Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18413
Revised: September 15, 2014
Accepted: October 21, 2014
Published online: December 28, 2014
Processing time: 303 Days and 23 Hours
AIM: To investigate the clinical efficacy and toxic effects of neoadjuvant chemotherapy using docetaxel combined with oxaliplatin and fluorouracil for treating stage III/IV gastric cancer.
METHODS: A total of 53 stage III/IV gastric cancer patients were enrolled into the study and treated with neoadjuvant chemotherapy. Two of the cases were excluded. The program was as follows: 75 mg/m2 docetaxel and 85 mg/m2 oxaliplatin on day 1 and 1500 mg/m2 fluorouracil on days 1 to 3 for three weeks.
RESULTS: The tumour changes, postoperative remission rate, changes in the symptoms and adverse reactions were observed. The overall clinical efficacy (complete remission + partial remission) of the neoadjuvant chemotherapy was 62.7%. R0 radical resection was performed on 60.8% of the patients, with a remission rate (pathological complete response + pathological subtotal response + pathological partial response) of 74.2%. The Karnofksy score improved in 42 cases. The toxicity reactions mostly included myelosuppression, followed by gastrointestinal mucosal lesions, nausea, vomiting and diarrhoea.
CONCLUSION: Neoadjuvant chemotherapy consisting of docetaxel combined with oxaliplatin and fluorouracil is effective for stage III/IV gastric cancer. However, the treatment is associated with a high incidence of bone marrow suppression, which should be managed clinically.
Core tip: This study analysed the combination of docetaxel, oxaliplatin and fluorouracil in the neoadjuvant chemotherapy towards stage III/IV gastric cancer patients, and observed the changes of tumours after chemotherapy, as well as the surgical resection rate, postoperative pathological remission degree, clinical symptom remission degree and adverse reactions, aiming to verify the effectiveness and safety of this regimen in clinical applications.
- Citation: Yu YJ, Sun WJ, Lu MD, Wang FH, Qi DS, Zhang Y, Li PH, Huang H, You T, Zheng ZQ. Efficacy of docetaxel combined with oxaliplatin and fluorouracil against stage III/IV gastric cancer. World J Gastroenterol 2014; 20(48): 18413-18419
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18413.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18413
Gastric cancer has the second highest mortality rate in the world, and China has a high incidence of gastric cancer, with the highest mortality rate among all malignant cancers[1,2]. The five-year survival rate after the early radical resection of gastric cancer exceeds 90%. Since the majority of Chinese patients are diagnosed in the advanced stage, surgery alone is insufficient for treatment. The overall five-year survival rate is approximately 20%-25%. In recent years, studies on the pathogenesis of gastric cancer have changed the treatment methods from simple surgery to individualised chemotherapy. Comprehensive treatments are currently advocated for advanced gastric cancer, including neoadjuvant chemotherapy, standardised surgery, postoperative adjuvant chemoradiotherapy, and targeted therapy, among others[3,4]. Neoadjuvant chemotherapy is also known as preoperative chemotherapy. In 1989, Wilke et al[5] first reported the application of neoadjuvant chemotherapy as the treatment for gastric cancer and achieved good results. Since then, studies on neoadjuvant chemotherapy have indicated that the procedure progressively improves the prognosis of the patients with advanced gastric cancer, but the consistency among the results of studies on program choice, toxicity and tolerance of chemotherapy is still lacking. Most of the treatment regimens reported in the literature[6-12] are still based on fluorouracil and platinum. Considering that neoadjuvant chemotherapy lowers the tumour stage and improves the R0 resection rate, the toxicity of chemotherapy is acceptable and will not increase the perioperative complications and mortality. In this study, a neoadjuvant chemotherapy scheme based on fluorouracil and cisplatin (docetaxel combined with fluorouracil and oxaliplatin) was applied to stage III/IV gastric cancer patients at our hospital. The tumour changes after chemotherapy, the surgical resection rate and the postoperative remission rate were observed. In addition, the improvements in clinical symptoms and toxicity were observed. The efficacy of the neoadjuvant chemotherapy scheme in reducing the tumour stage and improving the R0 resection rate was verified, and the safety of this procedure in clinical application was evaluated.
A total of 53 patients with advanced gastric cancer who were admitted into our hospital from September 2007 to December 2012 were enrolled into this study, including 37 males and 16 females. They ranged in age from 28 to 81 years, with a mean age of 60.30 ± 9.41 years. The detailed patient information is shown in Table 1. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical College. Written informed consent was obtained from all participants.
| Item | Data |
| Gender | |
| Male | 37 |
| Female | 16 |
| Age (yr, mean ± SD) | 60.30 ± 9.41 |
| Karnofsky score | |
| 90 | 7 |
| 80 | 20 |
| 70 | 22 |
| 60 | 4 |
| Tumor stage | |
| III | 24 |
| IV | 29 |
| Pathological type | |
| Poorly differentiated adenocarcinoma | 23 |
| Undifferentiated adenocarcinoma | 12 |
| Signet-ring cell carcinoma | 18 |
| Tumor location | |
| Gastric fundus and cardia | 11 |
| Gastric corpus | 14 |
| Gastric antrum and pylorus | 25 |
| Sclerotic stomach | 3 |
| Tumor size (cm), mean ± SD | 5.6 ± 1.5 |
| Chemotherapeutic course | |
| 1 course | 2 |
| 2 courses | 20 |
| 3 courses | 16 |
| 4 courses | 15 |
Inclusion criteria were as follows: stage III (T3N0M0) or IV (T4N3M0) gastric cancer patients diagnosed through gastric endoscopy biopsy who underwent chest X-ray, computed tomography (CT) and double contrast-enhanced ultrasonography (DCUS); Karnofsky physical scores < 60; no other primary malignancies; had not received treatment or developed symptoms such as active gastrointestinal bleeding, diarrhoea and intestinal obstruction; did not have blood and hematopoietic system disease; had normal liver, cardiopulmonary and kidney functions; and provided informed consent.
The exclusion criteria were as follows: patients who completed less than two courses of treatment; patients who completed only two courses without testing and assessment; and patients who completed more than two courses but refused testing and assessment.
The patients were all treated as follows: 75 mg/m2 docetaxel injection (Hengrui Pharmaceutical Co., Ltd, Jiangsu, China) in 500 mL of 5% glucose for intravenous infusion on day 1; 85 mg/m2 oxaliplatin injection (Hengrui Pharmaceutical Co., Ltd, Jiangsu, China) in 500 mL of 5% glucose for intravenous infusion on day 1; and 1500 mg/m2 5-Fu (Xudong Haipu Pharmaceutical Co., Ltd, Shanghai, China) in 200 mL of normal saline for the continuous intravenous administration on days 1 to 3. Dexamethasone (DXM) at 10 mg was intramuscularly injected at 9:00 pm the night before the chemotherapy, and at least 1500 mL of liquid was intravenously administered for hydration on the same day. DXM at 10 mg was injected intramuscularly again at 3:00 am in the morning of the chemotherapy, and 25 mg of finasteride was intramuscularly injected before the chemotherapy. The anti-emetic, acid-generating and bone marrow-protecting drugs were intravenously infused, followed by the sequential infusion of docetaxel and oxaliplatin. 5-Fu was continuously infused through an alternative intravenous access. The course of chemotherapy was set every three weeks, and the routine blood parameters were reviewed on a weekly basis. In cases where the patient exhibited severe toxicity, the dose was appropriately postponed or reduced. Toxicity was evaluated according to the specific evaluation criteria in the WHO standards, and the design treatment was two to four courses. The clinical efficacy was evaluated three to four weeks after chemotherapy and, if the conditions were suitable, the surgery was performed immediately. The resected specimens were sent for pathological tests to evaluate histological efficacy.
Clinical examinations were performed to assess the physical conditions of the patients and any symptom changes before and after chemotherapy. Chest X-ray, CT and DCUS examination[13,14] were conducted to measure the size of the tumour for efficacy evaluation. Histopathological examinations of the resected specimens were performed to assess the objective remission rate of the lesion. Clinical efficacy evaluation according to the WHO criteria was as follows: complete remission (CR), the lesion completely disappeared for more than a month; partial remission (PR), shrinkage of the tumour by more than 50% within four weeks; stable disease (SD), the lesion remained unchanged or increased by less than 25%, or decreased by less than 50%; and progressive disease (PD), the lesion increased by more than 25%, or a new lesion formed. The pathological evaluation criteria[15,16] were as follows: no residual tumour cells in the specimen (pathological complete response, pCR); 10% residual tumour cells (pathological subtotal response, pSR); 10% to 50% residual tumour cells (pathological partial response, pPR); > 50% residual tumour cells (pathologic minor response, pMR); and no tumour cell necrosis (pathologic no response, pNR).
After each treatment course, the toxicity of the chemotherapy was evaluated as grade 0 to grade IV according to the WHO criteria. Grade III to IV myelosuppression patients were treated by subcutaneous injection of granulocyte colony stimulating factor (Filgrastim, Kirin Kunpeng, China; Bio-Pharmaceutical Co., Ltd., Shanghai, China). Patients with severe gastrointestinal mucosal lesions were given an intravenous antibiotic drip. Oral gentamycin and chlorhexidine gargle (Nanyue Pharmaceutical Co., Ltd., Shenzhen, China) were given after fasting. Patients with gastrointestinal reactions such as nausea, vomiting and diarrhoea were given intravenous ondansetron (Qilu Pharmaceutical Co., Ltd., Jinan, China) and oral montmorillonite granules [Smecta, Beaufour Ipsen (Tianjin) Pharmaceutical Co Ltd., Tianjin, China]. Alopecia and peripheral neuritis were not treated.
Data are expressed as mean ± SD. Statistical analyses were performed using SPSS 13.0 statistical software. A χ2 test was performed to compare the incidence of toxicity among chemotherapy schemes. Differences with P < 0.05 were considered statistically significant.
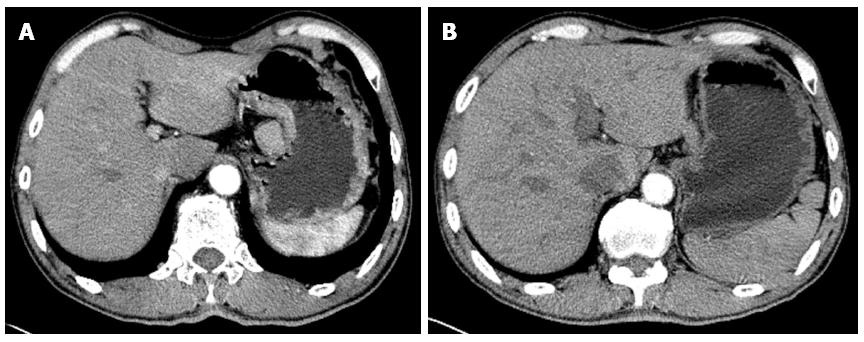
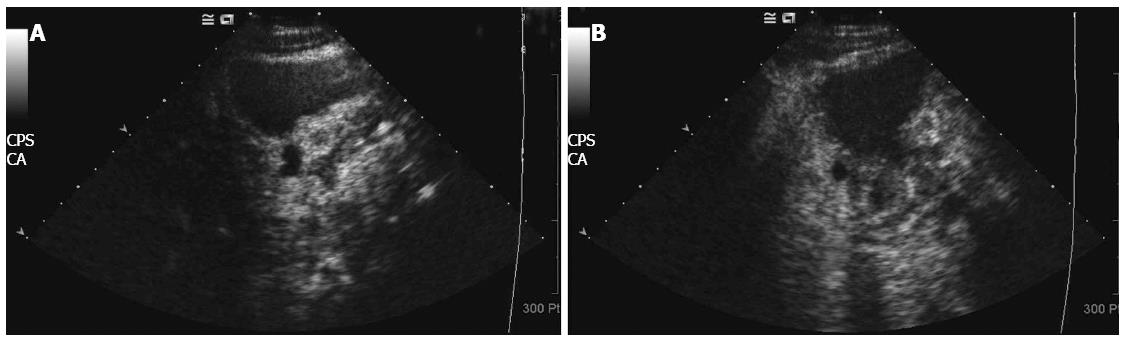
Among the 53 patients, two developed liver metastasis after completing one course. These patients were switched to other treatment options, and were removed from the study. The remaining 51 patients all completed two to four courses of chemotherapy. The clinical efficacy was as follows: two cases achieved CR, 30 cases achieved PR, 10 cases achieved SD, and 9 cases had PD. The clinical efficiency (CR + PR) was 62.7%. The typical pathological CT images are shown in Figure 1, and the DCUS images are shown in Figure 2. The Karnofksy scoring indicated that 42 cases significantly improved, whereas the rest did not change significantly. Bone marrow suppression was the most common toxic effect of chemotherapy, affecting a higher proportion of patients with stage III to IV gastric cancer. Gastrointestinal mucosa lesions were the second most common adverse reaction. Gastrointestinal reactions such as nausea, vomiting and diarrhoea were the third most common toxicity. Alopecia and peripheral neuritis were the least common toxic effects of the chemotherapy. Serious liver toxicity was not observed, and no patient died because of chemotherapeutic toxicity. The detailed toxicity data are shown in Table 2.
| Type | Side effects degree | Sum | |||
| I | II | III | IV | ||
| Bone marrow suppression | |||||
| leukopenia | 6 | 9 | 10 | 13 | 38 |
| Thrombocytopenia | 5 | 3 | 4 | 1 | 13 |
| anemia | 6 | 5 | 2 | 1 | 14 |
| Gastrointestinal reactions | |||||
| Nausea, vomiting | 23 | 14 | 8 | 3 | 48 |
| SGOT/SGPT | 5 | 4 | 2 | 0 | 11 |
| Diarrhea | 8 | 4 | 0 | 0 | 12 |
| Oral mucositis | 7 | 8 | 4 | 1 | 20 |
| Baldness | 17 | 5 | 3 | 0 | 25 |
| Peripheral neuritis | 12 | 1 | 0 | 0 | 13 |
| Urea nitrogen | 4 | 2 | 0 | 0 | 6 |
| Creatinine | 3 | 1 | 0 | 0 | 4 |
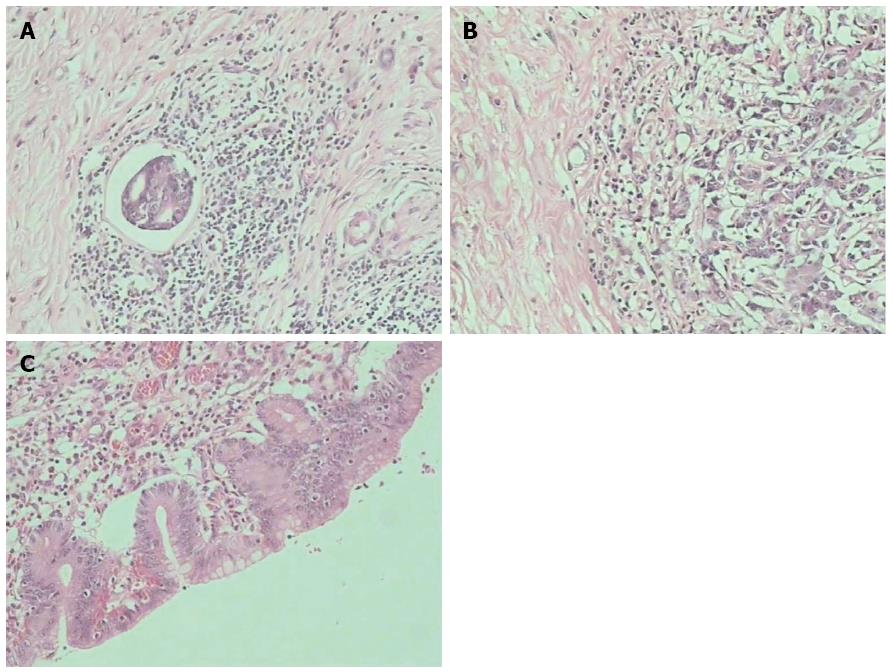
R0 resection was performed in 31 patients (60.8%), 14 of whom had distal gastrectomy, 8 had proximal gastrectomy, and 9 had total gastrectomy. The histopathological results of the resected specimens were as follows: 1 case of pCR, 6 cases of pSR, 16 cases of pPR, 8 cases of pMR, and no pNR. The images of the typical lesions are shown in Figure 3.
Treating advanced gastric cancer by simple surgery, especially in patients with stage III/IV disease, does not provide satisfactory results. With developments in gastric cancer research, simple surgery has been transformed into comprehensive treatment using a combination of methods, among which neoadjuvant chemotherapy has attracted increasing attention and recognition from clinicians.
Neoadjuvant chemotherapy is also known as preoperative chemotherapy, and its curative effect has remained the focus of controversy among the majority of gastric cancer researchers. In 1989, Wilke et al[5] first reported the efficacy of neoadjuvant chemotherapy for gastric cancer, which was later questioned by other scholars[17,18] who believe that neoadjuvant chemotherapy has limited curative effect and may cause tumour progression. Surgery should be performed as early as possible for gastric cancer patients with surgical indications. With the continuous development of new chemotherapeutic agents and the introduction of new chemotherapy schemes, many clinicians have unanimously affirmed the role of neoadjuvant chemotherapy in the treatment of advanced gastric cancer, especially 5-fluorouracil- and platinum-based chemotherapy schemes[8-12,19-21]. Neoadjuvant chemotherapy lowers the tumour stage, improves the surgical success rate and has tolerable toxicity. The results of this study are consistent with those of previous reports.
Neoadjuvant chemotherapy exhibited an overall clinical efficiency (CR + PR) of 62.7%, with an R0 radical resection rate of 60.8%. A total of 42 patients exhibited improved Karnofsky scores. The histopathological results of 31 patients indicated that 23 had pCR + pSR + pPR, which suggests the destruction of cancer tissues in most patients. The clinical efficiency and R0 resection rate obtained in our study are consistent with previously reported results. In addition, the improvement in clinical symptoms and histopathological results of patients were satisfactory, which indicates that neoadjuvant chemotherapy has better efficacy against stage III/IV gastric cancer. Therefore, neoadjuvant chemotherapy has a significant therapeutic effect against stage III/IV gastric cancer, with a strong tumour cell killing effect. Neoadjuvant chemotherapy has the following advantages[22]: (1) it reduces tumour volume, lowers the tumour stage and increases the success rate of surgical resection. As shown in the CT examination (Figure 1), the tumour tissues and peripheral lymph nodes were reduced to about 1/4 of their original size. The interspace between the tumour tissue and peripheral lymph nodes becomes clear, thereby lowering the tumour stage and improving the conditions for surgery; (2) neoadjuvant chemotherapy retards tumour growth, reduces tumour burden and improves the nutritional status of patients, thereby reducing the incidence of postoperative complications and increasing the surgical treatment effect. The DCUS results (Figure 2) show that the tumour tissues and swollen lymph nodes shrank by more than 50%, and the general condition of patients obviously improved, thereby enhancing surgical tolerance; and (3) neoadjuvant chemotherapy destroys the tumour cell bed, kills the micrometastatic foci and prevents and retards metastasis, thereby increasing the proportion of radical resection. The postoperative histopathological examination shows that neoadjuvant chemotherapy caused tumour cell necrosis and karyopyknosis (Figure 3C), with surrounding inflammatory cell infiltration and fibrous connective tissue hyperplasia (Figure 3B). In addition, neoadjuvant chemotherapy reduced intravascular thrombus tissue (Figure 3A) and prevented the haematogenous metastasis of tumour, thereby improving the surgical treatment effect.
Liver metastasis occurred in two patients after one course of chemotherapy. This finding indicates that neoadjuvant chemotherapy is not effective for all patients, and that drug resistance should be assessed during clinical treatment. Neoadjuvant chemotherapy[22] can also be used for initial treatment to assess the sensitivity and resistance of tumour cells to chemotherapy drugs. This process can prevent unnecessary surgery, and facilitates the selection of the appropriate therapeutic scheme and prognostication.
Toxicity effect is another important result of this scheme. A total of 38 patients (74.5%) developed leukopenia, and 23 cases (45.1%) were at least grade III. Thrombocytopenia occurred in 13 patients (25.5%), and 14 patients (27.4%) developed different degrees of anaemia. The patients improved with the subcutaneous injection of granulocyte colony stimulating factor, erythropoietin or other drugs for an average of 7 d (the longest was for 14 d). A total of 48 patients developed gastrointestinal reactions such as malignant vomiting, but the reaction was mild (grade III, 8 cases; grade IV, 3 cases), and improved with central anti-emetics. A total of 25 cases developed alopecia and 13 cases reported mild hand and foot numbness, but no cases of severe liver function damage or death developed. The rate of myelosuppression in this study is higher than in other reported results[8-12,19-21], with most patients exhibiting grades III and above. The reported neoadjuvant chemotherapy schemes often use two combined drugs which may have caused the low rate of myelosuppression, but this study uses three combined drugs, which increases the myelosuppression reaction. Other toxic effects such as gastrointestinal reaction, alopecia, peripheral neuritis and liver and kidney dysfunctions were relatively mild, with no significant differences with the reported results. Mild toxicity may be associated with the chemotherapy drugs used. Adjuvant chemotherapy of advanced gastric cancer using a regimen based on paclitaxel, oxaliplatin, irinotecan and high-dose fluorouracil achieves a good curative effect with few adverse reactions[11,12,23].
In conclusion, neoadjuvant chemotherapy using docetaxel combined with oxaliplatin and fluorouracil has a significant therapeutic effect on stage III/IV gastric cancer, with good clinical tolerance. Myelosuppression is the most common toxicity, with a high incidence of leukopenia. Clinical efficacy and toxicity should be evaluated in a timely manner. This scheme is safe and effective with full attention and proper treatment of the complications. However, the sample size in this study was small, and no control design or multicentre study was conducted. Moreover, patient survival was not statistically analysed. Therefore, this study can be considered a preliminary experiment for a multicentre controlled study on the role of neoadjuvant chemotherapy in the treatment of advanced gastric cancer. Nevertheless, this study has at least confirmed the efficacy of neoadjuvant chemotherapy in the treatment of advanced gastric cancer. The study summarises the application of neoadjuvant chemotherapy for treating advanced gastric cancer, and has particular significance for clinicians.
Neoadjuvant chemotherapy might improve the prognosis of patients with advanced gastric cancer, and the new national comprehensive cancer network guidelines have already included it as one of the comprehensive treatments towards advanced gastric cancer; however, there is no unified standard towards the choice of chemotherapy program and toxicity tolerability, and it was previously reported that the programs were more based on fluorouracil and platinum drugs. In this study, the combination of docetaxel, oxaliplatin and fluorouracil was used as the neoadjuvant chemotherapy towards stage III/IV gastric cancer patients, aiming to evaluate its efficacy and safety in clinical applications.
Not only were the iconographical changes of tumours observed before and after chemotherapy, but the degrees of postoperative pathologic remission and surgical resection rate were also evaluated, and the improvements of patients’ clinical symptoms and toxicity were observed, thus validating its efficacy in reducing the tumour stage and increasing the R0 resection rate.
The efficacy and safety of neoadjuvant chemotherapy were evaluated from both radiological and pathological examinations. It exhibited a better clinical reference value.
This study evaluated the efficacy and safety of specific chemotherapy, thus exhibiting certain clinical reference values towards the clinical promotion of these programs, and offered guidance for clinicians to choose the programs of neoadjuvant chemotherapy towards advanced gastric cancer.
This study evaluated the efficacy and safety of neoadjuvant chemotherapy using a three-drug combination towards advanced gastric cancer. The sample size was large, and the research had some innovation, while similar research studies are rare.
P- Reviewer: Yoon DH S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Wang YC, Wei LJ, Liu JT, Li SX, Wang QS. Comparison of Cancer Incidence between China and the USA. Cancer Biol Med. 2012;9:128-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 3. | Price TJ, Shapiro JD, Segelov E, Karapetis CS, Pavlakis N, Van Cutsem E, Shah MA, Kang YK, Tebbutt NC. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol. 2012;6:199-208; quiz 209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [PubMed] |
| 5. | Wilke H, Preusser P, Fink U, Gunzer U, Meyer HJ, Meyer J, Siewert JR, Achterrath W, Lenaz L, Knipp H. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol. 1989;7:1318-1326. [PubMed] |
| 6. | Wang LB, Shen JG, Xu CY, Chen WJ, Song XY, Yuan XM. Neoadjuvant chemotherapy versus surgery alone for locally advanced gastric cancer: a retrospective comparative study. Hepatogastroenterology. 2008;55:1895-1898. [PubMed] |
| 7. | Graziosi L, Bugiantella W, Gunnellini M, Qweider NA, Cavazzoni E, Donini A. [Preliminary experience in treatment of locally advanced gastric adenocarcinoma with peri-operative chemotherapy]. G Chir. 2010;31:147-150. [PubMed] |
| 8. | Moehler M, Gockel I, Roessler HP, Arnold D, Trarbach T, Thomaidis T, Klautke G, Rödel C, Brenner B, Lang H. Prospective, open, multi-centre phase I/II trial to assess safety and efficacy of neoadjuvant radiochemotherapy with docetaxel and oxaliplatin in patients with adenocarcinoma of the oesophagogastric junction. BMC Cancer. 2013;13:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Pepek JM, Chino JP, Willett CG, Palta M, Blazer Iii DG, Tyler DS, Uronis HE, Czito BG. Preoperative chemoradiotherapy for locally advanced gastric cancer. Radiat Oncol. 2013;8:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Molina R, Lamarca A, Martínez-Amores B, Gutiérrez A, Blázquez A, López A, Granell J, Álvarez-Mon M. Perioperative chemotherapy for resectable gastroesophageal cancer: a single-center experience. Eur J Surg Oncol. 2013;39:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Tsuburaya A, Nagata N, Cho H, Hirabayashi N, Kobayashi M, Kojima H, Munakata Y, Fukushima R, Kameda Y, Shimoda T. Phase II trial of paclitaxel and cisplatin as neoadjuvant chemotherapy for locally advanced gastric cancer. Cancer Chemother Pharmacol. 2013;71:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Bayraktar UD, Bayraktar S, Hosein P, Chen E, Koniaris LG, Rocha-Lima CM, Montero AJ. Preoperative docetaxel/cisplatin/5-fluorouracil chemotherapy in patients with locally advanced gastro-esophageal adenocarcinoma. Med Oncol. 2012;29:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Ang J, Hu L, Huang PT, Wu JX, Huang LN, Cao CH, Zheng YX, Chen L. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol. 2012;18:7026-7032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Zheng Z, Yu Y, Lu M, Sun W, Wang F, Li P, Zhang Y, Lin L, Huang P, Chen J. Double contrast-enhanced ultrasonography for the preoperative evaluation of gastric cancer: a comparison to endoscopic ultrasonography with respect to histopathology. Am J Surg. 2011;202:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Becker K, Fumagalli U, Mueller JD, Fink U, Siewert JR, Höfler H. Neoadjuvant chemotherapy for patients with locally advanced gastric carcinoma: effect on tumor cell microinvolvement of regional lymph nodes. Cancer. 1999;85:1484-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Lee MS, Mamon HJ, Hong TS, Choi NC, Fidias PM, Kwak EL, Meyerhardt JA, Ryan DP, Bueno R, Donahue DM. Preoperative cetuximab, irinotecan, cisplatin, and radiation therapy for patients with locally advanced esophageal cancer. Oncologist. 2013;18:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 17. | Wu AW, Xu GW, Wang HY, Ji JF, Tang JL. Neoadjuvant chemotherapy versus none for resectable gastric cancer. Cochrane Database Syst Rev. 2007;CD005047. [PubMed] |
| 18. | Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Chen SS, Yang XC, Chi F, Yu WZ, Wang ZB, Ning FL, Yu ZS, Hao YZ, Li ML, Wang F. A phase II study of preoperative chemotherapy with modified FOLFOX6 followed by surgery and postoperative chemoradiation in patients with localized gastric adenocarcinoma. Oncol Res. 2013;20:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Chen RX, Zhang J, Cai J, Meng H, Wu GC, Zhang ZT, Wang Y, Wang KL. Efficacy and safety of neoadjuvant chemotherapy with modified FOLFOX7 regimen on the treatment of advanced gastric cancer. Chin Med J (Engl). 2012;125:2144-2150. [PubMed] |
| 21. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1501] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 22. | D’Ugo D, Rausei S, Biondi A, Persiani R. Preoperative treatment and surgery in gastric cancer: friends or foes? Lancet Oncol. 2009;10:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Nakagawara H, Tajima H, Fujita H, Takamura H. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. 2012;105:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |