Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16381
Revised: June 19, 2014
Accepted: July 16, 2014
Published online: November 21, 2014
Processing time: 223 Days and 22.9 Hours
Adenosquamous carcinoma rarely occurs in the pancreas, and is characterized by the presence of cellular components from both duct adenocarcinoma and squamous carcinoma. Here, we describe a rare case of pancreatic adenosquamous carcinoma with sarcomatous change. Immunohistochemistry showed that the sarcomatous lesion lost the epithelial marker and aberrantly expressed of acquired mesenchymal markers, which indicated that this special histological phenotype may be attributed to epithelial-mesenchymal transition. This case also indicated that a routine radical surgery without aggressive treatment strategies was still appropriate for adenosquamous carcinoma of the pancreas with sarcomatoid change.
Core tip: Adenosquamous carcinoma rarely occurs in the pancreas, and is characterized by the presence of cellular components from both duct adenocarcinoma and squamous carcinoma.
- Citation: Lu BC, Wang C, Yu JH, Shen ZH, Yang JH. A huge adenosquamous carcinoma of the pancreas with sarcomatoid change: An unusual case report. World J Gastroenterol 2014; 20(43): 16381-16386
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16381
Adenosquamous carcinoma always arises in organs that are predilection sites for adenocarcinoma, such as the gastrointestinal tract and uterus, or for squamous carcinoma, such as the esophagus and vagina[1]. Adenocarcinoma is the most common histopathology of pancreatic cancer, adenosquamous carcinoma also occur in the pancreas, making up only approximately 1%-4% of all pancreatic exocrine malignancies, including specimens from autopsy and clinical surgery[2,3]. As in other organs, pancreatic adenosquamous carcinoma is characterized by the presence of cellular components from both duct adenocarcinoma and squamous carcinoma. When a third cellular component is found, can the tumor still be defined as adenocarcinoma and squamous carcinoma?
Epithelial-mesenchymal transition (EMT), a transition between different phenotypes, is regarded as essential process in tumor progression[4]. When EMT occurs, epithelial tumor cells lose their restricted phenotypes, concomitant with the loss of epithelial characteristics and the acquisition of motile behavior. These changes, which contribute to the transition from an epithelial morphology towards a more mesenchymal fibroblastic (e.g., sarcomatoid) phenotype, have been reported to occur in some special tumors, such as cholangiocarcinomas[5]; this change is also defined as sarcomatous change[6]. For many carcinomas, such as breast carcinoma and cholangiocarcinomas, EMT always comes with a poor prognosis, because it promotes the process of cancer invasion, metastasis and resistance to chemotherapy[7-9].
Three different cellular components synchronously arise in the lesion of carcinoma has been reported in other organs, including thymus, esophagus, and stomach[10-12], however, the similar neoplasm has not been reported. In this report, we describe a case of huge pancreatic adenosquamous carcinoma with sarcomatous change. This case indicated that a routine radical surgery without aggressive treatment strategies was still appropriate for adenosquamous carcinoma of the pancreas with sarcomatoid change.
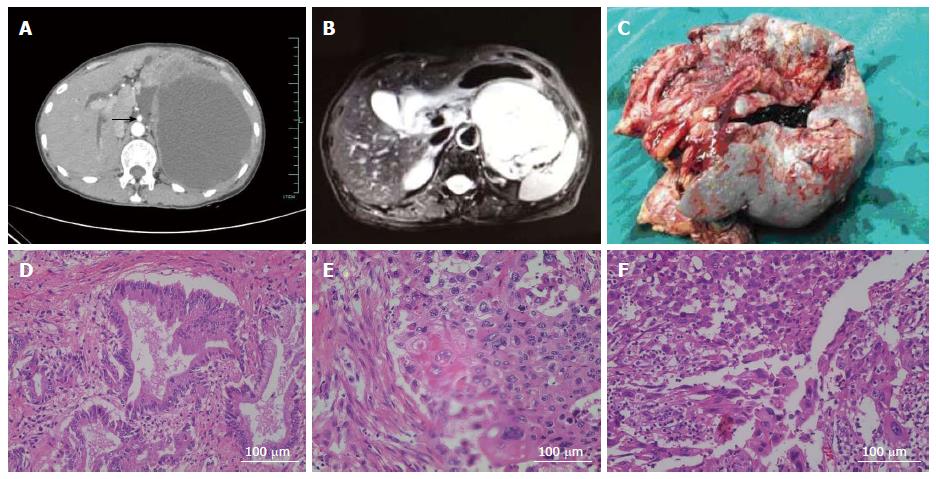
A 58-year-old woman was hospitalized with a 2-wk history of epigastric fullness and poor appetite on July 26, 2013. She did not complain of jaundice, vomiting, fever, or weight loss. Three years previously, the patient had undergone left lateral hepatic lobectomy and common bile duct exploration for left hepatolithiasis and choledocholithiasis. At that time, no pancreatic neoplasia was found on a contrast-enhanced computed tomography (CECT) scan of the abdomen. On examination at our hospital, a 12 cm × 10 cm mass with obscure boundaries, a smooth surface, and poor mobility was palpated. No other remarkable findings were detected on physical examination except for slight tenderness in the left and right upper quadrants. Laboratory tests showed a white blood cell count of 12.2 × 109/L, a C-reactive protein level of 188 mg/L (normal value, 0-8 mg/L), carcinoma antigen 19-9 (CA 19-9) of 3630 U/mL (normal value, 0-37 U/mL), carcinoma antigen 125 of 645 U/mL (normal value, 0-35 U/mL), carcino embryonic antigen of 7.43 ng/mL (normal value, 0-10 ng/mL), and squamous cell carcinoma antigen of 2.5 ng/mL (normal value, 0-2.5 ng/mL). Other routine tests were normal, including liver function analysis, bilirubin, amylase, and blood glucose. A CECT scan of the abdomen showed a huge solid-cystic neoplasm of 15 cm × 12 cm located amidst the pancreas, spleen, and stomach. The lesion was well circumscribed with heterogeneous enhancement, and it displaced the surrounding organs, including the stomach, spleen, tail of the pancreas, and left kidney (Figure 1A). Magnetic resonance imaging showed the lesion to possibly originate from the tail of the pancreas; retroperitoneal lymph nodes were enlarged, and other findings correlated with the CECT (Figure 1B). Gastroscopy showed upheaval of the posterior gastric wall because of pressure from the mass. The patient was presumptively diagnosed with pancreatic cystadenocarcinoma prior to surgery.
On surgical exploration, the left upper quadrant of the abdomen was full of the solid-cystic neoplasm, and a small volume of ascites was present. The mass measured 16 cm × 18 cm, and it invaded and displaced the surrounding organs, including the gastric fundus, the entire spleen, and the tail of the pancreas. Celiac artery and superior mesenteric artery (Figure 1A) were displaced but without involvement, however, splenic artery had been enclosed by the mass. Approximately 2500 mL of dull red liquid was extracted from the solid-cystic mass, and a frozen section of the capsule wall was read as a “malignant tumor” arising from the pancreas. We performed an en bloc resection of the distal pancreatic tumor, including the neoplasm, tail of the pancreas, fundus of the stomach, spleen, and peripheral lymph nodes (Figure 1C). Surgical margins were negative.
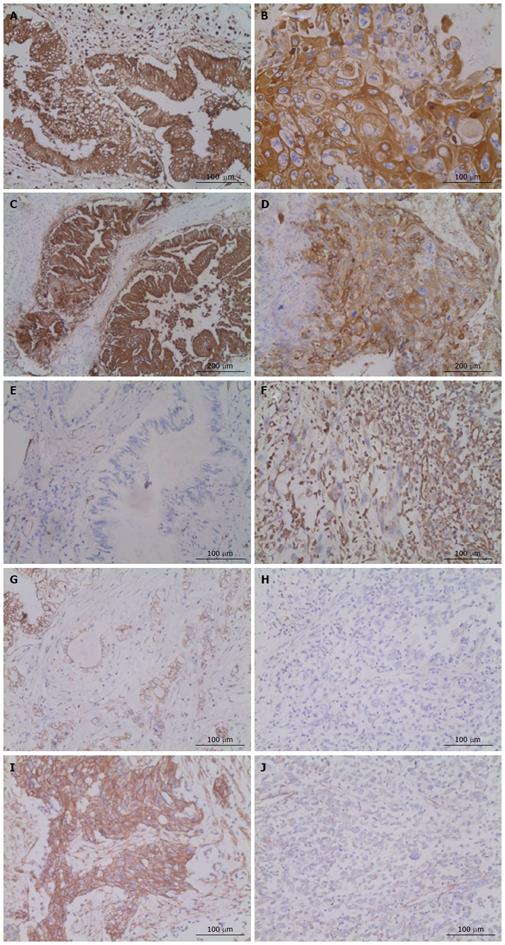
Pathological examination by Image-pro Plus 5.1 identified three different cellular components within the lesion. One component showed irregularity, glands, and tracts typical of a carcinoma (Figure 1D); another part of the lesion was characterized by clumps and streaks, with keratin pearls and intercellular desmosomes (Figure 1E). The final cellular component consisted of spindle cells, with multiplicative division of the nuclei and an immature appearance (Figure 1F). The principal pathologic diagnosis for the pancreatic neoplasm was adenosquamous carcinoma of the pancreas. In the extrapancreatic lesion, invasive squamous carcinoma appeared in the gastric wall, sarcomatoid change arose in the lymph nodes, and squamous carcinoma with sarcomatoid change arose on the spleen. Lymphatic invasion occurred in 12 of the 12 nodes around the splenic hilum, 3 of 5 nodes around the pancreas, 1 of 2 nodes adjacent to mesenteric vessels, and 1 of 3 nodes around the celiac trunk. On immunohistochemistry (IHC), E-cadherin and CA 19-9 were positive in the lesions containing cellular components of squamous carcinoma, vimentin was positive in the sarcomatoid lesions, and cytokeratin 7 (CK7) was positive in all lesions (Table 1 and Figure 2). The patient had an uneventful recovery and left the hospital 10 d after the operation; she received traditional Chinese medicine and thymosin as post-discharge treatment. Five months later, multiple metastases were found in the left upper quadrant and the liver. However, the patient was alive and self-sufficient as of this writing.
| Location | Cell component | CK7 | CA 19-9 | E-cadherin | Vim |
| Gastric wall | Squamous carcinoma | + | + | + | - |
| Pancreas | Adenosquamous carcinoma with sarcomatous change | + | + | + | - |
| Spleen | Squamous carcinoma | + | + | + | - |
| Sarcomatous change | + | - | - | + | |
| Lymph nodes | Sarcomatous change | + | - | - | + |
In this case, the neoplasm unexpectedly consisted of three different histopathological types, including not only adenocarcinoma and squamous carcinoma but also sarcoma. The question of how this special neoplasm arose is a significant one. To explore the origin of the three different cellular components, IHC staining was performed. In a previous study, CK7, a common keratin marker for gastrointestinal tumors, and CA 19-9, a classical tag protein for pancreatic tumors, were aberrantly expressed in adenosquamous carcinoma of the pancreas[13]. Given that CK7 was positive in all the different parts of our patient’s neoplasm and that CA 19-9 was positive in the adenocarcinoma and squamous cellular components, it is reasonable that the neoplasm should be defined as adenosquamous carcinoma of the pancreas. However, this does not answer the question of how the mesenchymal sarcomatoid lesions arose. In our opinion, EMT is a possible explanation. Loss of the epithelial marker E-cadherin and aberrant expression of acquired mesenchymal markers (such as vimentin) are important characteristics in the process of EMT[14]. To verify our hypothesis, IHC was performed to look for E-cadherin and vimentin expression. Loss of membranous E-cadherin expression and acquired vimentin expression were observed in tumor cells of the sarcomatoid lesion, while strong expression of E-cadherin and negative expression of vimentin were observed in the adenocarcinoma and squamous carcinoma portions of the lesion (Table 1 and Figure 2). p63, a central transcription factor is constitutively expressed at high levels in a variety of epithelial tissues[15], high expressed in the squamous carcinoma portions of the lesion but low expressed in sarcomatoid lesion (Figure 2I and J). Another pathologic characteristic of note was that the sarcomatous change generally presented as squamous cellular lesions in sites such as the spleen and lymph nodes. Overall, these findings indicate that the adenosquamous carcinoma of the pancreas was undergoing a special development process, which maybe influence the biological behaviour of the neoplasm.
Adenosquamous carcinoma of the pancreas is associated with high rates of recurrence and metastasis and carries a poor prognosis, much worse than even that of pancreatic adenocarcinoma[3,16], which has a reported median overall survival of no more than 5 mo[2]. Surgery is still the first-line treatment for adenocarcinoma of the pancreas, and the median survival can reach 14.4 mo for R0 resection and 8 mo for R1 resection[17]. Chemoradiotherapy is unable to achieve a similar or better curative effect. In our case, sarcomatoid change arose from adenosquamous carcinoma of the pancreas, which is regarded as a key step during cancer invasion and metastasis[7], and has been associated with resistance to chemotherapy and radiotherapy[8,9]. Given that the particulars of our case and because the surgical margins were negative, no postoperative chemotherapy or radiotherapy were used except for traditional Chinese medicine and thymosin. Metastases were found 5 mo after surgery, but the patient was alive as of this writing. In our opinion, although adenosquamous carcinoma of the pancreas with sarcomatoid change may indicate a poor prognosis, radical surgery without following aggressive treatment strategies may be more appropriate for the rare case.
The neoplasm in pancreas consisted of three different histopathological types, including not only adenocarcinoma and squamous carcinoma but also sarcoma.
Symptomatic cases may present as epigastric discomfort, such as epigastric fullness and poor appetite, similar to common adenosquamous carcinoma of the pancreas.
With aid of contrast-enhanced computed tomography (CECT) and magnetic resonance imaging (MRI), the neoplasm can be distinguished as solid-cystic mass, similar to common adenosquamous carcinoma of the pancreas.
Some serum tumor marker may elevate, such as carcinoma antigen (CA) 19-9 and CA-125, and didn’t have a significant different from common adenosquamous carcinoma of the pancreas. However, squamous cell carcinoma antigen may be normal.
Solid-cystic neoplasm in the CECT and MRI revealed adenosquamous carcinoma of the pancreas.
Three different histopathological types, including adenocarcinoma, squamous cellular components and sarcomatoid lesions could be found in neoplasm. Loss of membranous E-cadherin expression and acquired vimentin expression were observed in tumor cells of the sarcomatoid lesion.
Aggressive treatment strategies may be not appropriate for adenosquamous carcinoma of the pancreas with sarcomatoid change, however, R0 resection was still necessary.
R0 resection is still the first-line treatment for common adenocarcinoma of the pancreas.
Although no postoperative chemotherapy or radiotherapy was used, a relatively good prognosis still could be got on the base of R0 resection.
Adenocarcinoma of the pancreas is a rare disease, and adenosquamous carcinoma of the pancreas with sarcomatoid change has not been reported. This report provided a reference case and indicated that a routine radical surgery without aggressive treatment strategies was still appropriate for adenosquamous carcinoma of the pancreas with sarcomatoid change.
P- Reviewer: Azhar R, Bazhin AV, Nakai Y, Yee NS S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Hsu JT, Chen HM, Wu RC, Yeh CN, Yeh TS, Hwang TL, Jan YY, Chen MF. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol. 2008;6:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134:599-603. [PubMed] |
| 3. | Rahemtullah A, Misdraji J, Pitman MB. Adenosquamous carcinoma of the pancreas: cytologic features in 14 cases. Cancer. 2003;99:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 5. | Yoo HJ, Yun BR, Kwon JH, Ahn HS, Seol MA, Lee MJ, Yu GR, Yu HC, Hong B, Choi K. Genetic and expression alterations in association with the sarcomatous change of cholangiocarcinoma cells. Exp Mol Med. 2009;41:102-115. [PubMed] |
| 6. | Boyer B, Roche S, Denoyelle M, Thiery JP. Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J. 1997;16:5904-5913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 8. | Sabbah M, Emami S, Redeuilh G, Julien S, Prévost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, Wouters BG, Lammering G, Vooijs M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Liu YG, Sun KK, Sui XZ, Li JF, Wang J. Thymic carcinosarcoma consisting of sarcomatous and adenosquamous carcinomatous component. Chin Med J (Engl). 2012;125:4154-4155. [PubMed] |
| 11. | Sato Y, Shimozono T, Kawano S, Toyoda K, Onoe K, Asada Y, Hayashi T. Gastric carcinosarcoma, coexistence of adenosquamous carcinoma and rhabdomyosarcoma: a case report. Histopathology. 2001;39:543-544. [PubMed] |
| 12. | Zhao S, Xue Q, Ye B, Lu H, He J, Zhao H. Synchronous primary carcinosarcoma and adenosquamous carcinoma of the esophagus. Ann Thorac Surg. 2011;91:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7750] [Article Influence: 484.4] [Reference Citation Analysis (0)] |
| 15. | Olsen JR, Oyan AM, Rostad K, Hellem MR, Liu J, Li L, Micklem DR, Haugen H, Lorens JB, Rotter V. p63 attenuates epithelial to mesenchymal potential in an experimental prostate cell model. PLoS One. 2013;8:e62547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest Cancer Res. 2013;6:75-79. [PubMed] |
| 17. | Smoot RL, Zhang L, Sebo TJ, Que FG. Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg. 2008;207:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |