role played by immune mechanisms in the pathogenesis of both disorders and by fact that immune system inhibitors that impair cell-mediated immunity are effective for treating both MS and IBD. Disturbances in functional T-cell subsets as well as in antigen-presenting cells have also been implicated. Studies suggest that aberrant proinflammatory activity in particular may be a common pathway leading to the destruction of target tissue in both diseases[3-5].
Studies have reported an association between epilepsy and IBD; however, the possible relationship between epilepsy and IBD has not been completely addressed[12,15,21]. Furthermore, seizures may be symptoms of systemic or brain processes such as hypomagnesemia or cerebral venous thrombosis[54,55]. Vasculitis of the CNS and transverse myelitis associated with Jo-1 antibody syndrome are additional complications that have been described in IBD patients[56-58].
In a prospective multicenter study in patients with CD, incidence of restless legs syndrome (RLS) was 43% and prevalence was 30%. This prevalence rate exceeds that of the general population, and the incidence figure is higher than the incidence of many known EIMs of CD. Furthermore, RLS symptoms occurred during or after the onset of CD symptoms in most patients, suggesting a link between CD and RLS[59]. Recent studies have shown that sleep disorders are more common in IBD patients, and treating sleep disruption with a melatonin supplement has been shown to improve sleep in animal models of immune colitis[60].
Some authors suggest that the autonomic nervous system plays a role in the pathogenesis of IBD and that IBD may be a potential neurologic complication of chronic inflammation. On the other hand, autonomic dysfunctions have been reported in patients with IBD; although earlier reports suggested autonomic hypofunction, later studies also identified autonomic hyperreflexia. Sympathetic autonomic neuropathy is an early systemic feature of CD and one that is independent from disease activity. Moreover, clinically manifest autonomic dysfunction is associated with lower quality of life and more intensive healthcare use in IBD patients[61-63].
Sensorineural hearing loss (SNHL), first described in 1982, is currently a well-recognized EIM of UC[64]. In a study of 38 patients with a history of IBD, 59% had inner ear dysfunction that was probably related to gastrointestinal disease. Of the 19 patients with hearing loss (14 patients had UC and 5 had CD), 16 patients had bilateral SNHL; unfortunately, only one patient experienced clinical improvement following medical treatment[65]. The pathogenesis of SNHL associated with UC is not fully understood, and several immune mechanisms may lead to inner ear dysfunction[66]. Bilateral anterior optic neuritis and ischemic optic neuropathy due to UC and CD have been described[67,68]. Lastly, researchers have reported peripheral facial palsy and Melkersson-Rosenthal syndrome associated with CD[69].
Furthermore, myasthenia gravis (MG), whether in its ocular or generalized form, has been reported in association with both UC and CD. Intriguingly, two case reports of patients with MG and IBD describe clinical improvement of neuromuscular disease and IBD following different surgical procedures, one after thymectomy and the other following proctocolectomy. These reports emphasize the immunological link between MG and IBD[21,70-74]. In addition to MG, cases of IBD associated with polymyositis or dermatomyositis have also been described[75-77].
TNF inhibitors are currently approved as treatment for IBD, including both CD and UC. The proinflammatory cytokine TNF-α has been identified as playing a pivotal role in the inflammatory cascade that causes chronic intestinal inflammation in IBD. Synthetic anti-TNF-α antibodies have been shown to mitigate this inflammatory process. Infliximab was the first TNF inhibitor successfully used to treat IBD. Other drugs with demonstrated efficacy as IBD treatments include adalimumab and golimumab (humanized monoclonal antibodies), and certolizumab pegol (humanized anti-TNF-α antibody Fab’ fragment conjugated with a polyethylene glycol molecule). Conversely, etanercept (non-antibody soluble recombinant TNF receptor-Fc fusion protein) is not an effective treatment for IBD[78].
Since TNF inhibitors were first used in 1996[79], researchers have reported an increased risk of CNS events (new onset or exacerbation of demyelinating events, including optic neuritis and MS); in addition, PNs have also been reported in small clinical series. Information regarding management and clinical significance of such events is very limited because the incidence of neurologic events is estimated at less than 1 event per 1000 patients during TNF-inhibitor treatment[78,80-82].
The FDA’s most recent report on adverse events related to TNF-α inhibitors from dates to December 31, 2009. Here, the second-highest number of reported adverse events, after patients with rheumatoid arthritis, was in patients with IBD (140 cases, 18.1% of the total reports). These figures show that IBD may be a risk factor for neurologic disease. Most of the cases involving infliximab and adalimumab are linked to PN (42%), followed by CNS or spinal demyelination (17.2%), optic neuritis (17.4%) and facial palsy (7.8%). In addition, authors reported 19 patients diagnosed with transverse myelitis, leukoencephalopathy, unspecified demyelinating disease, encephalopathy, meningitis due to Listeria, or right cerebellar neuroglial cyst. Last of all, one case of PN and another case of CNS demyelination associated with certolizumab (17.2%) have also been reported[22]. These data are consistent with results from the BIOEGAS registry of the Spanish Society of Internal Medicine which reported a total of 175 cases of central demyelinating processes and 44 cases of PNs resulting from TNF-inhibitor therapy as of July 2009[83]. Recent studies have highlighted the development of posterior reversible encephalopathy syndrome in pediatric patients with IBD treated with infliximab[84]. Although several hypotheses have been proposed in an attempt to explain the possible relationship between TNF inhibitor treatment and CNS events, none is considered to be completely satisfactory. Some authors propose that systemic administration of TNF-α inhibitors may enhance different functions of the immune system which are known to activate demyelinating processes[85-87]. However, other authors prefer to seek genetic connections, such as haplotypes of TNF-α or signal transducer and activator of transcription 3 (STAT3), which could explain the emergence of neurologic events in IBD patients treated with TNF inhibitors[88-90].
Regarding PNS disease, most reported types of neuropathy involve demyelination. Cases of Guillain-Barré syndrome account for the majority, but cases of multifocal motor neuropathy with conduction block, chronic inflammatory demyelinating polyradiculoneuropathy, and Lewis-Summer syndrome have also been reported. Moreover, mononeuropathy multiplex and axonal sensorimotor polyneuropathies have been described, which adds an axonal process to the physiopathology of nervous system damage elicited by anti-TNF agents[83,91,92]. The proposed pathogenesis of PNs associated with TNF inhibitors includes a T-cell and humoral immune attack against peripheral nerve myelin, vasculitis-induced nerve ischemia, and inhibition of signaling support for axons[91].
Prognosis in cases of TNF inhibitor-induced neurologic events is usually good if the treatment is discontinued. Gastroenterologists should therefore be mindful of the neurologic complications of biological treatments in order to recognize them immediately; neurological consultations may play a key role in the assessment of these patients[11,22]. In light of the data reported here, use of TNF inhibitors should be avoided in patients diagnosed with PN or MS.
Natalizumab is an IgG4/κ humanized monoclonal antibody which interferes with the interaction between very late antigen-4, expressed on leukocytes, and vascular adhesion molecule-1, expressed on endothelial cells, thus preventing leukocyte extravasation in inflamed sites[93]. Since natalizumab’s mechanism of action differs from that of other biological agents, it represents an important therapeutic option for CD patients who cannot tolerate other treatments or who are experiencing decreased treatment efficacy, particularly when TNF inhibitor treatment does not achieve remission.
The main neurologic adverse event in patients with CD treated with natalizumab is progressive multifocal leukoencephalopathy (PML). The incidence reported in these patients is 2.13 out of 1000 patients. However, it seems possible to stratify risk of PML among natalizumab-treated patients given that risk mainly tends to be higher in cases of more than 2 years of natalizumab therapy, positivity for anti-JC virus antibodies, and combined use of natalizumab and immunosuppressive drugs[94]. No cases of PML were reported in three recent reports including 154 CD patients on natalizumab[95-97].
Cyclosporine is a well-recognized treatment for acute severe UC. Between 10% and 28% of the patients on cyclosporine develop neurotoxicity. The most common associated CNS disorder is a postural tremor that affects the upper extremities and responds to beta-blockers. Severe symptoms affect up to 5% of patients and include psychoses, hallucinations, blindness, seizures, cerebellar ataxia, motor weakness and leukoencephalopathy[98-100].
A pure sensory or autonomic neuropathy is a well-known PNS complication following metronidazole treatment. The neuropathy usually resolves completely once the antibiotic is discontinued[101].
Sulfasalazine (sulphasalazopyrine) is prescribed as treatment for IBD due to its immunomodulatory action. Reported serious adverse reactions include transverse myelitis and encephalopathy[102,103].
Finally, it is well known that psychiatric symptoms can develop in association with administration of corticosteroids in IBD patients, although some authors have reported unusual cases of psychotic episodes in UC and CD patients who were not receiving steroids[104-108].
In conclusion, reports about the incidence and type of neurologic diseases associated with CD and UC are controversial. Therefore, prospective studies are needed to clarify these points, as well as basic studies addressing the multiple mechanisms responsible for nervous system damage.
P- Reviewers: Caccaro R, Sachar DB, Tsai HH S- Editor: Cui XM L- Editor: A E- Editor: Ma S
| 1. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1508] [Article Influence: 83.8] [Reference Citation Analysis (2)] |
| 2. | Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 915] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 3. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2201] [Article Influence: 137.6] [Reference Citation Analysis (6)] |
| 4. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1529] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 5. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1544] [Article Influence: 118.8] [Reference Citation Analysis (5)] |
| 6. | Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 510] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol. 2011;17:2702-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227-7236. [PubMed] |
| 9. | Scheid R, Teich N. Neurologic manifestations of ulcerative colitis. Eur J Neurol. 2007;14:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wills AJ, Pengiran Tengah DS, Holmes GK. The neurology of enteric disease. J Neurol Neurosurg Psychiatry. 2006;77:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Singh S, Kumar N, Loftus EV, Kane SV. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis. 2013;19:864-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Benavente L, Morís G. Neurologic disorders associated with inflammatory bowel disease. Eur J Neurol. 2011;18:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Zois CD, Katsanos KH, Kosmidou M, Tsianos EV. Neurologic manifestations in inflammatory bowel diseases: current knowledge and novel insights. J Crohns Colitis. 2010;4:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Oliveira GR, Teles BC, Brasil EF, Souza MH, Furtado LE, de Castro-Costa CM, Rola FH, Braga LL, Gondim Fde A. Peripheral neuropathy and neurological disorders in an unselected Brazilian population-based cohort of IBD patients. Inflamm Bowel Dis. 2008;14:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Elsehety A, Bertorini TE. Neurologic and neuropsychiatric complications of Crohn’s disease. South Med J. 1997;90:606-610. [PubMed] |
| 16. | de Lau LM, de Vries JM, van der Woude CJ, Kuipers EJ, Siepman DA, Sillevis Smitt PA, Hintzen RQ. Acute CNS white matter lesions in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Babali A, Terzoudi A, Vadikolias K, Souftas V, Kleitsas K, Pitiakoudis M, Piperidou H, Lirantzopoulos N, Kouklakis G. Peripheral neuropathy electrophysiological screening in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2013;25:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Figueroa JJ, Loftus EV, Harmsen WS, Dyck PJ, Klein CJ. Peripheral neuropathy incidence in inflammatory bowel disease: a population-based study. Neurology. 2013;80:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Gondim FA, Brannagan TH, Sander HW, Chin RL, Latov N. Peripheral neuropathy in patients with inflammatory bowel disease. Brain. 2005;128:867-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Shen TC, Lebwohl B, Verma H, Kumta N, Tennyson C, Lewis S, Scherl E, Swaminath A, Capiak KM, DiGiacomo D. Peripheral neuropathic symptoms in celiac disease and inflammatory bowel disease. J Clin Neuromuscul Dis. 2012;13:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Lossos A, River Y, Eliakim A, Steiner I. Neurologic aspects of inflammatory bowel disease. Neurology. 1995;45:416-421. [PubMed] |
| 22. | Deepak P, Stobaugh DJ, Sherid M, Sifuentes H, Ehrenpreis ED. Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther. 2013;38:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Sassi SB, Kallel L, Ben Romdhane S, Boubaker J, Filali A, Hentati F. Peripheral neuropathy in inflammatory bowel disease patients: a prospective cohort study. Scand J Gastroenterol. 2009;44:1268-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | de la Torre RG, Morís G, Martínez DP, Montes IC. Guillain-Barré syndrome, tuberculosis and inflammatory bowel disease: a multiple association. Int Arch Med. 2010;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Boylu E, Toğrol E, Doğan T, Saraçoğlu M. Crohn disease and chronic inflammatory demyelinating polyneuropathy; a case report. Electromyogr Clin Neurophysiol. 2010;50:181-185. [PubMed] |
| 26. | Krystallis CS, Kamberoglou DK, Cheilakos GB, Maltezou MN, Tzias VD. Guillain-Barré syndrome during a relapse of ulcerative colitis: a case report. Inflamm Bowel Dis. 2010;16:555-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zitomersky NL, Levine AE, Atkinson BJ, Harney KM, Verhave M, Bousvaros A, Lightdale JR, Trenor CC. Risk factors, morbidity, and treatment of thrombosis in children and young adults with active inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Tsiolakidou G, Koutroubakis IE. Thrombosis and inflammatory bowel disease-the role of genetic risk factors. World J Gastroenterol. 2008;14:4440-4444. [PubMed] |
| 29. | Koutroubakis IE. Therapy insight: Vascular complications in patients with inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 30. | Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of Cerebrovascular Accidents and Ischemic Heart Disease in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2013;Aug 24; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Sibai M, El Moutawakil B, Chourkani N, Bourezgui M, Rafai MA, Slassi I. [Neurological manifestations of chronic inflammatory bowel disease]. Rev Neurol (Paris). 2008;164:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Bermejo PE, Burgos A. [Neurological complications of inflammatory bowel disease]. Med Clin (Barc). 2008;130:666-675. [PubMed] |
| 33. | Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Nogami H, Iiai T, Maruyama S, Tani T, Hatakeyama K. Common carotid arterial thrombosis associated with ulcerative colitis. World J Gastroenterol. 2007;13:1755-1757. [PubMed] |
| 36. | Zitomersky NL, Verhave M, Trenor CC. Thrombosis and inflammatory bowel disease: a call for improved awareness and prevention. Inflamm Bowel Dis. 2011;17:458-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1414] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 38. | Cognat E, Crassard I, Denier C, Vahedi K, Bousser MG. Cerebral venous thrombosis in inflammatory bowel diseases: eight cases and literature review. Int J Stroke. 2011;6:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Rodríguez S, Calleja S, Morís G. Cluster-like headache heralding cerebral venous thrombosis. Cephalalgia. 2008;28:906-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Guédon C, Le Cam-Duchez V, Lalaude O, Ménard JF, Lerebours E, Borg JY. Prothrombotic inherited abnormalities other than factor V Leiden mutation do not play a role in venous thrombosis in inflammatory bowel disease. Am J Gastroenterol. 2001;96:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Oussalah A, Guéant JL, Peyrin-Biroulet L. Meta-analysis: hyperhomocysteinaemia in inflammatory bowel diseases. Aliment Pharmacol Ther. 2011;34:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Aichbichler BW, Petritsch W, Reicht GA, Wenzl HH, Eherer AJ, Hinterleitner TA, Auer-Grumbach P, Krejs GJ. Anti-cardiolipin antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1999;44:852-856. [PubMed] |
| 44. | Vrij AA, Rijken J, van Wersch JW, Stockbrügger RW. Coagulation and fibrinolysis in inflammatory bowel disease and in giant cell arteritis. Pathophysiol Haemost Thromb. 2003;33:75-83. [PubMed] |
| 45. | Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Rang EH, Brooke BN, Hermon-Taylor J. Association of ulcerative colitis with multiple sclerosis. Lancet. 1982;2:555. [PubMed] |
| 47. | Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Geissler A, Andus T, Roth M, Kullmann F, Caesar I, Held P, Gross V, Feuerbach S, Schölmerich J. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet. 1995;345:897-898. [PubMed] |
| 49. | Dolapcioglu C, Guleryuzlu Y, Uygur-Bayramicli O, Ahishali E, Dabak R. Asymptomatic brain lesions on cranial magnetic resonance imaging in inflammatory bowel disease. Gut Liver. 2013;7:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 50. | Ramagopalan SV, Dyment DA, Valdar W, Herrera BM, Criscuoli M, Yee IM, Sadovnick AD, Ebers GC. Autoimmune disease in families with multiple sclerosis: a population-based study. Lancet Neurol. 2007;6:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Sadovnick AD, Paty DW, Yannakoulias G. Concurrence of multiple sclerosis and inflammatory bowel disease. N Engl J Med. 1989;321:762-763. [PubMed] |
| 52. | Nielsen NM, Frisch M, Rostgaard K, Wohlfahrt J, Hjalgrim H, Koch-Henriksen N, Melbye M, Westergaard T. Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: a nationwide cohort study in Denmark. Mult Scler. 2008;14:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Dobson R, Giovannoni G. Autoimmune disease in people with multiple sclerosis and their relatives: a systematic review and meta-analysis. J Neurol. 2013;260:1272-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Menon B, Goyal R, Nihal L, Reddy R. Cerebral venous thrombosis in ulcerative colitis. J Neurosci Rural Pract. 2013;4:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Fernández-Rodríguez E, Camarero-González E. [Patient with Crohn’s disease and seizures due to hypomagnesemia]. Nutr Hosp. 2007;22:720-722. [PubMed] |
| 56. | Schluter A, Krasnianski M, Krivokuca M, Spielmann RP, Neudecker S, Hirsch W. Magnetic resonance angiography in a patient with Crohn’s disease associated cerebral vasculitis. Clin Neurol Neurosurg. 2004;106:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Pandian JD, Henderson RD, O’Sullivan JD, Rajah T. Cerebral vasculitis in ulcerative colitis. Arch Neurol. 2006;63:780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Ray DW, Bridger J, Hawnaur J, Waldek S, Bernstein RM, Dornan TL. Transverse myelitis as the presentation of Jo-1 antibody syndrome (myositis and fibrosing alveolitis) in long-standing ulcerative colitis. Br J Rheumatol. 1993;32:1105-1108. [PubMed] |
| 59. | Weinstock LB, Bosworth BP, Scherl EJ, Li E, Iroku U, Munsell MA, Mullen GE, Walters AS. Crohn’s disease is associated with restless legs syndrome. Inflamm Bowel Dis. 2010;16:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. 2011;7:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 61. | Ananthakrishnan AN, Issa M, Barboi A, Jaradeh S, Zadvornova Y, Skaros S, Johnson K, Otterson MF, Binion DG. Impact of autonomic dysfunction on inflammatory bowel disease. J Clin Gastroenterol. 2010;44:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Boissé L, Chisholm SP, Lukewich MK, Lomax AE. Clinical and experimental evidence of sympathetic neural dysfunction during inflammatory bowel disease. Clin Exp Pharmacol Physiol. 2009;36:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Ohlsson B, Sundkvist G, Lindgren S. Subclinical sympathetic neuropathy appears early in the course of Crohn’s disease. BMC Gastroenterol. 2007;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Summers RW, Harker L. Ulcerative colitis and sensorineural hearing loss: is there a relationship? J Clin Gastroenterol. 1982;4:251-252. [PubMed] |
| 65. | Karmody CS, Valdez TA, Desai U, Blevins NH. Sensorineural hearing loss in patients with inflammatory bowel disease. Am J Otolaryngol. 2009;30:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Morís G, Milla A, Ribacoba R, González C. Acute deafness as an extraintestinal manifestation of ulcerative colitis. Eur J Intern Med. 2005;16:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Romero Aroca P, Salvat Serra M, Perena Soriano F, Martínez Salcedo I. [Anterior optic neuritis do to ulcerative colitis]. Arch Soc Esp Oftalmol. 2001;76:189-191. [PubMed] |
| 68. | Heuer DK, Gager WE, Reeser FH. Ischemic optic neuropathy associated with Crohn's disease. J Clin Neuroophthalmol. 1982;2:175-181. [PubMed] |
| 69. | Lloyd DA, Payton KB, Guenther L, Frydman W. Melkersson-Rosenthal syndrome and Crohn’s disease: one disease or two? Report of a case and discussion of the literature. J Clin Gastroenterol. 1994;18:213-217. [PubMed] |
| 70. | Foroozan R, Sambursky R. Ocular myasthenia gravis and inflammatory bowel disease: a case report and literature review. Br J Ophthalmol. 2003;87:1186-1187. [PubMed] |
| 71. | McCann P, Pramanik A. Dysphagia and unexpected myasthenia gravis associated with primary biliary cirrhosis, ulcerative colitis and vitiligo. J Am Geriatr Soc. 2004;52:1407-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Finnie IA, Shields R, Sutton R, Donnelly R, Morris AI. Crohn’s disease and myasthenia gravis: a possible role for thymectomy. Gut. 1994;35:278-279. [PubMed] |
| 73. | Martin RW, Shah A. Myasthenia gravis coexistent with Crohn’s disease. J Clin Gastroenterol. 1991;13:112-113. [PubMed] |
| 74. | Gower-Rousseau C, Reumaux D, Bellard M, Delecourt L, Ribet M, Colombel JF. Remission of myasthenia gravis after proctocolectomy in a patient with ulcerative colitis. Am J Gastroenterol. 1993;88:1136-1138. [PubMed] |
| 75. | Szabo N, Lukacs S, Kulcsar I, Gunasekera W, Nagy-Toldi A, Dezso B, Danko K. Association of idiopathic inflammatory myopathy and Crohn’s disease. Clin Rheumatol. 2009;28:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Leibowitz G, Eliakim R, Amir G, Rachmilewitz D. Dermatomyositis associated with Crohn’s disease. J Clin Gastroenterol. 1994;18:48-52. [PubMed] |
| 77. | Hayashi T, Nakamura T, Kurachi K, Asai Y, Nakajima A, Suzuki S, Konno H. Ulcerative colitis accompanied with sarcoidosis and dermatomyositis: report of a case. Dis Colon Rectum. 2008;51:474-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 79. | van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, Woody JN, Hartung HP, Polman CH. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531-1534. [PubMed] |
| 80. | Faivre A, Franques J, De Paula AM, Gutierrez M, Bret S, Aubert S, Attarian S, Pouget J. Acute motor and sensory axonal neuropathy and concomitant encephalopathy during tumor necrosis factor-alpha antagonist therapy. J Neurol Sci. 2010;291:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Carrilho PE, Araújo AC, Alves O, Kotze PG. Motor neuropathy with multiple conduction blocks associated with TNF-alpha antagonist. Arq Neuropsiquiatr. 2010;68:452-454. [PubMed] |
| 82. | Lozeron P, Denier C, Lacroix C, Adams D. Long-term course of demyelinating neuropathies occurring during tumor necrosis factor-alpha-blocker therapy. Arch Neurol. 2009;66:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Ramos-Casals M, Roberto-Perez-Alvarez C, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 84. | Haddock R, Garrick V, Horrocks I, Russell RK. A case of posterior reversible encephalopathy syndrome in a child with Crohn’s disease treated with Infliximab. J Crohns Colitis. 2011;5:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev. 2014;13:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 86. | Fromont A, De Seze J, Fleury MC, Maillefert JF, Moreau T. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Cohen M, Baldin B, Thomas P, Lebrun C. [Neurological adverse events under anti-TNF alpha therapy]. Rev Neurol (Paris). 2012;168:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Cénit MC, Alcina A, Márquez A, Mendoza JL, Díaz-Rubio M, de las Heras V, Izquierdo G, Arroyo R, Fernández O, de la Concha EG. STAT3 locus in inflammatory bowel disease and multiple sclerosis susceptibility. Genes Immun. 2010;11:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Jakkula E, Leppä V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, Purcell S, Koivisto K, Tienari P, Sumelahti ML. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Genet. 2010;86:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 90. | Matesanz F, González-Pérez A, Lucas M, Sanna S, Gayán J, Urcelay E, Zara I, Pitzalis M, Cavanillas ML, Arroyo R. Genome-wide association study of multiple sclerosis confirms a novel locus at 5p13.1. PLoS One. 2012;7:e36140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Stübgen JP. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve. 2008;37:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 92. | Burger DC, Florin TH. Peripheral neuropathy with infliximab therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Diotti RA, Nakanishi A, Clementi N, Mancini N, Criscuolo E, Solforosi L, Clementi M. JC polyomavirus (JCV) and monoclonal antibodies: friends or potential foes? Clin Dev Immunol. 2013;2013:967581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 909] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 95. | Juillerat P, Wasan SK, Fowler SA, Friedman S, Pabby VK, Coukas JA, Barto AE, Pellish R, Germansky KA, Cheifetz AS. Efficacy and safety of natalizumab in Crohn’s disease patients treated at 6 Boston academic hospitals. Inflamm Bowel Dis. 2013;19:2457-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Kane SV, Horst S, Sandborn WJ, Becker B, Neis B, Moscandrew M, Hanson KA, Tremaine WJ, Bruining DH, Faubion WA. Natalizumab for moderate to severe Crohn’s disease in clinical practice: the Mayo Clinic Rochester experience. Inflamm Bowel Dis. 2012;18:2203-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Sakuraba A, Keyashian K, Correia C, Melek J, Cohen RD, Hanauer SB, Rubin DT. Natalizumab in Crohn’s disease: results from a US tertiary inflammatory bowel disease center. Inflamm Bowel Dis. 2013;19:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol. 1999;246:339-346. [PubMed] |
| 99. | Durai D, Hawthorne AB. Review article: how and when to use ciclosporin in ulcerative colitis. Aliment Pharmacol Ther. 2005;22:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Sternthal MB, Murphy SJ, George J, Kornbluth A, Lichtiger S, Present DH. Adverse events associated with the use of cyclosporine in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Boyce EG, Cookson ET, Bond WS. Persistent metronidazole-induced peripheral neuropathy. DICP. 1990;24:19-21. [PubMed] |
| 102. | Schoonjans R, Mast A, Van Den Abeele G, Dewilde D, Achten E, Van Maele V, Pauwels W. Sulfasalazine-associated encephalopathy in a patient with Crohn’s disease. Am J Gastroenterol. 1993;88:1416-1420. [PubMed] |
| 103. | Olenginski TP, Harrington TM, Carlson JP. Transverse myelitis secondary to sulfasalazine. J Rheumatol. 1991;18:304. [PubMed] |
| 104. | Alcena V, Alexopoulos GS. Ulcerative colitis in association with chronic paranoid schizophrenia: a review of steroid-induced psychiatric disorders. J Clin Gastroenterol. 1985;7:400-404. [PubMed] |
| 105. | Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289-294. [PubMed] |
| 106. | Mullen RS, Romans-Clarkson SE. Behavioural sensitisation and steroid-induced psychosis. Br J Psychiatry. 1993;162:549-551. [PubMed] |
| 107. | Triantafillidis JK, Vagianos K, Rontos I. Psychotic reaction as a cardinal first clinical manifestation in a patient with Crohn’s disease. J Crohns Colitis. 2013;7:e76-e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Reimer J, Fink T, Bläker M, Schäfer I, Otte C. Successful treatment of psychosis with infliximab in a patient with Crohn’s disease. Schizophr Res. 2009;109:194-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |