Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17568
Revised: May 15, 2014
Accepted: May 26, 2014
Published online: December 14, 2014
Processing time: 291 Days and 10.7 Hours
AIM: To evaluate the incidence, surgery, mortality, and readmission of upper gastrointestinal bleeding (UGIB) secondary to peptic ulcer disease (PUD).
METHODS: Administrative databases identified all hospitalizations for UGIB secondary to PUD in Alberta, Canada from 2004 to 2010 (n = 7079) using the International Classification of Diseases Codes (ICD-10). A subset of the data was validated using endoscopy reports. Positive predictive value and sensitivity with 95% confidence intervals (CI) were calculated. Incidence of UGIB secondary to PUD was calculated. Logistic regression was used to evaluate surgery, in-hospital mortality, and 30-d readmission to hospital with recurrent UGIB secondary to PUD. Co-variants accounted for in our logistic regression model included: age, sex, area of residence (i.e., urban vs rural), number of Charlson comorbidities, presence of perforated PUD, undergoing upper endoscopy, year of admission, and interventional radiological attempt at controlling bleeding. A subgroup analysis (n = 6356) compared outcomes of patients with gastric ulcers to those with duodenal ulcers. Adjusted estimates are presented as odds ratios (OR) with 95%CI.
RESULTS: The positive predictive value and sensitivity of ICD-10 coding for UGIB secondary to PUD were 85.2% (95%CI: 80.2%-90.2%) and 77.1% (95%CI: 69.1%-85.2%), respectively. The annual incidence between 2004 and 2010 ranged from 35.4 to 41.2 per 100000. Overall risk of surgery, in-hospital mortality, and 30-d readmission to hospital for UGIB secondary to PUD were 4.3%, 8.5%, and 4.7%, respectively. Interventional radiology to control bleeding was performed in 0.6% of patients and 76% of these patients avoided surgical intervention. Thirty-day readmission significantly increased from 3.1% in 2004 to 5.2% in 2010 (OR = 1.07; 95%CI: 1.01-1.14). Rural residents (OR rural vs urban: 2.35; 95%CI: 1.83-3.01) and older individuals (OR ≥ 65 vs < 65: 1.57; 95%CI: 1.21-2.04) were at higher odds of being readmitted to hospital. Patients with duodenal ulcers had higher odds of dying (OR = 1.27; 95%CI: 1.05-1.53), requiring surgery (OR = 1.73; 95%CI: 1.34-2.23), and being readmitted to hospital (OR = 1.54; 95%CI: 1.19-1.99) when compared to gastric ulcers.
CONCLUSION: UGIB secondary to PUD, particularly duodenal ulcers, was associated with significant morbidity and mortality. Early readmissions increased over time and occurred more commonly in rural areas.
Core tip: In our population-based study the overall risk of surgery, in-hospital mortality, and 30-d readmission for hospitalized upper gastrointestinal bleeding (UGIB) secondary to peptic ulcer disease (PUD) was 4.3%, 8.5%, and 4.7%, respectively. Duodenal ulcers had a worse prognosis than gastric ulcers. Readmission was more common among rural residents, which might be due to decreased access to resources or practice differences between urban and rural centers. Interventional radiology was uncommonly utilized (0.6%) and limited to urban centers, but prevented surgery in 3/4 of patients. These findings suggest that greater access to medical services may improve outcomes for UGIB secondary to PUD.
- Citation: Quan S, Frolkis A, Milne K, Molodecky N, Yang H, Dixon E, Ball CG, Myers RP, Ghosh S, Hilsden R, van Zanten SV, Kaplan GG. Upper-gastrointestinal bleeding secondary to peptic ulcer disease: Incidence and outcomes. World J Gastroenterol 2014; 20(46): 17568-17577
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17568.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17568
Despite advances in management, upper gastrointestinal bleeding (UGIB) secondary to peptic ulcer disease (PUD) remains a prominent medical emergency associated with substantial morbidity, mortality, and healthcare expenditures[1-4]. It is assumed that the burden of PUD has lessened due to advancements in endoscopic techniques, reduced prevalence of Helicobacter pylori (H. pylori), and increased utilization of acid suppressive drug therapy[5-7]. However, advances in the treatment of PUD have not necessarily translated to reduced admissions to hospital for UGIB secondary to PUD[8-10] nor reduced the risk for undesirable outcomes, including mortality[6,9,11-14].
Inconsistencies in reported outcomes among previous studies may be explained by several factors. The epidemiology of PUD has evolved and is no longer primarily driven by H. pylori[2,5]. An aging society[15] has led to a rise in the use of non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin[16]. This contributed to UGIB secondary to PUD becoming more common among the elderly population. As elderly patients often have more comorbidities and more complicated PUD, they may also experience worse outcomes than young PUD patients[17]. Healthcare systems have provided more effective treatment for PUD, but these advances in health systems may be restricted to large urban centres[1]. Rural areas may face barriers to access specialist care and to timely interventions (e.g., therapeutic endoscopy). Furthermore, most prior studies did not validate codes used to identify UGIB secondary to PUD in their administrative databases, which may have impaired interpretability of findings.
Consequently, we analyzed population-based data to assess the disease burden, utilization of surgical intervention, in-hospital mortality and readmission associated with UGIB secondary to PUD in the province of Alberta, Canada. To ensure rigorousness of our study, we validated the coding in the administrative hospital discharge abstract database (DAD).
The province of Alberta, Canada, has a public, single payer healthcare system, which provides medical and surgical care to its population of 3.6 million residents. The DAD captures all discharges in Alberta and contains rich and high-quality information including: hospital admission and discharge dates, in-hospital mortality, up to 25 International Classification of Disease, Tenth Revision (ICD-10) diagnostic codes, up to 25 procedural codes [based on the Canadian Classification of Health Interventions (CCI) coding system], and patient demographic information including age, sex, and residential area (i.e., urban vs rural). The Alberta Health Insurance Registry contains date of birth, sex, and mailing address for 99% of the resident population[18].
Using the DAD we identified patients hospitalized for UGIB secondary to PUD in the fiscal years of 2002 to 2010 in the province of Alberta, Canada. We included all adult patients (≥ 18 years) with UGIB secondary to PUD (ICD-10: K2X.0, K2X.2, K2X.4, and K2X.6, where X = 5-8 in any of 25 diagnosis coding fields) (Table 1). These ICD-10 codes were selected for validation because they have previously been used to investigate ulcer-related causes of hemorrhage using administrative databases[5]. We chose 2002 as the start of our washout period because that was the year when Alberta switched from ICD-9 to ICD-10 codes[19]. Unique personal health numbers were used to identify the multiple admissions in the study period and the first admission was assigned to each patient as the index date. Transfers between hospitals were combined into a single admission event. A two-year washout period for prior admissions for an UGIB secondary to PUD event was used to define incident cases. Thus, our study population ranged from 2004 to 2010. Population size used to calculate the incidence was obtained from the Alberta Health Insurance Registry.
| ICD-10 codes used to define perforation with UGIB secondary to PUD | |
| Perforated ulcer | K25.2, K25.6, K26.2, K26.6, K27.2, K27.6, K28.2, K28.6 |
| Non-perforated ulcer | K25.0, K25.4, K26.0, K26.4, K27.0, K27.4, K28.0, K28.4 |
| CCI codes used to define surgery | |
| Stomach repair | 1.NF.80.DA, 1.NF.80.DA-XX-E, 1.NF.80.DA-XX-N, 1.NF.80.LA, 1.NF.80.LA-XX-E, 1.NF.80.LA-XX-N |
| Partial excision of stomach | 1.NF.87.BA, 1.NF.87.DA, 1.NF.87.DG, 1.NF.87.DH, 1.NF.87.DJ, 1.NF.87.DL, 1.NF.87.DQ, 1.NF.87.GX, 1.NF.87.LA, 1.NF.87.RG, 1.NF.87.RH, 1.NF.87.RJ, 1.NF.87.RK, 1.NF.87.RP, 1.NF.87.SH |
| Total excision of stomach | 1.NF.89.DZ, 1.NF.89.GW, 1.NF.89.SG, 1.NF.89.TH |
| Small intestine repair | 1.NK.80.DA, 1.NK.80.DA-W2, 1.NK.80.DA-XX-E, 1.NK.80.LA, 1.NK.80.LA-W2, 1.NK.80.LA-XX-E |
| Partial excision of small intestine | 1.NK.87.BA, 1.NK.87.DA, 1.NK.87.DN, 1.NK.87.DP, 1.NK.87.DX, 1.NK.87.DY, 1.NK.87.LA, 1.NK.87.RE, 1.NK.87.RF, 1.NK.87.TF, 1.NK.87.TG |
| CCI codes used to define interventional radiology | |
| Control of bleeding in stomach using percutaneous transluminal (transarterial) approach | 1.NF.13.GP-C2, 1.NF.13.GP-GE, 1.NF.13.GP-WO |
| Control of bleeding in small and large intestine using percutaneous transluminal (transarterial) approach | 1.NP.13.GQ-C2, 1.NP.13.GQ-GE, 1.NP.13.GQ-WO |
| CCI codes used to define an upper endoscopic procedure | |
| Control of bleeding in stomach | 1.NF.13.BA, 1.NF.13.BA-AG, 1.NF.13.BA-BD, 1.NF.13.BA-C2, 1.NF.13.BA-FA, 1.NF.13.BA-GX, 1.NF.13.BA-KK, 1.NF.13.BA-W4, 1.NF.13.BA-X7 |
| Stomach repair | 1.NF.80.BA |
| Inspection or biopsy of stomach | 2.NF.70.BA, 2.NF.70.BN, 2.NF.71.BA, 2.NF.71.BP, 2.NF.71.BR |
| Inspection or biopsy of small intestine | 2.NK.70.BA, 2.NK.70.BA-BL, 2.NK.70.BN-BL, 2.NK.71.BA, |
| 2.NK.71.BA-BL, 2.NK.71.BR-BL | |
| Control of bleeding in small and large intestine | 1.NP.13.BA-C2, 1.NP.13.BA-KK, 1.NP.13.BA-GX |
We studied three outcomes for UGIB secondary to PUD: (1) surgical intervention; (2) in-hospital mortality; and (3) 30-d readmission for recurrent UGIB secondary to PUD. Management of UGIB secondary to PUD by surgery was defined using the CCI codes (Table 1). In-hospital mortality was ascertained using the death flag in the DAD. The incident patients with UGIB secondary to PUD were linked with DAD to determine readmission within 30 d after discharge.
The following covariates were extracted from the DAD: age, sex, urban vs rural residence (based on residence postal codes), Charlson comorbidities[20] (excluding PUD, stratified as 0, 1-2, or ≥ 3 comorbidities), perforated vs non-perforated PUD, upper endoscopy, interventional radiological attempt at controlling bleeding, and fiscal year of the hospital admission (Table 1).
To calculate positive predictive value (PPV), we extracted patients with UGIB secondary to PUD from the DAD in the Calgary Health Zone of Alberta, Canada. The estimated population in the zone was 1.3 million in 2009[21]. The DAD was searched using ICD-10 codes in all diagnostic coding positions to identify adult patients (≥ 18 years) admitted to hospital between January 1 and December 31, 2008. The DAD data were linked to our gold standard, EndoPRO, which is a centralized electronic endoscopy database. Physicians in the tertiary care centres in the Calgary Health Zone record all endoscopic procedures in EndoPRO. Each record contains patient demographics, a brief medical history, indication for the procedure, endoscopic findings, impressions, and recommendations. Individuals whose endoscopic reports were missing or incomplete underwent an inpatient chart review. Based on history and endoscopy findings, patients with confirmed cases of UGIB secondary to PUD were defined as true positives. UGIB due to other causes (e.g., gastritis or variceal bleeds) were defined as negative cases.
To calculate sensitivity, we randomly selected 110 endoscopic reports of patients with UGIB secondary to PUD and linked their personal health numbers to the original DAD and determined presence of ICD-10 codes for UGIB secondary to PUD.
For the validation study, sensitivity and PPV with 95% confidence intervals (CI) were calculated. Annual incidence per 100000 persons with age and sex standardization was calculated for the entire province. Poisson regression was used to report incidence rate ratio (IRR) with 95%CI for the year of admission of an UGIB secondary to PUD after adjusting for age and sex. For the three outcomes of interest (i.e., surgery, in-hospital mortality, and readmission), logistic regression was used to evaluate covariates including age, sex, comorbidities, urban or rural residency, upper endoscopy, interventional radiology, and year. Perforation was included in the models describing mortality and 30-d readmission. The adjusted odds ratio (OR) with 95%CI were estimated from the model.
In a secondary analysis, we restricted the population to patients with UGIB due to a gastric (ICD-10: K25.0, K25.2, K25.4, and K25.6) or duodenal ulcer (ICD-10: K26.0, K26.2, K26.4, and K26.6). Patients coded with both gastric and duodenal ulcers, gastrojejunal ulcers, or non-specific location were excluded. We repeated the logistic regression models for mortality, surgery, and 30-d readmission and included a covariate that compared duodenal to gastric ulcers.
We conducted two sensitivity analyses to ensure the reproducibility of our results. For our first sensitivity analysis we removed patients with UGIB secondary to PUD that occurred as an in-hospital complication following admission for another medical condition. The study population was restricted to admissions for UGIB secondary to PUD with ICD-10 codes only in the primary diagnostic position (i.e., the main reason responsible for hospital stay). In a second sensitivity analysis, we restricted our study population to individuals with a CCI code for upper endoscopy. In this sensitivity analysis patients with an UGIB secondary to PUD underwent an upper endoscopy to confirm the diagnosis.
Statistical analysis was performed using SAS version 9.3. This study was approved by the Conjoint Health Research Ethics Board of the University of Calgary.
We identified 7079 incident cases of UGIB secondary to PUD within the province of Alberta between 2004 and 2010. The PPV and sensitivity for the ICD-10 definition of UGIB secondary to PUD were 85.2% (95%CI: 80.2%-90.2%) and 77.1% (95%CI: 69.1%-85.2%), respectively.
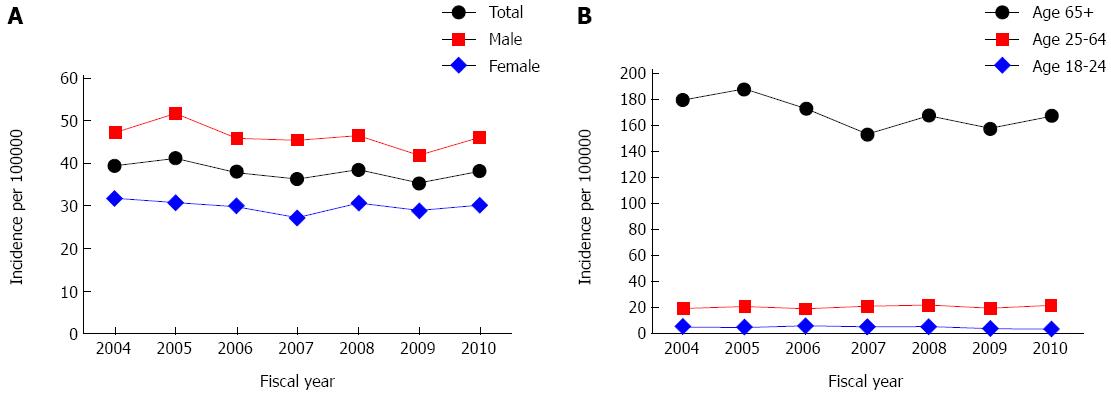
Patient characteristics, interventional procedures performed, and outcomes are reported in Table 2. Age and sex specific incidence for UGIB secondary to PUD from 2004 to 2010 ranged from 35.4 to 41.2 per 100000 persons (Figure 1). Age and sex adjusted annual incidence did not significantly change between 2004 and 2010 (IRR = 0.99, 95%CI: 0.98-1.02).
| All hospitalized patients with UGIB Secondary to PUD | Patients admitted for UGIB Secondary to PUD | |
| n =7079 | n = 4713 | |
| Female | 2784 (39.3) | 1841 (39.1) |
| Age ≥ 65 yr | 4307 (60.8) | 2720 (57.7) |
| Rural | 1318 (18.6) | 883 (18.7) |
| Underwent upper endoscopy | 5422 (76.6) | 3466 (73.5) |
| Underwent interventional radiology treatment | 42 (0.6) | 26 (0.6) |
| Underwent both interventional radiology and surgery1 | 10 (23.8) | 7 (26.7) |
| Comorbidities | ||
| No comorbidities | 3016 (42.6) | 2491 (52.9) |
| 1-2 comorbidities | 2329 (32.9) | 1460 (31.0) |
| ≥ 3 comorbidities | 1734 (24.5) | 762 (16.2) |
| In-hospital mortality | 601/7079 (8.5) | 175/4722 (3.7) |
| 30-d readmission with UGIB secondary to PUD2 | 301/6478 (4.7) | 229/4538 (5.1) |
| Surgical intervention | 305/7079 (4.3) | 200/4722 (4.2) |
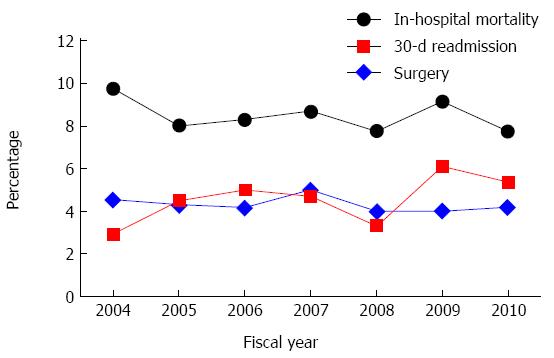
Overall risk of surgical intervention among patients with UGIB secondary to PUD was 4.3% (Figure 2, Table 2). The odds of surgery was higher in patients who were younger (adjusted OR ≥ 65 vs < 65: 0.74; 95%CI: 0.58-0.94) (Table 3). Only 0.6% of our population underwent an interventional radiological procedure to control bleeding (Table 2). While 76% of those who underwent interventional radiology avoided surgery, undergoing interventional radiology was associated with increased odds of surgery (adjusted OR = 7.18; 95%CI: 3.48-14.84) (Table 3).
| In-hospital mortality (95%CI) | 30-d readmission (95%CI)1 | Surgical intervention (95%CI) | |
| One year increase (yr) | 0.98 (0.94-1.02) | 1.07 (1.01-1.14) | 0.98 (0.93-1.04) |
| Female to male | 1.28 (1.07-1.53) | 1.16 (0.91-1.47) | 0.94 (0.74-1.20) |
| Age ≥ 65 yr to age < 65 yr | 1.68 (1.37-2.06) | 1.57 (1.21-2.04) | 0.74 (0.58-0.94) |
| Rural to urban | 0.90 (0.71-1.14) | 2.30 (1.79-2.95) | 0.90 (0.66-1.22) |
| Perforation to no perforation | 3.14 (2.03-4.85) | 1.59 (0.76-3.33) | Not applicable |
| Surgery2 | 2.23 (1.56-3.20) | 1.16 (0.62-2.15) | Not applicable |
| Interventional radiology2 | 2.41 (1.07-5.41) | 0.70 (0.09-5.16) | 7.18 (3.48-14.84) |
| Upper endoscopy2 | 1.08 (0.86-1.35) | 0.58 (0.45-0.74) | 0.86 (0.66-1.13) |
| Charlson comorbidities3 | |||
| 1-2 comorbidities | 3.13 (2.36-4.16) | 1.14 (0.86-1.49) | 1.07 (0.81-1.41) |
| ≥ 3 comorbidities | 9.51 (7.28-12.43) | 1.20 (0.88-1.64) | 1.04 (0.77-1.41) |
The overall risk of in-hospital mortality was 8.5% (Figure 2, Table 2). In-hospital mortality was associated with sex (adjusted OR female vs male: 1.28; 95%CI: 1.07-1.53), age (adjusted OR ≥ 65 vs < 65: 1.68; 95%CI: 1.37-2.06), comorbidities (adjusted OR ≥ 3 comorbidities vs no comorbidities: 9.51; 95%CI: 7.28-12.43), and interventional radiology (adjusted OR = 2.41; 95%CI: 1.07-5.41) (Table 3).
Risk of 30-d readmission with UGIB secondary to PUD significantly increased from 3.1% to 5.2% between 2004 and 2010 (adjusted OR per year: 1.07; 95%CI: 1.01-1.14) (Figure 2). The odds of readmission were higher among older patients (adjusted OR ≥ 65 vs < 65: 1.57; 95%CI: 1.21-2.04) and patients living in rural areas (adjusted OR rural vs urban: 2.30; 95%CI: 1.79-2.95). Patients who received an upper endoscopy were at lower odds of hospital readmission (adjusted OR = 0.58; 95%CI: 0.45-0.74) (Table 3).
Patients with duodenal ulcers were at higher odds of dying in-hospital (adjusted OR = 1.27; 95%CI: 1.05-1.53), needing surgery (adjusted OR = 1.73; 95%CI: 1.34-2.23), and being readmitted to hospital (adjusted OR = 1.54; 95%CI: 1.19-1.99) when compared to those with gastric ulcers (Table 4).
| In-hospital mortality (95%CI) | 30-d readmission (95%CI)1 | Surgical intervention (95%CI) | |
| Duodenal vs gastric ulcer | 1.27 (1.05-1.53) | 1.54 (1.19-1.99) | 1.73 (1.34-2.23) |
| One year increase (yr) | 0.98 (0.93-1.02) | 1.08 (1.02-1.15) | 0.99 (0.93-1.05) |
| Female to male | 1.22 (1.01-1.48) | 1.26 (0.97-1.63) | 0.96 (0.74-1.24) |
| Age ≥ 65 yr to age < 65 yr | 1.65 (1.33-2.05) | 1.59 (1.20-2.10) | 0.76 (0.59-0.98) |
| Rural to urban | 0.83 (0.64-1.08) | 2.36 (1.80-3.09) | 1.00 (0.73-1.38) |
| Perforation to no perforation | 2.32 (1.44-3.75) | 1.29 (0.58-2.84) | Not Applicable |
| Surgery2 | 2.52 (1.73-3.68) | 1.29 (0.69-2.43) | Not Applicable |
| Interventional radiology2 | 2.22 (0.99-4.99) | 0.59 (0.08-4.41) | 5.74 (2.69-12.27) |
| Upper endoscopy2 | 1.24 (0.96-1.59) | 0.59 (0.45-0.77) | 0.77 (0.58-1.01) |
| Charlson comorbidities3 | |||
| 1-2 comorbidities | 3.19 (2.35-4.32) | 1.29 (0.96-1.73) | 1.06 (0.79-1.41) |
| ≥ 3 comorbidities | 9.87 (7.42-13.14) | 1.33 (0.96-1.85) | 1.00 (0.73-1.37) |
In a sensitivity analysis we defined UGIB secondary to PUD with ICD-10 codes only in the primary diagnostic position. The risk of surgery was 4.2%, in-hospital mortality was 3.7%, and 30-d readmission was 5.1% (Table 2). In this sensitivity analysis endoscopy was associated with reduced in-hospital mortality (adjusted OR = 0.62; 95%CI: 0.44-0.88) and age was no longer associated with a significantly reduced odds of surgery (adjusted OR ≥ 65 vs < 65: 0.78; 95%CI: 0.58-1.06) (Table 5). Otherwise, the sensitivity analysis reported findings similar to the primary analysis that used all 25 diagnostic positions (Tables 3 and 5). Our second sensitivity analysis restricted the study population to patients with a CCI code for endoscopy; these analyses supported our primary findings; with the exception that interventional radiology was no longer associated with increased in-hospital mortality (adjusted OR = 2.30, 95%CI: 0.95-5.55) (Table 6).
| In-hospital mortality (95%CI) | 30-d readmission (95%CI)1 | Surgical intervention (95%CI) | |
| One year increase (yr) | 0.96 (0.89-1.04) | 1.09 (1.02-1.16) | 0.99 (0.93-1.07) |
| Female to male | 1.42 (1.03-1.96) | 1.02 (0.78-1.35) | 1.03 (0.76-1.38) |
| Age ≥ 65 yr to age < 65 yr | 2.90 (1.90-4.43) | 1.83 (1.35-2.47) | 0.78 (0.58-1.06) |
| Rural to urban | 0.89 (0.59-1.35) | 2.35 (1.77-3.14) | 1.10 (0.77-1.57) |
| Perforation to no perforation | 3.70 (2.05-6.69) | 1.35 (0.60-3.04) | |
| Surgery2 | 3.30 (1.89-5.74) | 1.42 (0.72-2.8) | |
| Interventional radiology2 | 4.89 (1.54-15.54) | 0.88 (0.11-6.7) | 8.59 (3.54-20.85) |
| Upper endoscopy2 | 0.62 (0.44-0.88) | 0.71 (0.53-0.95) | 0.79 (0.58-1.08) |
| Charlson comorbidities3 | |||
| 1-2 comorbidities | 2.56 (1.67-3.95) | 1.22 (0.90-1.66) | 1.30 (0.94-1.81) |
| ≥ 3 comorbidities | 6.63 (4.33-10.16) | 1.33 (0.92-1.94) | 1.24 (0.83-1.87) |
| In-hospital mortality | 30-d readmission1 | Surgical intervention | |
| One year increase (yr) | 0.98 (0.94-1.03) | 1.10 (1.02-1.18) | 0.98 (0.91-1.05) |
| Female to male | 1.35 (1.10-1.64) | 1.13 (0.84-1.51) | 0.92 (0.69-1.22) |
| Age ≥ 65 yr to age < 65 yr | 1.56 (1.24-1.95) | 1.70 (1.23-2.36) | 0.64 (0.49-0.85) |
| Rural to urban | 0.90 (0.69-1.17) | 2.51 (1.85-3.41) | 0.88 (0.61-1.27) |
| Perforation to no perforation | 1.96 (1.08-3.57) | 1.65 (0.65-4.16) | Not Applicable |
| Surgery2 | 2.04 (1.34-3.1) | 1.64 (0.85-3.18) | Not Applicable |
| Interventional radiology2 | 2.30 (0.95-5.55) | 0.74 (0.10-5.52) | 5.38 (2.32-12.47) |
| Charlson comorbidities3 | |||
| 1-2 comorbidities | 3.21 (2.3-4.47) | 1.28 (0.92-1.79) | 1.03 (0.75-1.41) |
| ≥ 3 comorbidities | 10.32 (7.55-14.1) | 1.12 (0.76-1.64) | 1.01 (0.72-1.43) |
Despite advances in medical management and endoscopic interventions during the 20th century, in the 21st century UGIB secondary to PUD continues to be a considerable burden to patients and to the healthcare system. Across the province the risk for surgery (4.3%), mortality (8.7%), and 30-d readmission to hospital (4.7%) for UGIB secondary to PUD was high. Annual incidence in Alberta ranged from 35.4 to 41.2 per 100000 persons between 2004 and 2010. By extrapolating our findings in Alberta, we estimate that every year over 12000 patients experience UGIB secondary to PUD in hospitals across Canada and 600 of these patients die in hospital.
Several studies have shown that the incidence of UGIB secondary to PUD diminished towards the end of the 20th century and has primarily stabilized during the turn of the 21st century. In Sweden the incidence declined from 64/100000 in 1987 to approximately 35/100000 in 1999, but was stable up to 2005[9]. Incidence was stable in the United States from 1999-2004[10]. Spain reported a steady decline in incidence from approximately 55/100000 in 1996 to approximately 25/100000 in 2005[12]. The decreasing incidence of UGIB secondary to PUD from the late 20th century to the early 21st century may be partially explained by the decreased prevalence of H. pylori[22].
Mortality continues to be a prevalent outcome for UGIB secondary to PUD. In our study, overall in-hospital mortality for Alberta, Canada, was 8.5%. However, the risk of death varies between countries: Korea (2.2% in 2006-2007)[23], United States (2.5% in 2006)[10], Turkey (2.8% in 2009)[24], Spain (3.1% in 1996-2005)[12], Sweden (6.2% in 2005)[9], Denmark (11% in 2010-2011)[13], and the Netherlands (14% in 2000)[11]. Heterogeneity between countries may be explained by the time period of study, the age distribution of populations, prevalence of comorbidities, and differences in UGIB management practices, which may be influenced by factors such as availability of endoscopy and utilization of pharmacotherapies such as proton pump inhibitors and prokinetics. Methodological factors may additionally explain heterogeneity, including different definitions of mortality. For example, in our study the risk of in-hospital mortality was high (8.5%) when we defined UGIB secondary PUD using any of diagnosis coding fields. However, when we restricted the study population to those coded in the primary diagnostic position (i.e., the primary reason for hospital stay was due to UGIB secondary to PUD), then in-hospital mortality dropped to 3.7%. The drop in mortality may also be due to fewer patients with multiple comorbidities or fewer secondary complications associated with prolonged hospital stay.
Epidemiologic studies have shown that the average age of patients who experience UGIB secondary to PUD is increasing[25]. Current evidence suggests that the cause of death is less often attributable directly to the bleeding ulcer[26]. Instead, the most predominant causes of death are related to cardiopulmonary deterioration as a result of exacerbated comorbidities[26]. Similarly, in our study, older women with multiple comorbidities were at the highest risk for mortality following an UGIB secondary to PUD. A recent meta-analysis reported UGIB secondary to PUD patients with comorbidities were at several-fold higher risk of overall mortality when compared to patients without comorbidities[27]. Thus, our data supports a growing body of literature that highlights the importance of optimizing the timely management of elderly individuals with multiple comorbidities who experience UGIB secondary to PUD.
The 30-d risk of readmission for a recurrent UGIB increased from 3% to 5% in Alberta during the study period. We showed that elderly patients were at increased odds of readmission. Similarly, in Sweden 6% of elderly patients were readmitted to hospital following their initial bleed[28]. Additionally, we demonstrated that patients who lived in rural areas and those patients who did not undergo endoscopy were at greater odds of being readmitted to hospital for a recurrent bleed. The reasons for the odds of readmission for UGIB secondary to PUD to be higher in rural areas is likely multi-factorial. A Canadian multicenter study showed that several factors contributed to readmissions including management errors, complications, and inappropriate medical management[29]. A previous study showed that endoscopy utilization in Alberta was similar in rural and urban regions; however, in rural areas endoscopy for UGIB secondary to PUD is most commonly performed by surgeons[30]. Lack of gastroenterologist in rural areas may result in decreased utilization of therapeutic endoscopy and improper medical management such as failure to eradicate H. pylori[31,32]. Future studies are needed to assess whether greater access to gastroenterologists in rural areas would improve outcomes for PUD.
An upper endoscopy procedure was performed in over 70% of our study population. Methodological considerations may explain the lack of endoscopy reporting in our administrative database. The subpopulation that was validated included 15% false positives and some of these patients did not undergo upper endoscopy. Additionally, within our database some true cases of UGIB secondary to PUD underwent endoscopy, but the CCI procedure code was missing from the database (3% in our validation subpopulation). Reassuringly, our predictors of surgery, in-hospital mortality, and 30-d readmission remained similar to our primary analysis when we restricted our study population to patients with a concurrent upper endoscopy procedural code.
The risk of surgery for UGIB secondary to PUD in Alberta was 4.3%, which was similar to other studies. For example, the risk of surgery was 3.8% in Los Angeles from 2000-2004[33], 5.7% in Malmö City, Sweden from 1999-2004[14], 4.2% in France from 2005-2006[34], and ranged between 4% and 7% in Demark from 2007-2011[13]. Thus, the role of surgery in treating UGIB secondary to PUD remains important in the 21st century[13,35-37].
Less than 1% of patients underwent an interventional radiology procedure in Alberta, which is comparable to the United Kingdom (1%)[3]. Low utilization of interventional radiology was likely due to the natural history of bleeding PUD, success of endoscopic and medical therapies, and restricted access to large urban hospitals. Among those patients receiving an interventional radiological procedure 76% avoided surgery. While interventional radiology increased the odds of surgery and mortality, these findings likely reflected disease severity. Consequently, central regionalization of patients with severe UGIB secondary to PUD to centers with access to specialized interventions may improve province-wide health outcomes.
In our study patients with a bleeding duodenal ulcer had a worse prognosis than those with a bleeding gastric ulcer. Duodenal ulcers were associated with higher odds of mortality, surgery, and readmission to hospital. Bleeding duodenal ulcers were associated with a higher risk of mortality and surgery in some[10,33], but not all prior studies[9,28]. Duodenal ulcers may be associated with a worse prognosis because ulcers located within the duodenum can be technically more difficult to manage; particularly, for endoscopy performed in rural areas with reduced volume of experience in managing UGIB secondary to PUD[38].
Limitations to our study should be considered. Our study period was only seven years, which may be too short to meaningfully assess temporal trends. Also, misclassification of some cases is unavoidable in studies using administrative database. Thus, we validated the ICD-10 definition for UGIB secondary to PUD. In our validation study, we discovered that most of the false positive cases represented UGIB due to other causes (e.g., variceal bleed) or PUD without clinical evidence of UGIB. Additionally, our administrative dataset missed patients who died before presenting to hospital and patients with bleeding from low risk ulcers that were not admitted to hospital. Furthermore, the administrative database lacked clinically meaningful information that likely influenced the outcomes of surgery, mortality, and readmissions. Specifically, we did not explore: (1) disease severity at clinical presentation (e.g. risk stratification scores such as the Glasgow-Blatchford); (2) the prevalence and eradication of H. pylori; (3) endoscopic evidence of high-risk stigmata of re-bleeding such as a visible vessel; and (4) medications such as NSAIDs, proton pump inhibitors, prokinetic agents, or vasoactive drugs. Additionally, we were not able to identify therapeutic endoscopic interventions such as injection of epinephrine, cauterizing vessels, and placement of clips. Finally, we studied the province of Alberta, Canada and thus, additional epidemiological studies are necessary to confirm generalizability in other regions.
In conclusion, we used validated ICD-10 codes to identify a province-wide population-based cohort of over 7000 hospitalized patients with an UGIB secondary to PUD from 2004 to 2010. The administrative database tracked patients through multiple admissions, allowing us to evaluate readmissions to hospital. Our findings showed that UGIB secondary to PUD continues to remain an important health problem in the 21st century. The incidence of UGIB secondary to PUD, need for surgical management, and mortality observed in Alberta was similar to findings around the world that have published outcomes since 2000. Our data also highlights that older women with comorbidities are at the highest risk of mortality. Further, we demonstrated that patients with a bleeding duodenal ulcer have a worse prognosis than those with gastric ulcers. Readmission to hospital for a recurrent UGIB was more common in rural areas, where access to gastroenterologists is lacking. Future studies should focus on identifying additional factors that may decrease incidence of UGIB secondary to PUD, as well as surgical interventions and mortality. Furthermore, to improve the quality of care for rural residents, we need to explore ways to increase their access to gastrointestinal health services.
We acknowledge the Alberta Health Services and Mr. Bing Li for providing administrative data.
Peptic ulcer disease (PUD) is a medical condition where the lining of the stomach or duodenum is damaged, which can lead to upper gastrointestinal bleeding (UGIB). UGIB secondary to PUD is a medical emergency, and despite the very best management, patients often die of this condition. Due to its severity, UGIB secondary to PUD places a substantial burden on patients, their families, and the healthcare system.
Medical care has advanced to better manage UGIB secondary to PUD with the use of therapies such as proton pump inhibitors, therapeutic endoscopy, interventional radiology, and surgery. However, previous studies investigating the epidemiological characteristics of UGIB secondary to PUD were limited to large urban centres and lack data on rural outcomes. This is important as rural areas may face additional challenges in accessing healthcare services. This population-based study includes both urban and rural settings to investigate the burden of UGIB secondary to PUD.
This study investigated the incidence of UGIB secondary to PUD along with three outcomes: in-hospital mortality, need for surgery, and 30-d readmission. UGIB secondary to PUD is associated with a high risk of mortality (8.5%) and need for surgery (4.5%). Readmission to hospital was more common among rural residents as compared to urban residents. Interventional radiology used to control bleeding was limited to specialized urban centers and these procedures prevented the need for surgery in 76% of treated patients.
Outcome data can aid in the care of patients in multiple ways. Healthcare workers can use our findings to guide patient management. By understanding the PUD prognosis, patients and their families can make more informed decisions. Further, healthcare administrators can use this data to estimate disease burden and to allocate healthcare resources. Also, this data can be used to compare outcomes from UGIB secondary to PUD in Alberta to other jurisdictions. Finally, this data can be used for surveillance of outcomes as therapeutic advances in the management of UGIB secondary to PUD evolve.
International Classification of Diseases is a set of codes used by hospital health records to document diseases. These coded data can be used for research and surveillance purposes. Interventional radiology is a less-invasive medical procedure using image-guided catheters to perform tasks such as controlling bleeding.
In this manuscript, the authors tried to evaluate the incidence, surgery, mortality, and readmission of upper gastrointestinal bleeding secondary to peptic ulcer disease in the province of Alberta, Canada. The study was uniquely performed and the results are very interesting.
P- Reviewer: Shimatani T S- Editor: Ding Y L- Editor: A E- Editor: Liu XM
| 1. | Sung JJ. Marshall and Warren Lecture 2009: peptic ulcer bleeding: an expedition of 20 years from 1989-2009. J Gastroenterol Hepatol. 2010;25:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang YS, Chan FK. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013;381:2033-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 432] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 4. | Khamaysi I, Gralnek IM. Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract Res Clin Gastroenterol. 2013;27:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Trawick EP, Yachimski PS. Management of non-variceal upper gastrointestinal tract hemorrhage: controversies and areas of uncertainty. World J Gastroenterol. 2012;18:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Suo BJ, Zhou LY, Ding SG, Guo CJ, Gu F, Zheng YA. [Analysis of etiological and related factors responsible for acute gastrointestinal hemorrhage]. Zhonghua Yixue Zazhi. 2011;91:1757-1761. [PubMed] |
| 8. | Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 9. | Ahsberg K, Ye W, Lu Y, Zheng Z, Staël von Holstein C. Hospitalisation of and mortality from bleeding peptic ulcer in Sweden: a nationwide time-trend analysis. Aliment Pharmacol Ther. 2011;33:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalizations for peptic ulcer disease in the United States, 1993 to 2006. Ann Surg. 2010;251:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Tytgat GN. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 365] [Article Influence: 16.6] [Reference Citation Analysis (37)] |
| 12. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Muñoz M. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Rosenstock SJ, Møller MH, Larsson H, Johnsen SP, Madsen AH, Bendix J, Adamsen S, Jensen AG, Zimmermann-Nielsen E, Nielsen AS. Improving quality of care in peptic ulcer bleeding: nationwide cohort study of 13,498 consecutive patients in the Danish Clinical Register of Emergency Surgery. Am J Gastroenterol. 2013;108:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Sadic J, Borgström A, Manjer J, Toth E, Lindell G. Bleeding peptic ulcer - time trends in incidence, treatment and mortality in Sweden. Aliment Pharmacol Ther. 2009;30:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Canada HRaSD. Canadians in Context - Aging Population. 2012;. |
| 16. | Hogan DB, Campbell NR, Crutcher R, Jennett P, MacLeod N. Prescription of nonsteroidal anti-inflammatory drugs for elderly people in Alberta. CMAJ. 1994;151:315-322. [PubMed] |
| 17. | Musa SA, Brecker SJ, Rahman TM, Kang JY. Upper gastrointestinal haemorrhage in the acute cardiac care setting: antiplatelets and endoscopy. Scott Med J. 2012;57:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Jin Y, Ellenhoj E, Sanderson M, Malo S, Haan M, Odynak D. Comparison of Alberta Population Counts Between the AHCIP Registry and the 2006 Census. Edmonton: Alberta Health and Wellness 2009; . |
| 19. | Health Surveillance System Series. Edmonton: Alberta Health and Wellness 2006; 2. |
| 20. | Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [PubMed] |
| 21. | Alberta Health Services Annual Report, April 1, 2009 - March 31, 2010. Available from: http://www.albertahealthservices.ca/publications/ahs-pub-annual-rpt.pdf. |
| 22. | Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: a nation-wide study in The Netherlands. Aliment Pharmacol Ther. 2006;23:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Bae S, Kim N, Kang JM, Kim DS, Kim KM, Cho YK, Kim JH, Jung SW, Shim KN. Incidence and 30-day mortality of peptic ulcer bleeding in Korea. Eur J Gastroenterol Hepatol. 2012;24:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mungan Z. An observational European study on clinical outcomes associated with current management strategies for non-variceal upper gastrointestinal bleeding (ENERGIB-Turkey). Turk J Gastroenterol. 2012;23:463-477. [PubMed] |
| 25. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Sostres C, Lanas A. Epidemiology and demographics of upper gastrointestinal bleeding: prevalence, incidence, and mortality. Gastrointest Endosc Clin N Am. 2011;21:567-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Leontiadis GI, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:331-345; quiz 346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (37)] |
| 28. | Hasselgren G, Blomqvist A, Eriksson S, Henningsson A, Lundell L. Short and long term course of elderly patients with peptic ulcer bleeding--analysis of factors influencing fatal outcome. Eur J Surg. 1998;164:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | van Walraven C, Jennings A, Taljaard M, Dhalla I, English S, Mulpuru S, Blecker S, Forster AJ. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Hilsden RJ. Patterns of use of flexible sigmoidoscopy, colonoscopy and gastroscopy: a population-based study in a Canadian province. Can J Gastroenterol. 2004;18:213-219. [PubMed] |
| 31. | Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Kaplan GG, Bates D, McDonald D, Panaccione R, Romagnuolo J. Inappropriate use of intravenous pantoprazole: extent of the problem and successful solutions. Clin Gastroenterol Hepatol. 2005;3:1207-1214. [PubMed] |
| 33. | Smith BR, Stabile BE. Emerging trends in peptic ulcer disease and damage control surgery in the H. pylori era. Am Surg. 2005;71:797-801. [PubMed] |
| 34. | Zeitoun JD, Rosa-Hézode I, Chryssostalis A, Nalet B, Bour B, Arpurt JP, Denis J, Nahon S, Pariente A, Hagège H. Epidemiology and adherence to guidelines on the management of bleeding peptic ulcer: a prospective multicenter observational study in 1140 patients. Clin Res Hepatol Gastroenterol. 2012;36:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Lee CW, Sarosi GA. Emergency ulcer surgery. Surg Clin North Am. 2011;91:1001-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Paimela H, Paimela L, Myllykangas-Luosujärvi R, Kivilaakso E. Current features of peptic ulcer disease in Finland: incidence of surgery, hospital admissions and mortality for the disease during the past twenty-five years. Scand J Gastroenterol. 2002;37:399-403. [PubMed] |
| 37. | Schwesinger WH, Page CP, Sirinek KR, Gaskill HV, Melnick G, Strodel WE. Operations for peptic ulcer disease: paradigm lost. J Gastrointest Surg. 2001;5:438-443. [PubMed] |
| 38. | Lou HY, Lin HC, Chen KY. Hospital case volume and clinical outcomes for peptic ulcer treatment. J Gen Intern Med. 2008;23:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |