Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15682
Revised: May 4, 2014
Accepted: July 24, 2014
Published online: November 14, 2014
Processing time: 271 Days and 0.8 Hours
Resistance to 5-fluorouracil (5-FU), an important anticancer drug, is a serious challenge in the treatment of pancreatic cancer. Equilibrative nucleoside transporter 1 and multidrug-resistance protein (MRP) 5 and MRP8, rather than P-glycoprotein, play important roles in 5-FU transport. Thymidylate synthase, dihydropyrimidine dehydrogenase, methylenetetrahydrofolate reductase and thymidine phosphorylase are four key enzymes involved in 5-FU metabolism. Other metabolic enzymes, including uridine monophosphate synthetase, also contribute to chemoresistance. Intracellular signaling pathways are an integrated network, and nuclear factor kappa-light-chain-enhancer of activated B cells, AKT and extracellular signal-regulated kinases are signaling pathways that are particularly relevant to 5-FU resistance. In addition, recent reports indicate that STAT-3 is a crucial survival protein. Proteomic assays provide a powerful tool for identifying target proteins and understanding the role of microRNAs and stromal factors to facilitate the development of strategies to combat 5-FU resistance.
Core tip: 5-fluorouracil (5-FU) is one of the most important drugs for human pancreatic cancer. Although recent studies have questioned the effectiveness of 5-FU against pancreatic cancer, it remains a good choice for pancreatic cancer. Our paper discusses recent studies that provide novel insights into 5-FU chemotherapy in pancreatic cancer.
- Citation: Wang WB, Yang Y, Zhao YP, Zhang TP, Liao Q, Shu H. Recent studies of 5-fluorouracil resistance in pancreatic cancer. World J Gastroenterol 2014; 20(42): 15682-15690
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15682.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15682
Pancreatic cancer is one of the most fatal types of cancer worldwide, accounting for 3% of new cancer cases and 6% of all cancer-related deaths in the United States[1]. The annual death rate for pancreatic cancer patients has remained stable over the past 10 years, and approximately 4% of patients survive for 5 years after diagnosis[2]. 5-fluorouracil (5-FU), a widely accepted anti-cancer drug, was first introduced in 1957[3]. As a pyrimidine analog, 5-FU exerts its anticancer effects through the inhibition of thymidylate synthase (TS) and the incorporation of its metabolites into RNA and DNA[4,5]. Despite initial doubts concerning the efficacy of 5-FU, numerous studies have since demonstrated a valuable role for 5-FU in combined treatment protocols compared with single gemcitabine chemotherapy[6,7]. However, 5-FU chemoresistance, which may result from deficient drug uptake, alterations of targets, activation of DNA repair pathways, resistance to apoptosis and the tumor microenvironment, and other serious problems have been reported[8]. In this review, we will discuss recent studies that provide novel insights into the mechanisms of 5-FU resistance.
5-FU targets intracellular enzymes, and thus, the efficiency of 5-FU treatment depends on transport systems. However, there is little information regarding the role of transporters in mediating 5-FU resistance in pancreatic cancer. Nucleoside transporter systems, including human equilibrative nucleoside transporters (hENTs) and concentrative nucleoside transporters (hCNTs), particularly hENT1, play important roles in the cellular uptake and supply of nucleosides and nucleoside analogues. Tsujie et al[9] reported that high expression of hENT1 mRNA led to low sensitivity to 5-FU in pancreatic cancer, which suggests that hENT1 plays an important role in 5-FU resistance and that hENT1 mRNA levels might be a useful marker to predict 5-FU sensitivity in pancreatic cancer. Furthermore, Gao et al[10] observed that inhibition of hENT1 by dipyridamole (DP) could increase the intracellular concentration of 5-FU, thereby enhancing cytotoxicity in human pancreatic cancer cell lines. High expression of hENT1 may preferentially facilitate the uptake of nucleosides relative to 5-FU. Alternatively, hENT1 may provide a bilateral channel for 5-FU, whereas other transporters actively pump 5-FU into the cell. For example, 5-FU is a substrate of the human organic anion transporter 2 (hOat2, SLC22A7)[11] but not hCNT1[12]. Members of the ATP-binding cassette (ABC) transporter superfamily facilitate drug resistance via their role as efflux pumps. Interestingly, P-glycoprotein (P-gp, ABCB1), which is encoded by the multidrug resistance 1 gene (MDR1) and is the most common drug resistance ABC transporter, is not involved in 5-FU resistance[13], but the expression of multidrug-resistance protein 5 (MRP5, ABCC5)[14-16] and MRP8 (ABCC11)[17] is correlated with cellular 5-FU sensitivity.
The role of breast cancer resistance protein (BCRP, ABCG2) remains controversial. ABCG2 can transport the nucleotide CdAMP, similar to several other ATP-binding cassette transporters of the ABCC (multidrug resistance protein) family, and the nucleoside cladribine. In addition, the expression of ABCG2, a target gene of MSX2, correlates with chemoresistance in pancreatic cancer[18,19].
Previous studies focused primarily on genes involved in 5-FU metabolism. Four intracellular enzymes are considered key determinants in controlling 5-FU sensitivity or resistance: thymidylate synthase (TS, TYMS), dihydropyrimidine dehydrogenase (DPD), methylenetetrahydrofolate reductase (MTHFR) and thymidine phosphorylase (TP)[20]. The majority of studies have focused on gene polymorphisms or expression, and few have examined pancreatic cancer.
5-fluorodeoxyuridine monophosphate (5-FdUMP), the metabolite of 5-FU, directly binds to TS and inhibits its activity, catalyzing the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) using 5,10-methylene tetrahydrofolate (CH2THF) as the methyl donor[4]. Immunohistochemical analysis of paraffin-embedded tissues from 212 patients with pancreatic head or periampullary cancer following 5-FU-based adjuvant treatment revealed a significantly increased median survival of patients with low intratumoral TS expression compared with those patients with high TS expression[21]. Furthermore, high TS expression was an independent predictor of poor prognosis. The TS gene can be classified into two different “alleles” based on the expression of a 28-bp variable number tandem repeat (VNTR) in the 5’ untranslated region (UTR) of TS: either two repeats (2R) or three repeats (3R) (with three common genotypes, 2R/2R, 2R/3R and 3R/3R)[22]. In addition, the 3R allele can be subclassified according to the presence of a single nucleotide polymorphism (SNP) replacing a cytosine with a guanine (G/C) in 3R (3C or 3G)[23]. An analysis of a panel of seven pancreatic cancer cell lines revealed that cells of the 3R/3R genotype, which express high levels of the TS protein, exhibit lower sensitivity to 5-FU compared with cells of the 2R/2R or 2R/3R genotype[24]. In a clinical study of patients with metastatic gastrointestinal cancer, Cui et al[25] observed that patients with the 2R/3R genotype may be more sensitive to chemotherapeutic regimens, including 5-FU, than those with 3R/3R. By contrast, Hur et al[26] observed no significant difference in the tumor responses of 3R/3R and 2R/3R patients. In a meta-analysis of 20 studies, Wang et al[27] observed a significant increase in the overall survival of rectal cancer patients exhibiting the TS 3R/3R genotype. Taken together, these data highlight the need for further studies investigating the role of TS polymorphisms in 5-FU resistance.
TS, FdUMP and CH2FH4 form an inactive ternary complex that is stabilized by high CH2FH4 levels. MTHFR, a key regulatory enzyme involved in intracellular folate metabolism, converts CH2FH4 to 5-methyltetrahydrofolate (CH3FH4) and reduces 5-FU efficacy. The two most common polymorphisms linked to altered enzymatic activity are C677T and A1298C. In preclinical studies, the C677T mutation was associated with increased chemosensitivity of colon and breast cancers to 5-FU[28], and mutated A1298C variants exhibited enhanced 5-FU efficacy[29]. Clinical studies performed by Delgado-Plasencia et al[30] demonstrated that colorectal cancer (CRC) patients expressing variant T genotypes (CT or TT) at the C677T polymorphism exhibited a higher survival rate after chemotherapy than the homozygote CC variant. No significant associations between the MTHFR c.1298 genotypes or MTHFR diplotypes and survival were observed[31].
More than 80% of administered 5-FU is catabolized by DPD in the liver. Thus, patients with DPD enzyme deficiency are at risk for developing serious 5-FU toxicity. Previous studies have demonstrated an association between DPD expression and patient survival. Immunohistochemical analysis of DPD expression in 176 patients with upper tract urothelial carcinoma (UTUC) revealed no significant association between DPD levels and patient prognosis. However, significantly higher levels of cell growth inhibition and a higher IC50 value for 5-FU were observed in UMUC-3 cells following targeted silencing of DPD by siRNA compared with controls[32]. Ciaparrone et al[33] demonstrated that CRC patients receiving adjuvant, systemic 5-FU and exhibiting high DPD expression had significantly shorter disease-free survival and overall survival compared with patients with low DPD expression. An analysis of 15 human pancreatic cancer cell lines and two 5-FU-resistant sub-lines revealed a significant correlation between 5-FU IC50 values and the expression of TS × DPD (quantitative analyses of mRNA expression levels), suggesting that pancreatic cancer cells with high TS and/or DPD levels are more resistant to 5-FU[34].
The first step of activation of 5-FU in tumor tissues involves the conversion of 5-FU to fluorodeoxyuridine by TP. TP, also referred to as platelet-derived endothelial cell growth factor, is an angiogenic factor that promotes angiogenesis in vivo and stimulates the in vitro growth of a variety of endothelial cells. The role of TP in the clinical response to fluoropyrimidine-based chemotherapy is complex. In a clinical study involving 35 patients with newly diagnosed, locally advanced pancreatic cancer who received radiotherapy with capecitabine, which is metabolized to 5-FU by TP, Saif et al[35] revealed that a lower TP/DPD mRNA ratio was significantly associated with higher overall survival. Miyake et al[36] also observed this association in a cohort of 25 pancreatic cancer patients following immunohistochemical analysis of the TP/DPD ratio in their surgical specimens.
Furthermore, Griffith et al[37] observed differential expression of uridine monophosphate synthetase (UMPS) isoforms in the MIP101 and MIP/5-FU CRC cell lines and demonstrated that a low UMPS A/B isoform ratio, rather than the abundance of UMPS mRNA, might be predictive of 5-FU resistance.
Taken together, these studies indicate that intracellular nucleoside metabolic enzymes are promising candidates as mediators of 5-FU resistance.
DNA and/or RNA damage caused by 5-FU leads to the activation of DNA repair systems or apoptosis. Thus, the alteration of genes involved in cell cycle regulation, proliferation, repair and apoptosis plays an important role in 5-FU resistance.
To investigate genes involved in 5-FU resistance, Wang et al[38] performed gene expression analysis using HG-U133A arrays in five breast cancer cell lines, including the 5-FU resistant cell lines MCF-7FU1, MCF-7FU5 and T47DFU2.5 and their drug-sensitive parental counterparts, MCF-7WT and T47DWT. Significant down-regulation of key genes involved in 5-FU activation was observed in 5-FU resistant cells, including TK, UMPK and OPRT. Furthermore, overexpression of genes involved in cell cycle regulation, proliferation, repair and apoptosis, including TS, c-YES, NF-ES, p65 and c-Flip, was detected in the resistant cell lines. Cotransfection of NF-κB p50 and p65 cDNA induced 5-FU resistance in MCF-7 cells and reduced the expression of genes governing the G1-S and S-phase transitions. Cotransfection of NF-κB p50 and p65 cDNA induced 5-FU resistance in MCF-7 cells. Both NF-κB- and 5-FU-induced resistant cell lines exhibited reduced expression of genes governing G1-S and S-phase transitions. The expression of genes involved in DNA replication was also down-regulated in resistant cell lines. These findings were highly consistent with the slower growth rate, higher proportion of G1 cells and lower proportion of S-phase cells in the resistant cell lines. This phenotype may protect resistant cells from cell death induced by the incorporation of 5-FU into DNA chains by allowing time to repair 5-FU-induced damage[38].
Ischenko et al[39] tested the hypothesis that inhibition of Src tyrosine kinase could augment the chemosensitivity of the drug-resistant human pancreatic cancer cell lines AsPC5-FU RES and L3.6pl5-FU RES to 5-FU. The authors observed the following: (1) inhibition of Src tyrosine kinase activity by PP2 enhances 5-FU-induced cytotoxicity and induces apoptosis in 5-FU-resistant cells following 5-FU treatment; (2) Src specifically regulates 5-FU chemosensitivity in both the parental and chemotherapy-resistant cell lines; (3) overexpression of TS in chemotherapy-resistant cell lines is suppressed by PP2; and (4) 5-FU-induced EGFR–AKT pathway activation is affected by PP2 in chemotherapy-resistant cell lines, and PP2 restores the chemosensitivity of AsPC5-FU RES pancreatic tumors to 5-FU in vivo[39]. Taken together, these studies indicate that 5-FU resistance may be reversed by PP2, a Src tyrosine kinase inhibitor, via the EGFR-Akt pathway, by overcoming TS regulation. Zhao et al[40] also observed that pERK expression levels were noticeably increased in 5-FU-resistant SW1990/FU cells compared with their parental cell line. Treatment of SW1990/FU cells with the ERK inhibitor PD98059 sensitized cells to 5-FU by activating caspase-8 and reducing phospho-Bcl-2. Yoon et al[41] also reported that the AKT and ERK1/2 signaling pathways were activated in the 5-FU-resistant intrahepatic cholangiocarcinoma cell line SCKR. Bcl-2 expression was also elevated in these cells, and the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 was capable of altering this phenotype.
Can et al[42] demonstrated the importance of a Ca2+-calmodulin (CaM)-p53 axis in 5-FU-induced extrinsic apoptosis. Inhibition of this pathway using a Ca2+-chelator or inhibitors of CaM abrogated the ability of 5-FU to activate caspase-8 and inhibited subsequent cell death. Furthermore, both TS inhibition and misincorporation of 5-FU metabolites into RNA result in p53 stabilization, and p53 may be involved in downstream signaling pathways in response to 5-FU[4].
Dicitore et al[43] reported that aberrant constitutive activation of STAT3 protein is frequently detected in pancreatic adenocarcinoma, and type I interferons (IFNs), especially IFN-α, activated the JAK-2/STAT-3 pathway. Dicitore et al[44] also reported the therapeutic role of peroxisome proliferator-activated receptor γ (PPAR-γ) in combination with other drugs (IFNs, gemcitabine and COX-2 inhibitors), highlighting molecular interactions and signaling pathways involved in pancreatic cancer cells, including Ras/Raf/MAPK pathway, Akt/PKB signaling, and Erk-1/2 pathway. Vitale et al[45] treated human pancreatic cell line BxPC-3 with combination of recombinant IFN-β and PPAR-γ agonist troglitazone, and found a synergistic growth inhibition by MTT assay. Westen blot analysis showed that IFN-β-induced activation of STAT-3, MAPK, and Akt could be counteracted by TGZ-induced inactivation of STAT-3. The combination also decreased anti-autophagic bcl-2/beclin-1 complex formation due to inactivation of the Akt-mTOR-dependent pathway. Spitzner et al[46] recently demonstrated that STAT-3 inhibition sensitizes colorectal cancer to 5-FU–based chemoradiotherapy (CT/RT) both in vitro and in vivo. Inhibition of STAT3 by RNAi-mediated silencing in both SW480 and SW837 cell lines exposed to 3 μmol/L of 5-FU and irradiation, and a subcutaneous xenograft model led to profound CT/RT sensitization. The inhibitory effect of STAT-3 in pancreatic cancer is worth expecting.
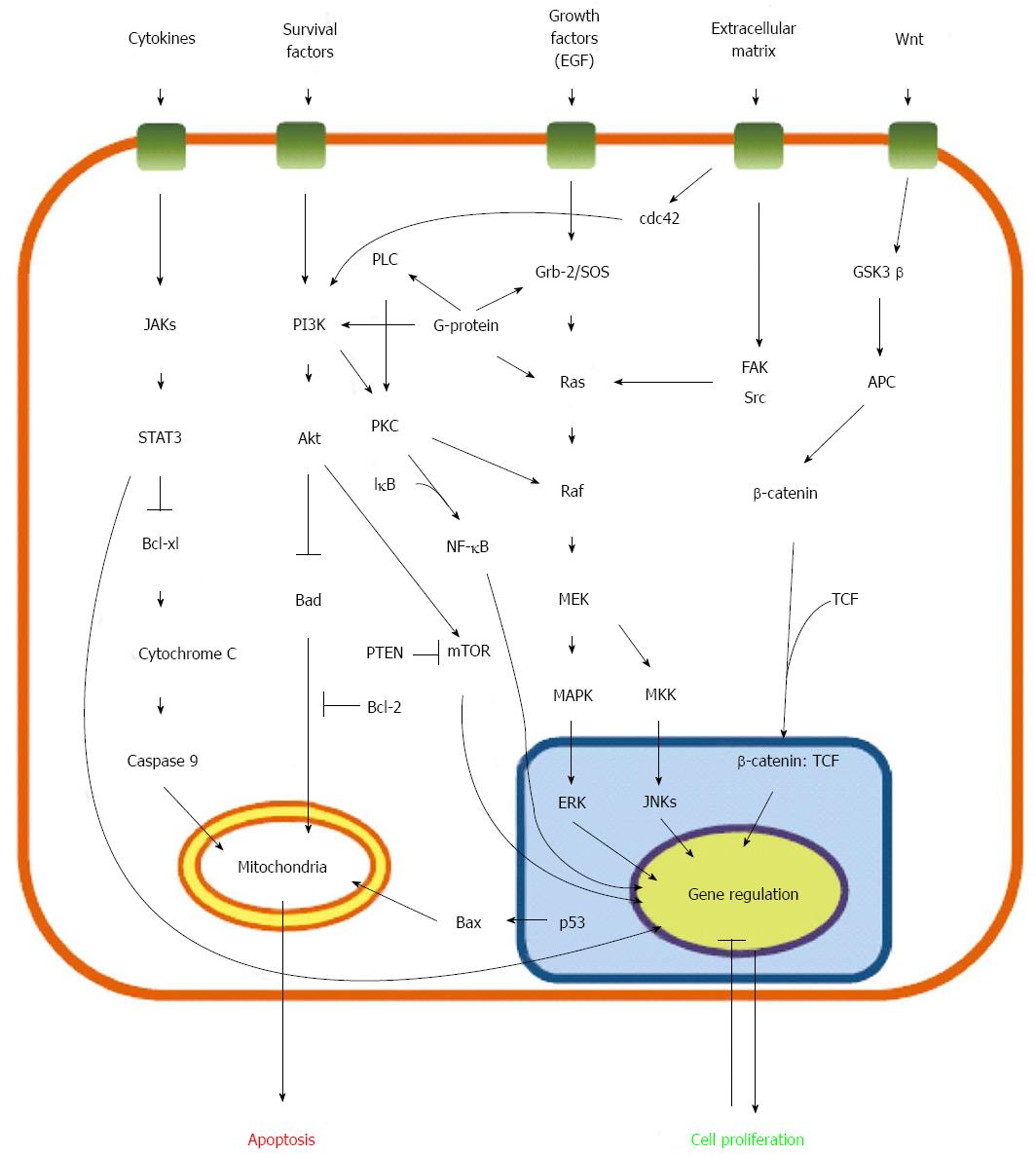
Cell signaling networks encompass numerous complicated pathways that involve significant crosstalk. To describe the main molecular mechanisms of 5-FU chemoresistance, we created an illustration based on many related studies; further studies are necessary to elucidate the roles of these networks (Figure 1).
Pancreatic cancer cells are typically surrounded by dense stroma. Stromal factors contribute significantly to the tumor microenvironment, but the role of the cancer microenvironment in 5-FU chemoresistance is just beginning to be explored. Sato et al[47] tested the sensitivity of MiaPaCa-2 and AsPC-1 cells to 5-FU following pre-incubation with recombinant annexin II (rANX II). In MiaPaCa-2 cells, treatment with rANX II led to the suppression of caspase-3 activation and increased Bcl-2/Bax ratio. Pre-incubation of cells with rANX II increased 5-FU resistance. Chen et al[48] demonstrated that the expression of focal adhesion kinase (FAK) related to 5-FU chemosensitivity involves an Akt/NF-kappaB signaling pathway in human CRC cells. Suppression of FAK expression significantly decreased 5-FU resistance and markedly increased the apoptosis of multicellular spheroid culture cells. Thus, 5-FU chemoresistance also requires the FAK/Akt/NF-κB survival signaling pathway. Expression of the obesity hormone leptin[49], stromal cell-derived factor-1α (SDF-1α)/CXCR4 cross-talk[50] and β6-integrin expression[51] have also been associated with 5-FU resistance in colon cancer cells. In addition to these classical signaling pathways, a new membrane receptor, calcium sensing receptor (CaSR), has also been shown to regulate drug resistance. Activation of CaSR by extracellular Ca2+ or its agonists enhanced the sensitivity of human colon carcinoma cells to 5-FU and down-regulated TS expression and the anti-apoptotic protein survivin[52]. Furthermore, the tumor-suppressive function of vitamin D in human colon carcinoma cells requires functional CaSR and promotes a cytotoxic response to 5-FU in a CaSR-dependent manner by suppressing the expression of TS and survivin[53]. Recent increased interest in pancreatic stellate cells should provide novel findings related to 5-FU resistance and the tumor microenvironment.
MicroRNAs (miRNAs) are small, 19-25 nucleotide (nt), non-coding RNAs that function as post-transcriptional regulators capable of blocking the translation of mRNAs into protein and/or promoting the degradation of target mRNAs. Kurokawa et al[54] profiled the expression of miRNAs in DLD-1/R and KM12C/R cells, two 5-FU-resistant colon cancer sub-lines derived from the DLD-1 and KM12C cell lines, using Agilent human miRNA microarrays (G4471A) that included 723 human and 76 human viral miRNAs from the Sanger miRBase release 10.1. The authors identified the specific up-regulation of eight miRNAs in DLD-1/R cells and 22 miRNAs in KM12C/R cells, in particular, miR-19b and miR-21. Subsequent miRNA: mRNA immunoprecipitation (RIP)-Chip analysis demonstrated that 66 mRNAs were recruited following the transfection of miR-19b into DLD-1 and DLD-1/R cells, including SFPQ (splicing factor proline and glutamate-rich), which has been linked to cell cycle function. SFPQ functions at different cell cycle stages to maintain sister chromatid interactions[55], and depletion of this gene has been shown to cause abnormal cell accumulation in the S phase of the cell cycle[56]. Similarly, Rossi et al[57] observed up-regulation of miR-19a (a paralog of miR-19b) and miR-21 in HT29 and HCT-119 colon cancer cells in response to 5-FU exposure. The majority of miR-21 targets are tumor suppressors, including PTEN[58,59], PDCD4[59] and Bcl-2[59]. By performing in silico analysis coupled to experimental validation, Boni et al[60] determined that miR-192 and miR-215 target TYMS expression in CRC cell lines; however, down-regulation of TYMS by miR-192/215 did not sensitize CRC cell lines to 5-FU treatment. Based on these results, the authors hypothesized that additional events induced by the knockdown of proteins targeted by miR-192 are likely involved in the cellular response to 5-FU independent of TYMS inhibition. Ectopic expression of miR-192 in RKO and LoVo cells transfected with siRNA targeting TYMS decreased 5-FU chemosensitivity. The functions of other miRNAs are currently under investigation, and the use of online databases will likely be critical in understanding their roles in mediating 5-FU resistance[61].
Proteomics is a powerful tool for detecting and identifying drug resistance-related proteins. Two-dimensional gel electrophoresis (2-DE) followed by mass spectrometry (MS), such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), is now a commonly employed method. A significant number of studies have investigated the mechanisms underlying gemcitabine resistance in pancreatic cancer cells[62-64], and similar studies related to 5-FU resistance are now underway. Yoshida et al[65] identified 40 differentially expressed protein spots between TS-1-resistant cells, PK45p and KLM-1, and TS-1-sensitive cells, Panc-1, BxPC-3, MiaPaCa-2 and PK59, using 2-DE and LC-MS/MS. TS-1 is a mixture of 5-FU and tegafur (FT), a metabolically activated prodrug of 5-FU. Among the 40 differentially expressed proteins, 29 were up-regulated, including hypoxia up-regulated protein 1 (oxygen regulated protein, ORP150) and annexin A1. Kimura et al[66] identified two ribosomal proteins, L15 and L37, by proteomic analysis of DLD-1 and DLD-1/5-FU cells by 2-DE and MALDI-TOF/TOF-MS/MS. Tan et al[67] identified 102 unique proteins, including p16, Maspin, PRDX6, PSMB7, MYL6, PHB and HSP27, in the altered hepatocellular carcinoma (HCC) cell line SMMC-7721/5-FU compared with parent cells. Furthermore, down-regulation of PRDX6 and PSMB7 enhanced 5-FU sensitivity in SMMC-7721/5-FU cells. Isobaric tags for relative and absolute quantitation (iTRAQ) is a non-gel-based technique used to quantify proteins from different sources in a single experiment followed by LC-MS/MS[68]. Using this technique, Tong et al[69] identified 52 proteins that were differentially expressed in the HCC cell line BEL7402/5-FU compared with its 5-FU-sensitive counterpart, BEL7402. Of these 52 differentially expressed proteins, 26 were increased in BEL7402/5-FU, notably annexin A3 (ANX3), one of the least-studied members of the annexin family. Importantly, suppression of ANX3 led to the enhancement of 5-FU sensitivity in BEL7402/5-FU cells. Although these experiments are associated with inherent technical variability, proteomic studies provide new targets for investigating novel mechanisms of 5-FU resistance.
More than 60 years after its development, 5-FU continues to be an important anticancer drug. However, more significant studies are required to understand the mechanisms underlying 5-FU resistance in pancreatic cancer. Screening at the genomic and proteomic levels has provided an abundance of candidate targets, and summarizing these studies and applying this knowledge for the development of successful 5-FU-based treatment strategies are essential. With comprehensive databases, the analysis of signaling networks, protein-protein interactions and intracellular-extracellular crosstalk is now possible. Multi-drug resistance studies have increased interest in drug-directed research. The study of chemoresistance mechanisms is likely to promote the application of novel, successful combination chemotherapy protocols for improved outcomes for cancer patients.
P- Reviewer: Di Lorenzo G, Vitale G S- Editor: Ma N L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2111] [Article Influence: 150.8] [Reference Citation Analysis (3)] |
| 3. | Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1023] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 4. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3616] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 5. | Phua LC, Mal M, Koh PK, Cheah PY, Chan EC, Ho HK. Investigating the role of nucleoside transporters in the resistance of colorectal cancer to 5-fluorouracil therapy. Cancer Chemother Pharmacol. 2013;71:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 997] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 7. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5612] [Article Influence: 400.9] [Reference Citation Analysis (1)] |
| 8. | Sheikh R, Walsh N, Clynes M, O’Connor R, McDermott R. Challenges of drug resistance in the management of pancreatic cancer. Expert Rev Anticancer Ther. 2010;10:1647-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Tsujie M, Nakamori S, Nakahira S, Takahashi Y, Hayashi N, Okami J, Nagano H, Dono K, Umeshita K, Sakon M. Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007;27:2241-2249. [PubMed] |
| 10. | Gao YM, Liu SL. The effect of human equilibrative nucleoside transport (hENTs) in pancreatic cancer cell membrane on the cytotoxicity of 5-fluorouracil. Xiandai Yixue. 2006;34:149-153. |
| 11. | Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005;57:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Mata JF, García-Manteiga JM, Lostao MP, Fernández-Veledo S, Guillén-Gómez E, Larrayoz IM, Lloberas J, Casado FJ, Pastor-Anglada M. Role of the human concentrative nucleoside transporter (hCNT1) in the cytotoxic action of 5[Prime]-deoxy-5-fluorouridine, an active intermediate metabolite of capecitabine, a novel oral anticancer drug. Mol Pharmacol. 2001;59:1542-1548. [PubMed] |
| 13. | Guo JC, Zhao YP, Liao Q, Zhu Y. [Significance and reversal of MDR1/P-gp in pancreatic cancer chemotherapy]. Zhonghua Waike Zazhi. 2007;45:1488-1490. [PubMed] |
| 14. | Hagmann W, Jesnowski R, Faissner R, Guo C, Löhr JM. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Nambaru PK, Hübner T, Köck K, Mews S, Grube M, Payen L, Guitton J, Sendler M, Jedlitschky G, Rimmbach C. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos. 2011;39:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Li Y, Revalde JL, Reid G, Paxton JW. Modulatory effects of curcumin on multi-drug resistance-associated protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol. 2011;68:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Oguri T, Bessho Y, Achiwa H, Ozasa H, Maeno K, Maeda H, Sato S, Ueda R. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther. 2007;6:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, Beijnen J, Borst P. Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008;7:3092-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H, Masamune A, Kikuta K, Kume K, Shimosegawa T. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2012;227:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Scartozzi M, Maccaroni E, Giampieri R, Pistelli M, Bittoni A, Del Prete M, Berardi R, Cascinu S. 5-Fluorouracil pharmacogenomics: still rocking after all these years? Pharmacogenomics. 2011;12:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | van der Zee JA, van Eijck CH, Hop WC, van Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM, Ten Hagen TL. Expression and prognostic significance of thymidylate synthase (TS) in pancreatic head and periampullary cancer. Eur J Surg Oncol. 2012;38:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kawakami K, Watanabe G. Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res. 2003;63:6004-6007. [PubMed] |
| 23. | Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz HJ, Ladner RD. A novel single nucleotide polymorphism within the 5’ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res. 2003;63:2898-2904. [PubMed] |
| 24. | Zhang Q, Zhao YP, Liao Q, Hu Y, Xu Q, Zhou L, Shu H. Associations between gene polymorphisms of thymidylate synthase with its protein expression and chemosensitivity to 5-fluorouracil in pancreatic carcinoma cells. Chin Med J (Engl). 2011;124:262-267. [PubMed] |
| 25. | Cui YH, Liu TS, Zhuang RY, Gao HJ, Li H. Polymorphism of thymidylate synthase gene and chemosensitivity of 5-fluorouracil regimen in metastatic gastrointestinal cancer. J Dig Dis. 2009;10:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Hur H, Kang J, Kim NK, Min BS, Lee KY, Shin SJ, Keum KC, Choi J, Kim H, Choi SH. Thymidylate synthase gene polymorphism affects the response to preoperative 5-fluorouracil chemoradiation therapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2011;81:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. BMC Gastroenterol. 2012;12:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J Natl Cancer Inst. 2004;96:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Etienne MC, Ilc K, Formento JL, Laurent-Puig P, Formento P, Cheradame S, Fischel JL, Milano G. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms: relationships with 5-fluorouracil sensitivity. Br J Cancer. 2004;90:526-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Delgado-Plasencia L, Medina-Arana V, Bravo-Gutiérrez A, Pérez-Palma J, Álvarez-Argüelles H, Salido-Ruiz E, Fernández-Peralta AM, González-Aguilera JJ. Impact of the MTHFR C677T polymorphism on colorectal cancer in a population with low genetic variability. Int J Colorectal Dis. 2013;28:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Sharma R, Hoskins JM, Rivory LP, Zucknick M, London R, Liddle C, Clarke SJ. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms and toxicity to capecitabine in advanced colorectal cancer patients. Clin Cancer Res. 2008;14:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Ide H, Kikuchi E, Hasegawa M, Kozakai N, Kosaka T, Miyajima A, Oya M. Prognostic significance of 5-fluorouracil metabolism-relating enzymes and enhanced chemosensitivity to 5-fluorouracil by 5-chloro 2,4-dihydroxy-pyridine in urothelial carcinoma. BMC Cancer. 2012;12:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ciaparrone M, Quirino M, Schinzari G, Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G, Barone C. Predictive role of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer patients receiving adjuvant 5-fluorouracil. Oncology. 2006;70:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Kurata N, Fujita H, Ohuchida K, Mizumoto K, Mahawithitwong P, Sakai H, Onimaru M, Manabe T, Ohtsuka T, Tanaka M. Predicting the chemosensitivity of pancreatic cancer cells by quantifying the expression levels of genes associated with the metabolism of gemcitabine and 5-fluorouracil. Int J Oncol. 2011;39:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Saif MW, Hashmi S, Bell D, Diasio RB. Prognostication of pancreatic adenocarcinoma by expression of thymidine phosphorylase/dihydropyrimidine dehydrogenase ratio and its correlation with survival. Expert Opin Drug Saf. 2009;8:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Miyake K, Imura S, Yoshizumi T, Ikemoto T, Morine Y, Shimada M. Role of thymidine phosphorylase and orotate phosphoribosyltransferase mRNA expression and its ratio to dihydropyrimidine dehydrogenase in the prognosis and clinicopathological features of patients with pancreatic cancer. Int J Clin Oncol. 2007;12:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Griffith M, Mwenifumbo JC, Cheung PY, Paul JE, Pugh TJ, Tang MJ, Chittaranjan S, Morin RD, Asano JK, Ally AA. Novel mRNA isoforms and mutations of uridine monophosphate synthetase and 5-fluorouracil resistance in colorectal cancer. Pharmacogenomics J. 2013;13:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Wang W, Cassidy J, O’Brien V, Ryan KM, Collie-Duguid E. Mechanistic and predictive profiling of 5-Fluorouracil resistance in human cancer cells. Cancer Res. 2004;64:8167-8176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 755] [Reference Citation Analysis (0)] |
| 39. | Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, Schwarz B, Graeb C, Eichhorn ME, Jauch KW. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212-7222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Zhao Y, Shen S, Guo J, Chen H, Greenblatt DY, Kleeff J, Liao Q, Chen G, Friess H, Leung PS. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J Surg Res. 2006;136:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Yoon H, Min JK, Lee JW, Kim DG, Hong HJ. Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun. 2011;405:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Can G, Akpinar B, Baran Y, Zhivotovsky B, Olsson M. 5-Fluorouracil signaling through a calcium-calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene. 2013;32:4529-4538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Dicitore A, Caraglia M, Gaudenzi G, Manfredi G, Amato B, Mari D, Persani L, Arra C, Vitale G. Type I interferon-mediated pathway interacts with peroxisome proliferator activated receptor-γ (PPAR-γ): at the cross-road of pancreatic cancer cell proliferation. Biochim Biophys Acta. 2014;1845:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Dicitore A, Caraglia M, Colao A, Zappavigna S, Mari D, Hofland LJ, Persani L, Vitale G. Combined treatment with PPAR-γ agonists in pancreatic cancer: a glimmer of hope for cancer therapy? Curr Cancer Drug Targets. 2013;13:460-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Vitale G, Zappavigna S, Marra M, Dicitore A, Meschini S, Condello M, Arancia G, Castiglioni S, Maroni P, Bendinelli P. The PPAR-γ agonist troglitazone antagonizes survival pathways induced by STAT-3 in recombinant interferon-β treated pancreatic cancer cells. Biotechnol Adv. 2012;30:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 46. | Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz J. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Sato T, Kita K, Sugaya S, Suzuki T, Suzuki N. Extracellular release of annexin II from pancreatic cancer cells and resistance to anticancer drug-induced apoptosis by supplementation of recombinant annexin II. Pancreas. 2012;41:1247-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Chen Y, Wang Z, Chang P, Xiang L, Pan F, Li J, Jiang J, Zou L, Yang L, Bian Z. The effect of focal adhesion kinase gene silencing on 5-fluorouracil chemosensitivity involves an Akt/NF-kappaB signaling pathway in colorectal carcinomas. Int J Cancer. 2010;127:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Heckmann D, Maier P, Laufs S, Wenz F, Zeller WJ, Fruehauf S, Allgayer H. CXCR4 Expression and Treatment with SDF-1α or Plerixafor Modulate Proliferation and Chemosensitivity of Colon Cancer Cells. Transl Oncol. 2013;6:124-132. [PubMed] |
| 51. | Liu S, Wang J, Niu W, Liu E, Wang J, Peng C, Lin P, Wang B, Khan AQ, Gao H. The β6-integrin-ERK/MAP kinase pathway contributes to chemo resistance in colon cancer. Cancer Lett. 2013;328:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Liu G, Hu X, Varani J, Chakrabarty S. Calcium and calcium sensing receptor modulates the expression of thymidylate synthase, NAD(P)H: quinone oxidoreductase 1 and survivin in human colon carcinoma cells: promotion of cytotoxic response to mitomycin C and fluorouracil. Mol Carcinog. 2009;48:202-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int J Cancer. 2010;126:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Kurokawa K, Tanahashi T, Iima T, Yamamoto Y, Akaike Y, Nishida K, Masuda K, Kuwano Y, Murakami Y, Fukushima M. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. J Gastroenterol. 2012;47:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Rajesh C, Baker DK, Pierce AJ, Pittman DL. The splicing-factor related protein SFPQ/PSF interacts with RAD51D and is necessary for homology-directed repair and sister chromatid cohesion. Nucleic Acids Res. 2011;39:132-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Salton M, Lerenthal Y, Wang SY, Chen DJ, Shiloh Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle. 2010;9:1568-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 57. | Rossi L, Bonmassar E, Faraoni I. Modification of miR gene expression pattern in human colon cancer cells following exposure to 5-fluorouracil in vitro. Pharmacol Res. 2007;56:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2191] [Cited by in RCA: 2183] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 59. | Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584-2595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 60. | Boni V, Bitarte N, Cristobal I, Zarate R, Rodriguez J, Maiello E, Garcia-Foncillas J, Bandres E. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Mol Cancer Ther. 2010;9:2265-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 61. | Rukov JL, Wilentzik R, Jaffe I, Vinther J, Shomron N. Pharmaco-miR: linking microRNAs and drug effects. Brief Bioinform. 2014;15:648-659. [PubMed] |
| 62. | Chen YW, Liu JY, Lin ST, Li JM, Huang SH, Chen JY, Wu JY, Kuo CC, Wu CL, Lu YC. Proteomic analysis of gemcitabine-induced drug resistance in pancreatic cancer cells. Mol Biosyst. 2011;7:3065-3074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Kuramitsu Y, Taba K, Ryozawa S, Yoshida K, Zhang X, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Identification of up- and down-regulated proteins in gemcitabine-resistant pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2010;30:3367-3372. [PubMed] |
| 64. | Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Taba K, Fujimoto M, Okita K, Nakamura K, Sakaida I. A proteomic profiling of gemcitabine resistance in pancreatic cancer cell lines. Mol Med Rep. 2008;1:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Yoshida K, Kuramitsu Y, Murakami K, Ryozawa S, Taba K, Kaino S, Zhang X, Sakaida I, Nakamura K. Proteomic differential display analysis for TS-1-resistant and -sensitive pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2011;31:2103-2108. [PubMed] |
| 66. | Kimura K, Wada A, Ueta M, Ogata A, Tanaka S, Sakai A, Yoshida H, Fushitani H, Miyamoto A, Fukushima M. Comparative proteomic analysis of the ribosomes in 5-fluorouracil resistance of a human colon cancer cell line using the radical-free and highly reducing method of two-dimensional polyacrylamide gel electrophoresis. Int J Oncol. 2010;37:1271-1278. [PubMed] |
| 67. | Tan Y, Qin S, Hou X, Qian X, Xia J, Li Y, Wang R, Chen C, Yang Q, Miele L. Proteomic-based analysis for identification of proteins involved in 5-fluorouracil resistance in hepatocellular carcinoma. Curr Pharm Des. 2014;20:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Zieske LR. A perspective on the use of iTRAQ reagent technology for protein complex and profiling studies. J Exp Bot. 2006;57:1501-1508. [PubMed] |
| 69. | Tong SW, Yang YX, Hu HD, An X, Ye F, Hu P, Ren H, Li SL, Zhang DZ. Proteomic investigation of 5-fluorouracil resistance in a human hepatocellular carcinoma cell line. J Cell Biochem. 2012;113:1671-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |