Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16702
Revised: August 3, 2014
Accepted: September 18, 2014
Published online: November 28, 2014
Processing time: 239 Days and 2.8 Hours
AIM: To investigate the expression of gastrokine 1 (GKN1) in normal gastric mucosa, precancerous lesions and gastric cancer tissues, and to analyse its correlations with tumour site and pathological pattern.
METHODS: Thirty gastric cancer patients (12 cases of diffuse type and 18 cases of intestinal type), 13 atrophic gastritis patients and 15 healthy volunteers with almost normal gastric mucosa (superficial gastritis) were enrolled in this study. Helicobacter pylori (H. pylori) infection was examined in all subjects. All gastric mucosa biopsy specimens were obtained. Cancer-adjacent specimens were taken from corresponding gastric cancer patients. Immunohistochemistry and real-time PCR were performed to determine the expressions of the GKN1 protein and mRNA, respectively.
RESULTS: H. pylori infection had no significant association with age, gender, tumour site or pathological pattern in all subjects. Compared with the superficial gastritis and atrophic gastritis groups, the expression of GKN1 protein (P = 0.011) and mRNA (P < 0.001) in gastric cancer was significantly decreased. The GKN1 mRNA level in diffuse type gastric cancer was significantly lower than in intestinal type gastric cancer (0.296 ± 0.076 vs 0.525 ± 0.164, P < 0.001).
CONCLUSION: Compared with almost normal gastric mucosa, GKN1 expression in the gastric mucosa of gastric cancer patients is decreased; this is associated with progression and prognosis of gastric cancer.
Core tip: The current study evaluated the expression of gastrokine 1 (GKN1) in normal gastric mucosa, gastric cancer tissues and gastric lesions. We found that GKN1 expression was decreased in gastric cancer tissues. Furthermore, low expression of GKN1 was particularly associated with diffuse types of gastric cancer patients. The role of GKN1 in gastric carcinogenesis requires further investigation.
- Citation: Guo XY, Dong L, Qin B, Jiang J, Shi AM. Decreased expression of gastrokine 1 in gastric mucosa of gastric cancer patients. World J Gastroenterol 2014; 20(44): 16702-16706
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16702.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16702
Gastric cancer is the fourth most common cancer and second leading cause of cancer death worldwide[1,2], with a high incidence in Asia[3]. In China, the incidence of gastric cancer ranks third among all malignant tumours[4]. According to a study conducted by Sun et al[5], the mortality rate of gastric cancer in China is 26.3 per 100000. The risk factors for gastric cancer include Helicobacter pylori (H. pylori) infection, geographical location, diet, and the population’ genetic background[2,6]. However, the aetiology of gastric cancer is unclear. Identifying the prevalent risk factors of gastric cancer may contribute to preventing tumour growth and spread, and could reduce the incidence and mortality. According to the classification criteria developed by Lauren in 1965[7], gastric cancer is frequently divided into the intestinal type and the diffuse type. The intestinal type is more common in males and older age groups, while the diffuse type is more common in younger age groups; the incidence has no association with sex difference[8]. The different histological types may have different molecular changes and aetiologies.
Gastrokine 1 (GKN1), also known as AMP18 or CA11, has been detected recently in normal gastric mucosa, but not in other regions of the gastrointestinal tract[9]. Decreased GKN1 expression is found in gastric cancer tissues and precancerous lesions[10,11]. In H. pylori infection-induced lesions, GKN1 is also downregulated[12,13]. Some studies have reported that GKN1 is involved in the protection of gastric mucosal integrity, has roles in mucosal healing after lesions, and that the overexpression of GKN1 in gastric cancer cells can induce apoptosis[14]. Although the function of GKN1 has not been clearly defined in previous studies, it is strongly suggested that GKN1 may play a role as a tumour suppressor in, and is a biomarker for, gastric cancer[15].
In the present study, we examined the expression of GKN1 in normal gastric mucosa, precancerous lesions and gastric cancer tissues. The relationships between GKN1 expression with gastric cancer histological type and H. pylori infection were investigated.
Patients with gastric cancer and precancerous lesions in the Second Affiliated Hospital of Xi’an Jiao Tong University of Medicine School (Xi’an, China) and People’ Hospital of Yan’an (Yan’an, China) were enrolled in this study from July 2010 to July 2012. There were 30 gastric cancer patients (12 cases of diffuse type and 18 cases of intestinal type) and 13 atrophic gastritis patients, who were confirmed through pathological diagnosis by at least two different specialists. No patient received preoperative chemotherapy or radiotherapy. Fifteen healthy volunteers with almost normal gastric mucosa (superficial gastritis), and without any history of gastric cancer, were selected as controls. Patients with other types of tumours were excluded. All subjects were examined for H. pylori infection using the rapid urea test, C13 urea breath test and serum antibody test. A positive diagnosis was defined when the results of two or more methods were positive. The clinical and pathological data are described in Table 1.
| Group | Superficial gastritis | Gastric cancer | Cancer- | Atrophic gastritis | |
| Diffuse type | Intestinal type | adjacent | |||
| Case (n) | 15 | 12 | 18 | 30 | 13 |
| Age (yr) | 54.3 ± 12.4 | 57.6 ± 10.9 | 59.8 ± 11.2 | 58.9 ± 11.08 | 60.5 ± 10.98 |
| Gender (male/female) | 10/5 | 8/4 | 12/6 | 20/10 | 8/5 |
| H. pylori infection (positive/negative) | 9/5 | 7/5 | 10/8 | 17/13 | 7/6 |
All subjects received gastroscopy and all gastric mucosa biopsy specimens were obtained. Cancer-adjacent biopsy specimens were taken from corresponding gastric cancer patients, with a distance of 5 cm to the visible edge of the tumour tissue. Each specimen was immediately frozen in liquid nitrogen for 1 h and subsequently stored at -80 °C for subsequent analysis. At least three slides were made for different areas of specimens in each group. The Ethics Committee of the Second Affiliated Hospital of Xi’an Jiao Tong University of Medicine College approved this study. Informed consent was obtained from all subjects.
After being fixed in 10% formaldehyde, the tissues were embedded in paraffin and then cut into slices, before being placed onto slides. Antigen retrieval was performed in citrate buffer (pH = 6.0 ± 0.1) using the microwave method. The slides with the primary antibody, which were diluted 1:100 (Abcam Inc., Cambridge, MA, United States), were left overnight at 4 °C. On the second day, after incubation with the second antibody for 25 min and streptavidin/peroxidase for 30 min, colouration analysis was performed. The results of staining were assessed quantitatively using IPP 6.0 software (Media Cybernetics, Silver Springs, MD, United States).
A Trizol reagent kit (Takara, Tokyo, Japan) was used to extract total cellular RNA from snap-frozen samples, according to the manufacturer’s protocol. Absorbance at 260 nm using an ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, United States) determined the concentration of the RNA. In addition, electrophoresis on an ethidium bromide-stained 1% agarose gel was used to assess RNA integrity. The PrimeScript™ RT reagent kit (Takara) was used to reverse transcribe the total cellular RNA at 37 °C for 15 min; the reaction was stopped by heat inactivation for 5 s at 85 °C. Primer sequences used for amplification were as follows: GKN1 upstream primer, 5’-GCTTGCCTACTCCTCTGTCCACTG-3’, downstream primer, 5’-CTCACTGACTGCTGCCCACTTCC-3’; β-actin upstream primer, 5’-AGGAAGGAAGGCTGGAAGAGTG-3’, downstream primer, 5’-AGGAAGGAAGGCTGGAAGAGTG-3’. An iCycler iQ system (Bio-Rad Laboratories Inc., Hercules, CA, United States) was used for mRNA expression analysis using quantitative real-time PCR. Real-time PCR was performed using an ABI PRISM 7500 sequence detector and SYBR green I chemistry (Applied Biosystems, Foster City, CA, United States). PCR conditions were as follows: 3 min denaturation at 94 °C in a final volume of 25 μL, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s. The PCR amplification products were determined using agarose gel electrophoresis. Melt curve analysis was performed to determine the specificity of PCR products. All cDNA samples were analysed in duplicate. The expression of β-actin was used as the internal control. Expression levels in various biopsy specimens were quantified by calculating the initial target concentrations using the obtained threshold cycle values. Calculation of the fold-change in mRNA expression was performed relative to the β-actin endogenous control using the 2-△△Ct method.
Data were expressed as means ± SD. Statistical analysis was performed using SPSS 16.0 statistical software (SPSS Inc., IL, United States). Tukey’s t-test or ANOVA were used to analyse the differences between the groups. P < 0.05 was considered statistically significant.
The clinical characteristics and pathological data of the subjects are shown in Tables 1 and 2. The relationship between H. pylori infection and clinical pathological factors was analysed using Logistic regression. H. pylori infection had no significant association with age, gender, tumour site or pathological pattern in all subjects.
| Index | Case (n) | P value | |
| Tumour site | Upper and middle | 20 | > 0.05 |
| Lower | 10 | ||
| Pathological pattern | Diffusion | 11 | > 0.05 |
| Intestines | 19 | ||
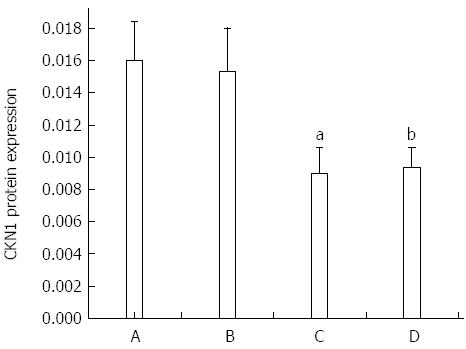
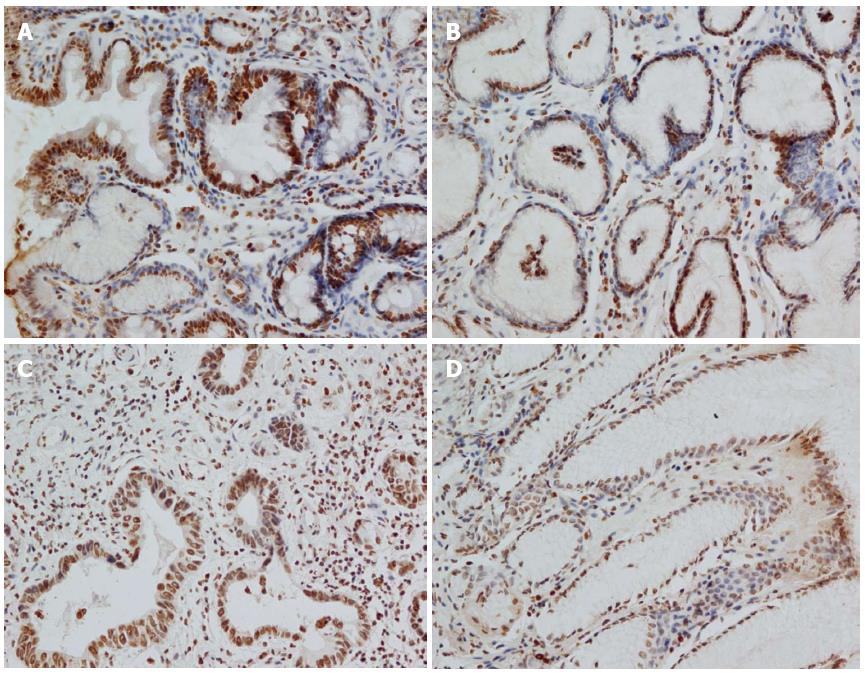
Expression of the GKN1 protein in gastric cancer was significantly decreased compared with the superficial gastritis group (0.009 ± 0.001 vs 0.016 ± 0.002, P = 0.011) and atrophic gastritis group (0.009 ± 0.001 vs 0.015 ± 0.002, P = 0.044, Figure 1). Expression of the GKN1 protein was also decreased in the cancer-adjacent group (0.009 ± 0.001). There was no significant difference between the atrophic gastritis and superficial gastritis groups (P = 0.803), or between the gastric cancer and cancer-adjacent groups (P = 0.354). The immunoreactions mainly appeared in the nucleus, with hardly any staining in the cytoplasm, of normal gastric epithelial cells, cancer cells, inflammatory cells and gland cells (Figure 2).
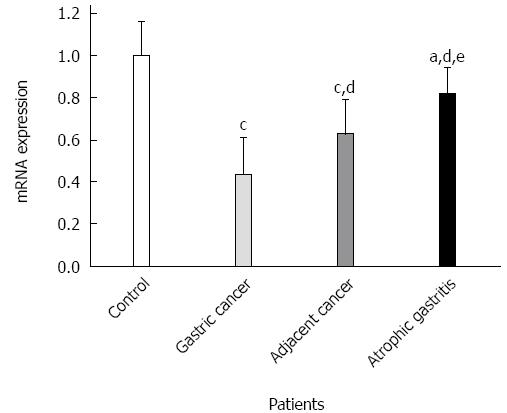
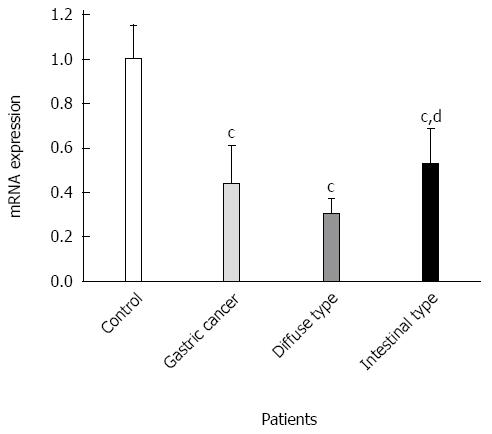
GKN1 mRNA was weakly expressed in the gastric cancer group (Figure 3). By contrast, GKN1 mRNA expression was abundant in the superficial gastritis group (0.989 ± 0.181), and was significantly higher than in the gastric cancer group (0.433 ± 0.176, P < 0.001) and cancer-adjacent group (0.626 ± 0.167, P < 0.001). In the atrophic gastritis group (0.819 ± 0.123), the expression level of GKN1 was significantly lower than in the superficial gastritis group (P = 0.011). The mRNA level of GKN1 in diffuse type gastric cancer patients was lower than in intestinal type gastric cancer patients (Figure 4) (0.296 ± 0.076 vs 0.525 ± 0.164, P < 0.001). Compared with the superficial gastritis group, the GKN1 mRNA levels in the two types of gastric cancer were both decreased (P < 0.001).
GKN1 is a novel protein that is exclusively expressed in normal gastric tissue, but absent in gastric cancer tissues. GKN1 is located in the superficial gastric epithelium, playing a significant role in protecting and maintaining gastric epithelium integrity after injury. If the protein is downregulated, the repair process may be impaired. Recently, a number of studies have found that deficiency of GKN1 can result in instability of the gastric epithelium. Invasive factors such as H. pylori contribute to the downregulation of GKN1, whilst inducing ulceration and cancer. GKN1 may play a key role in the progression of gastric cancer, and may be a potential biomarker for the early detection of gastric cancer.
The present study investigated GNK1 expression in different mucosa biopsy specimens from gastric cancer, cancer-adjacent lesions, atrophic gastritis and normal control subjects (superficial gastritis patients). We found that GKN1 mRNA expression was progressively downregulated from corresponding distant non-tumour tissues to tumour tissues, and was lower than in the control tissues of both groups. This suggested that low or absent expression of GKN1 may contribute to gastric carcinogenesis. This is consistent with a previous study that demonstrated decreased GKN1 expression in gastric cancer tissues[16]. In addition, we analysed GKN1 expression in two types of gastric cancer and found that the mRNA level of GKN1 was lower in patients with diffuse type gastric cancer. This indicates that GKN1 may be related to tumour classification.
In conclusion, we found differential expression of GKN1 in gastric cancer tissue, gastric lesions and normal gastric tissue. Low expression of GKN1 was especially detected in diffuse type gastric cancer patients. However, the role of GKN1 in gastric carcinogenesis requires further studies.
Gastric cancer remains the fourth most common cancer and second leading cause of cancer death worldwide. Gastrokine 1 (GKN1), detected recently in normal gastric mucosa, is involved in the development of gastric lesions and gastric cancer.
GKN1 has been found to be downregulated in gastric cancer, but the relationship between GKN1 expression and clinical characteristics is unclear. The current study aimed to evaluate the expression of GKN1 in normal gastric mucosa, gastric cancer tissues and matched tumour-adjacent tissues, and to investigate its relationship with Helicobacter pylori (H. pylori) infection and histological classification.
This study reported that GKN1 expression is decreased in gastric cancer tissue. Furthermore, low expression of GKN1 is especially found in diffuse type gastric cancer patients.
The authors found GKN1 was expressed differently in gastric cancer and normal gastric tissue, and it may be related to the tumour classification. The results provide a better understanding of the role of GKN1 in the development of gastric cancer.
The authors examined the expression of GKN1 in normal gastric mucosa, precancerous lesions and gastric cancer tissues. The relationships between GKN1 expression and gastric cancer histological type and H. pylori infection were also investigated. The results are interesting and may supply a new direction for GKN1 in gastric carcinogenesis.
P- Reviewer: Alsubai J, Jung DH S- Editor: Yu J L- Editor: Stewart G E- Editor: Liu XM
| 1. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 795] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 2. | Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249-258. [PubMed] |
| 3. | Shang J, Pena AS. Multidisciplinary approach to understand the pathogenesis of gastric cancer. World J Gastroenterol. 2005;11:4131-4139. [PubMed] |
| 4. | Yeoh KG. How do we improve outcomes for gastric cancer? J Gastroenterol Hepatol. 2007;22:970-972. [PubMed] |
| 5. | Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ, Qiao YL. [Analysis of mortality rate of stomach cancer and its trend in twenty years in China]. Zhonghua Zhongliu Zazhi. 2004;26:4-9. [PubMed] |
| 6. | Zabaleta J. Multifactorial etiology of gastric cancer. Methods Mol Biol. 2012;863:411-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 8. | Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Martin TE, Powell CT, Wang Z, Bhattacharyya S, Walsh-Reitz MM, Agarwal K, Toback FG. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285:G332-G343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, Burns S, Keith WN. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, May FE, Gao J, Meitner PA, Tavares R. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008;14:4161-4167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Nardone G, Rippa E, Martin G, Rocco A, Siciliano RA, Fiengo A, Cacace G, Malorni A, Budillon G, Arcari P. Gastrokine 1 expression in patients with and without Helicobacter pylori infection. Dig Liver Dis. 2007;39:122-129. [PubMed] |
| 13. | Nardone G, Martin G, Rocco A, Rippa E, La Monica G, Caruso F, Arcari P. Molecular expression of Gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic gastric lesions. Cancer Biol Ther. 2008;7:1890-1895. [PubMed] |
| 14. | Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, Arcari P. Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol. 2011;226:2571-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yoon JH, Kang YH, Choi YJ, Park IS, Nam SW, Lee JY, Lee YS, Park WS. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial-mesenchymal transition in gastric cancers. J Cancer Res Clin Oncol. 2011;137:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Mao W, Chen J, Peng TL, Yin XF, Chen LZ, Chen MH. Helicobacter pylori infection and administration of non-steroidal anti-inflammatory drugs down-regulate the expression of gastrokine-1 in gastric mucosa. Turk J Gastroenterol. 2012;23:212-219. [PubMed] |