Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1079
Revised: October 14, 2013
Accepted: November 18, 2013
Published online: January 28, 2014
Processing time: 163 Days and 7.3 Hours
AIM: To investigate the association between endogenous hydrogen sulfide (H2S) and portal hypertension as well as its effect on vascular smooth muscle cells.
METHODS: Portal hypertension patients were categorized by Child-Pugh score based on bilirubin and albumin levels, prothrombin time, ascites and hepatic encephalopathy. Plasma H2S concentrations and portal vein diameters (PVDs) were compared between portal hypertension patients and control participants, as well as between portal hypertension patients with varying degrees of severity. In addition, we established a rabbit hepatic schistosomiasis portal hypertension (SPH) model and analyzed liver morphology, fibrosis grade, plasma and liver tissue H2S concentrations, as well as cystathionine γ-lyase (CSE) activity and phosphorylated extracellular signal-regulated kinase (pERK)1/2, B cell lymphoma (Bcl)-2 and Bcl-XL expression in portal vein smooth muscle cells, in addition to their H2S-induced apoptosis rates.
RESULTS: In portal hypertension patients, endogenous H2S levels were significantly lower than those in healthy controls. The more severe the disease was, the lower were the H2S plasma levels, which were inversely correlated with PVD and Child-Pugh score. Liver tissue H2S concentrations and CSE expression were significantly lower in the SPH rabbit livers compared with the control animals, starting at 3 wk, whereas pERK 1/2 expressions gradually increased 12-20 wk after SPH model establishment. In portal vein smooth muscle cells, increasing H2S levels led to increased apoptosis, while Bcl-2 and Bcl-XL expression decreased.
CONCLUSION: H2S prevents vascular restructuring caused by excessive proliferation of smooth muscle cells via apoptosis induction, which helps to maintain normal vascular structures.
Core tip: In portal hypertension patients, endogenous hydrogen sulfide (H2S) levels were significantly lower than those in healthy controls. H2S plasma level reductions correlated with portal vein diameter and Child-Pugh score. In a rabbit hepatic schistosomiasis portal hypertension model, liver tissue H2S concentrations and cystathionine γ-lyase expression were significantly reduced and phosphorylated extracellular signal regulated kinase 1/2 expression gradually increased. Increasing H2S levels led to increased apoptosis of portal vein smooth muscle cells, while B-cell lymphoma-2 (Bcl-2) and Bcl-XL expression decreased. We suggest that H2S prevents portal hypertension though apoptosis induction of otherwise excessive proliferating smooth muscle cells.
- Citation: Wang C, Han J, Xiao L, Jin CE, Li DJ, Yang Z. Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World J Gastroenterol 2014; 20(4): 1079-1087
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1079.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1079
Cirrhosis is a common chronic liver disease with various causes, and in the late stage, the main symptoms are liver function impairment and portal vein hypertension, which eventually lead to esophagogastric junction vascular diseases. Major critical pathophysiological processes accompanying the progression of cirrhosis are structural changes in the liver tissue and changes in vasoactive substance activation[1]. Recent studies have shown that the gaseous molecule hydrogen sulfide (H2S) has biological signaling characteristics similar to NO and CO, and is involved in pathophysiological processes leading to portal hypertension through multiple mechanisms. H2S, following NO and CO, is the third discovered endogenous gas signaling molecule, while various studies since the 1990s have shown that endogenous H2S exists in many mammalian tissues and organs[2-5], serving as vascular and digestive tract smooth muscles relaxant, affecting long-term potentiation (LTP) induction in the hippocampus[6], regulating the secretion of corticotrophin-releasing hormone (CRH) in the hypothalamus[7], inhibiting the proliferation of vascular smooth muscle cells[8], and inducing smooth muscle relaxation in the human corpora cavernosum[9]. In mammals, there are two pathways for endogenous H2S production: enzymatic and non-enzymatic. In the enzymatic pathway, which is the major source of endogenous H2S, it is produced by cysteine breakdown through the actions of cystathionine-B-synthase (CBS) and cystathionine-γ-lyase (CSE). In the non-enzymatic pathway, glucose undergoes glycolysis and then combines with sulfur in the blood, thereby producing a small amount of H2S. CBS and CSE are expressed in a tissue-specific manner; hepatocytes express both enzymes, whereas hepatic stellate cells (HSCs) express only CSE, and sinusoidal endothelial cells express neither[10]. Endogenous H2S participates in the regulation of smooth muscle relaxation, therefore, it has been shown that it plays an important role in the development of primary hypertension and in the regulation of cardiovascular system functions and structures. The CSE/H2S pathway participates in the pathophysiological processes of cardiovascular diseases, such as hypoxic pulmonary arterial hypertension and high pulmonary blood flow, leading to pulmonary arterial hypertension[11]. H2S is an important vasoactive substance, thus, increasing attention has focused on its role in the development of portal hypertension. A 2003 study by Poliakova et al[12] found that a mixture of gases containing H2S could induce biochemical restructuring of rat livers. Both long-term exposure (> 2 wk) to a low dose and short-term exposure to a high dose of the H2S-containing gaseous mixture could lead to reversible changes in the liver. Although there have been studies about H2S involvement in cirrhosis-induced portal hypertension, detailed mechanisms remain unclear. Some researchers[13-15] have speculated that H2S participates in portal hypertension through the following three pathways: (1) regulation of smooth muscle relaxation; (2) inhibition of vascular smooth muscle proliferation; and (3) induction of vascular smooth muscle apoptosis. In this study we collected data about endogenous H2S levels and other clinical data from patients with portal hypertension and also established rabbit portal hypertension as well as primary portal vein smooth muscle cell models in order to study correlations between H2S and portal hypertension and its mode of action.
This study included 200 patients with cirrhosis-induced portal hypertension who were treated in the Department of General Surgery of the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, from December 2008 to December 2011. Clinical data and blood samples were collected from all 200 patients (140 males and 60 females, mean age 44 ± 14 years) and pathological studies identified 77 cases of hepatitis-induced cirrhosis, 82 of schistosomiasis-induced cirrhosis, 32 of cirrhosis of mixed causes, two of Caroli disease, four of portal vein cavernous transformation, and three of alcohol-induced cirrhosis. The control group comprised 100 healthy individuals examined at Tongji Hospital during the same period, consisting of 50 men and 50 women aged 47 ± 13 years. Exclusion criteria were chronic illnesses such as hypertension and pulmonary arterial hypertension, which could affect endogenous H2S levels. Diagnosis of portal hypertension was made according to published guidelines[16] and portal vein diameter (PVD) was determined by Doppler ultrasound; if PVD was ≥ 1.3 cm and accompanied with cirrhosis, the patient was diagnosed with portal hypertension. Child-Pugh scores were evaluated based on bilirubin and albumin levels, prothrombin time, and the presence of ascites and hepatic encephalopathy. All participants gave their written informed consent and the study was approved by the Ethical Committee of the Tongji Hospital.
Upon admission, 3 mL venous blood was collected from all participants in both groups, stored in sealed tubes for 2 h, and centrifuged at 3000 r/min for 10 min to separate the plasma and stored again at -70 °C in eppendorf (EP) tubes (Eppendorf, Hamburg, Germany). Plasma and liver tissue H2S contents were measured according to Chunyu et al[17]. The resultant supernatant was analyzed using a UV spectrophotometer at 665 nm and calculated with a standard curve obtained from NaHS solutions. Plasma H2S concentrations were expressed in μmol/L and liver tissue H2S contents were shown in nmol/mg wet tissue/min. All measurements were performed in triplicate and results were expressed as mean ± SD.
Thirty healthy adult long-eared white rabbits (purchased from Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) with a body weight of 2.5-3.5 kg, regardless of sex, were randomly divided into a control (n = 10) and model (n = 20) group.
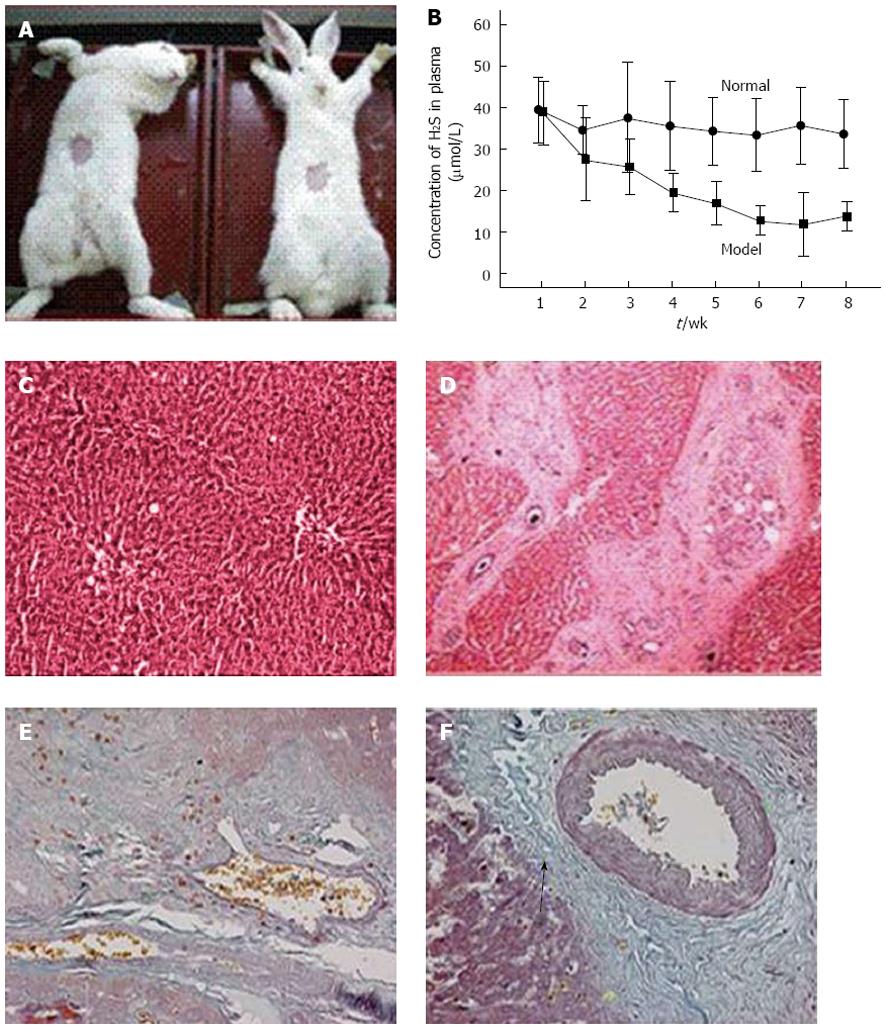
The animal model was established in the Schistosomiasis Research Facility, Hubei Provincial Disease Prevention and Control Center. Abdominal patches were used to infect the rabbits with schistosomal cercariae to induce a rabbit SPH model. The Schistosoma japonicum cercariae were collected from snails in Hubei Province. A 2 cm × 2 cm patch was collected into the abdominal region of the rabbits, creating a wound (Figure 1A). During wound cleansing, the cercariae were counted under a microscope (180 ± 10/rabbit) and the coverslips with the cercariae were located on the exposed skin for 15-20 min, leading to acute schistosomiasis infection. After infection, the animals were kept in rabbit rooms in the Tongji Hospital Laboratory Animal Facility. The control rabbits were treated similarly, apart from not being infected with the cercariae.
At 8, 12 and 16 wk after infection, five or six animals from the model group and three from the control group were randomly selected and peripheral venous blood samples were collected from the ear vein. The animals were sacrificed using air embolism; after which, portal vein blood was drawn and samples from liver and spleen tissues as well as portal vein blood vessels were taken. The tissue samples were stored in liquid nitrogen, while the blood samples were stored in a freezer.
Using the method reported by Stipanuk et al[18], CSE activity was determined in a 1.0-mL volume by incubation of 100 mmol/L potassium phosphate buffer, pH 7.5, 4.0 mmol/L L-cystathionine, 0.125 nmol/L pyridoxal 5’-phosphate, 0.32 nmol/L NADH, 1.5 U lactate dehydrogenase, and 25 or 50 μL of the 20000 g supernatant prepared from liver homogenate in a 1.5 mL semi-micro quartz cuvette ( 10 mm light path) at 37 °C. The decrease in OD340 gave a linear response to decreasing NADH concentrations, corresponding to lactate synthesis via L-cysteine-derived pyruvate accumulation and lactate dehydrogenase activity.
The rabbit liver tissues were fixed in a 10% formalin solution, embedded in paraffin, cut into 4-μm slices, stained with hematoxylin and eosin (HE), and sealed with neutral resin. Liver histology was observed under a microscope.
The rabbit liver tissues were fixed in a 10% neutral formalin solution, embedded in paraffin, sliced, stained with potassium dichromate, acid fuchsin and aniline blue, and sealed with neutral resin. Morphological changes in the liver tissue were observed under an optical microscope. Collagen fibers were stained blue-green, muscle fibers and cellulose were stained red, and nuclei were stained blue to black.
Portal vein smooth muscle cells were grown in complete culture medium at 37 °C for 1-2 d and cell growth was monitored under a microscope. Cells were replated at 80%-90% confluence. Culture medium was changed on the basis of the actual cell growth.
Portal vein smooth muscle cells were inoculated on glass slides after conventional digestion. On the next day, the cells were washed gently with PBS and 3.7% formaldehyde solution was added for 30 min at room temperature, after which they were washed three times with PBS for 2 min each. The cells were permeated for 10 min with 0.1% Triton-X-100 solution (prepared in PBS) and blocked with 10% goat serum (prepared in PBS) for 30 min, followed by incubation with rat α-smooth muscle actin (SMA) antibody (1:200 in PBS) at room temperature for 2 h. After washing three times with PBS, for 5 min each, the cells were incubated with fluorescent secondary antibody (1:200 dilute by PBS; Sigma, Shanghai, China) at room temperature in darkness for 1 h, followed by three times with PBS for 5 min each. Finally, we detected the fluorescence intensity using a fluorescence microscope (440/510 nm, excitation/emission) after adding fluorescent mounting medium.
Cultured portal vein smooth muscle cells at 80% confluence were harvested from 6-cm dishes, washed with PBS, and collected into 5 mL centrifuge tubes in triplicate. Cells (1 × 106) were prepared for apoptosis assays with an Annexin V/FITC Apoptosis Dectection Kit, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, United States) and then analyzed by flow cytometry.
Rabbit portal vein-tunica media dissections were prepared for detecting phosphorylated extracellular signal-regulated kinase (pERK)1/2 expression levels, and primary portal vein smooth muscle cells were used for analyzing B-cell lymphoma-XL (Bcl-XL) and Bcl-2, as well as β-actin expression. Protein concentrations were equalized after total protein extraction with lysate buffer and loading buffer was added, and samples were boiled and stored at -20 °C for further use. Ten microliters of the samples were subjected to SDS-PAGE and the protein was transferred to polyvinylidene difluoride membranes via wet electroblotting (21 V, 40 min). After 2 h blocking at room temperature in 5% bovine serum albumin, primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, United States) for Bcl-XL (1:400), Bcl-2 (1:400) and pERK1/2 (1:500) were added and the membranes were incubated for 2 h at room temperature and kept at 4 °C overnight. Samples were washed in Tris-buffered saline-Tween 20 (TBST), incubated with horseradish-peroxidase-conjugated secondary antibodies at room temperature for 90 min, and washed again with TBST. The membranes were placed in an imaging system to measure the signal intensities using chemiluminescent reagents.
Rabbit liver samples were cut into 1 mm3 pieces, fixed in 2.5% phosphate-buffered glutaraldehyde for 2 h, and washed in phosphate buffer for 30 min. Samples were fixed in osmium tetroxide for 2 h and then washed in phosphate buffer for 30 min. Samples were incubated in 50% ethanol for 15 min, 70% ethanol for 40 min, 90% ethanol for 15 min, 90% acetone/90% ethanol (1:1) for 15 min, 90% acetone for 15 min, 100% acetone for 3 × 10 min, 100% acetone/embedding medium (1:1) overnight, pure embedding medium for 5 h, and embedded overnight. Sample slices were stained with toluidine blue and cut into ultrathin 100 nm slices. Samples were stained with uranyl acetate and lead nitrate and observed under a transmission electron microscope to observe ultrastructural pathological changes.
Data were analyzed using SPSS version 12.0 software. Quantitative variables were described using mean ± SD in the case of normal distribution and each sample was measured at least three times. Possible differences between groups were evaluated using analysis of variance (ANOVA) in the case of continuous data. In the case of overall significant differences as a result of ANOVA, pair-wise t tests were conducted using the closed testing procedure. Analysis of PVD and endogenous H2S concentrations was done by linear correlation. Statistical significance was considered if P < 0.05.
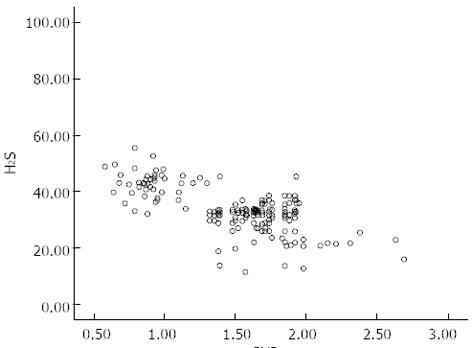
There was no significant difference in age between the control and portal hypertension groups. However, in the portal hypertension group, endogenous plasma H2S levels were significantly lower than those in the control group, correlating with disease severity. Furthermore, plasma H2S concentrations correlated inversely with PVD (r = -0.478, P < 0.05) (Table 1, Figure 1).
| Portal hypertension group (n = 200) | Control group (n = 100) | |
| Age (yr) | 43.6 ± 14.4 | 47.1 ± 12.6 |
| Albumin (g/dL) | 33.0 ± 3.7 | 38.4 ± 4.1 |
| TB (μmol/L) | 31 ± 24 | 14 ± 13 |
| ALT (U/L) | 37 ± 29 | 25 ± 26 |
| PVD (cm) | 1.5 ± 0.4a | 1.1 ± 0.3 |
| H2S | 43.5 ± 6.2 | |
| Child–Pugh score | ||
| A (H2S) n = 48 | 42.6 ± 4.7a | |
| B (H2S) n = 125 | 33.5 ± 7.7bc | |
| C (H2S) n = 27 | 22.2 ± 7.9cdf |
During the progression of schistosomiasis-induced cirrhosis in rabbits, plasma H2S concentrations showed a clearly descending trend and starting from week 3, there were significant differences compared to the control group (Figure 2B). Furthermore, HE staining of the SPH rabbit livers showed that the liver hepatocytes of the control group were neatly arranged with no significant proliferation of fibrous tissue in the portal areas and lobular structures were maintained (Figure 2C). In contrast, in the portal areas of the SPH rabbit livers, fibrous larval nodules were arranged in concentric rings and there were also significant proliferation and widening of the fibrous connective tissues in the portal areas with hyaline appearance (Figure 2D). There was a small amount of collagen fibers in the central venous tissue of normal livers (Figure 2E), whereas in the SPH rabbit livers, a larger amount of collagen fibers stained blue-green (arrow) (Figure 2F).
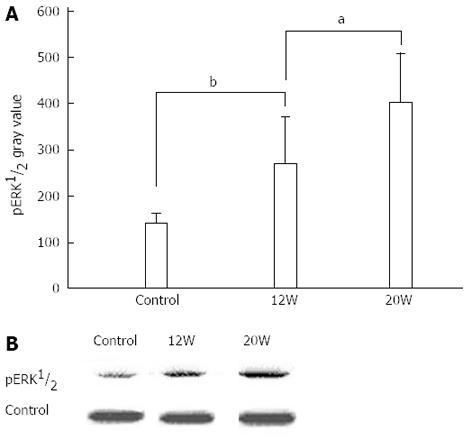
CSE activity and H2S concentrations were both lower in the SPH rabbit livers (Table 2). In order to elucidate whether the H2S-related differences had an effect on cell proliferation-related proteins, we measured pERK 1/2 expression levels in dissected SPH rabbit liver portal vein-tunica intima tissues and found that pERK 1/2 protein levels increased significantly with disease progression in the rabbit portal hypertension model (Figure 3).
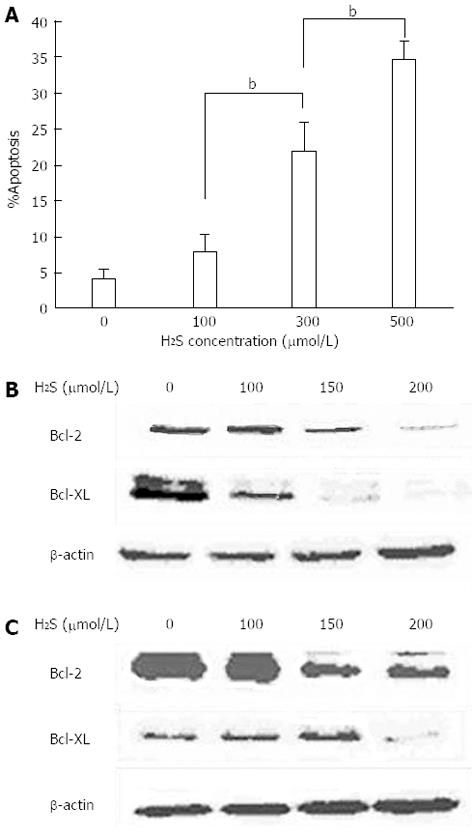
We collected primary rabbit portal vein smooth muscle cells and after cultivation, added H2S to the medium to observe its effect on apoptosis and Bcl-2 and Bcl-XL expression. Flow cytometry revealed that, with increasing concentrations of H2S in the cell culture medium, the apoptosis rate of the portal vein smooth muscle cells also increased (Figure 4A). In addition, with increasing H2S concentration, there was also decreased expression of the anti-apoptotic genes Bcl-2 and Bcl-XL (Figure 4B and C).
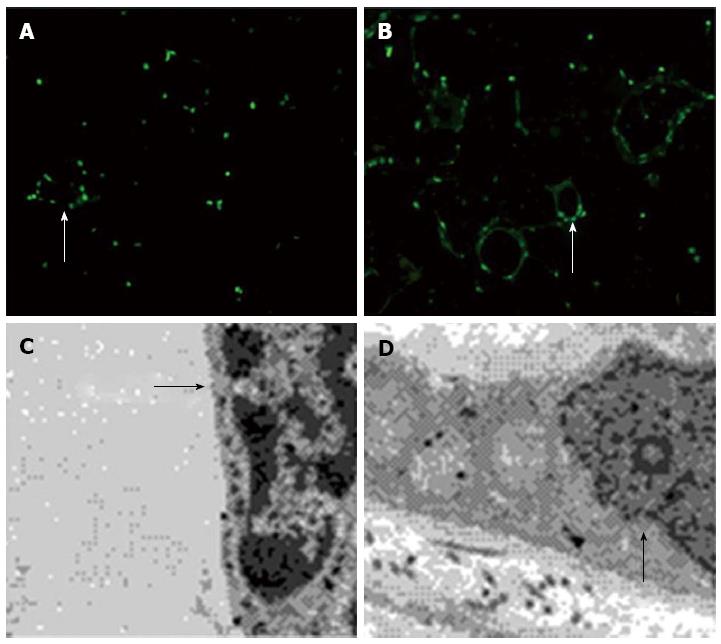
Our immunofluorescence apoptosis assay showed that vascular smooth muscle cells of the omentum underwent significant apoptosis under normal H2S concentrations (50 μmol.L), which was reduced without H2S (Figure 5A and B). Under the electron microscope, we observed that with 50 μmol/L H2S in the medium, the nuclear membrane in some cells disintegrated, the nucleoplasm condensed, and the cells clearly underwent irreversible cell death. In contrast, when smooth muscle cells were cultured without H2S, the nucleus was round or had minor indentations, there was abundant evenly distributed euchromatin and the nucleolus was clearly visible. The mitochondria were round or kidney-shaped and the overall structural integrity was maintained (Figure 5C and D). These results indicated that a certain concentration of H2S leads to vascular smooth muscle cell apoptosis.
Portal hypertension is one of the two most serious conditions resulting from cirrhosis. Signs include opening of the systemic portal collateral circulation, splenomegaly, hypersplenism and ascites. Portal hypertension arises when liver fibrosis and regenerative nodules create pressure on the liver sinusoids and hepatic veins, thus increasing portal vein resistance. Impaired liver function and an imbalance of various vasoactive substances, which can result from multiple causes, are important factors in maintaining and exacerbating portal hypertension[19]. The main cause of increased resistance is that contraction of sinusoidal endothelial cells increases resistance in the liver sinusoids and contraction reactivity of intrahepatic blood vessels, leading to increased contractility in the portal venous system. Increased liver sinusoidal resistance is in turn dependent on HSC contraction. It is reported that portal hypertension can be caused by increased production of vasoconstrictors and decreased production of vasodilators. H2S is an important vasodilator in the hepatic microcirculation and causes relaxation of vascular smooth muscles[20,21]. In a previous study, NaHS solutions were injected into the abdominal cavity of rats with cirrhosis for 5 d, and NaHS was perfused through the liver tissue outside the body. NaHS significantly relaxed vascular smooth muscles and reduced HSC contraction, thus reducing intrahepatic resistance[10]. These results showed to a certain extent that treatment with exogenous H2S can reverse the excessive intrahepatic resistance caused by decreased production of endogenous H2S in cirrhosis. Our study also showed in line with the previous findings that patients with portal hypertension had lower endogenous H2S concentrations than healthy controls, and that the lower the concentration, the more severe was the disease, while plasma H2S concentrations were inversely related with PVD. Our SPH results showed that rabbits with schistosomiasis-induced portal hypertension had lower plasma H2S concentrations after 3 wk than the control group had, and CSE activity in their tissue declined. A previous article reported that reduced CSE expression in cirrhotic liver contributed to the development of increased intrahepatic resistance and portal hypertension[10]. Hepatocyte apoptosis plays an important role in normal liver development and in various liver diseases[22,23]. When HSCs proliferate, they produce extracellular matrix and collagen, which leads to liver fibrosis, and it has been reported that H2S induces apoptosis[24] and inhibits HSC activation[25], which reduces vascular restructuring and aggravates hypertension. We found that H2S led to reduced expression of antiapoptotic Bcl-2 and Bcl-XL proteins, in addition to elevated apoptosis.
In summary, we found that portal hypertension patients had significantly lower serum H2S concentrations and that disease severity and PVD were correlated with H2S concentration. In addition, our SPH model revealed that liver cirrhosis led to low serum and liver tissue H2S concentrations, and reduced liver tissue CSE activity, while pERK1/2 expression gradually increased. In conclusion, we suggest that H2S deprivation may play a role in the initiation, progression and exacerbation of cirrhosis-related portal hypertension through reduction of portal vein smooth muscle cell apoptosis and concomitant pathological blood vessel restructuring.
We specially thank Jian-Li Wu for her help with the project design.
Major critical pathophysiological processes of cirrhosis are structural changes in the liver tissues leading to portal hypertension.
H2S, following NO and CO, is the third discovered endogenous gas signaling molecule, serving as a relaxant of vascular and digestive tract smooth muscles, inhibiting the proliferation of vascular smooth muscle cells, and inducing smooth muscle relaxation in the human corpora cavernosum.
This study found that portal hypertension patients had significant lower serum H2S concentrations and that the severity of the disease and portal vein diameter correlated with H2S concentration. H2S concentration and cystathionine γ-lyase (CSE) expression were significantly lower in schistosomiasis portal hypertension (SPH) rabbit livers, and phosphorylated extracellular signal-regulated kinase (pERK)1/2 expression was increased. In portal vein smooth muscle cells, increasing H2S levels led to increased apoptosis, while B-cell lymphoma (Bcl)-2 and Bcl-XL expression decreased.
H2S might be applied for treatment of liver cirrhosis.
pERK1/2, after stimulation by mitogens, hormones or growth factors, is involved in cell growth and differentiation, whereas Bcl-2 and Bcl-XL are antiapoptotic genes. Without H2S, high pERK1/2 and Bcl2 Bcl-XL activities lead to enhanced proliferation and reduced apoptosis rates in liver cells.
In their manuscript the authors first describe their clinical observation, that there is a correlation between H2S serum levels and portal vein diameter in portal hypertension patients. Then they extended their research on a rabbit hepatic schistosomiasis portal hypertension model and analyzed morphological changes and expression of the proliferation and apoptosis related genes. They concluded that H2S might be an important signal molecule for the integrity of hepatic veins. The research is interesting and might lead to a new approach for treatment of portal hypertension.
P- Reviewers: Gentilucci UV, Klinge U S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Laleman W. Role of vasoactive substances and cellular effectors in the pathophysiology of cirrhotic portal hypertension: the past, the present and the future--Georges Brohée Lecture. Acta Gastroenterol Belg. 2009;72:9-16. [PubMed] |
| 2. | Gharib-Naseri MK, Saberi S, Mard SA, Latifi SM. Bronchodilatory effect of hydrogen sulfide in rat. Iran J Basic Med Sci. 2012;15:907-915. [PubMed] |
| 3. | Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta. 2013;1832:1989-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Pan LL, Liu XH, Shen YQ, Wang NZ, Xu J, Wu D, Xiong QH, Deng HY, Huang GY, Zhu YZ. Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int J Cardiol. 2013;168:3770-3778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 961] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 6. | Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066-1071. [PubMed] |
| 7. | Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Chen XB, Du JB, Zhang CY, Tang CS, Zhou WJ. [Effect of hydrogen sulfide, a new gaseous signal molecule, on pulmonary vascular smooth muscle cell apoptosis in rats]. Beijing Da Xue Xue Bao. 2004;36:341-344. [PubMed] |
| 9. | d’Emmanuele di Villa Bianca R, Sorrentino R, Maffia P, Mirone V, Imbimbo C, Fusco F, De Palma R, Ignarro LJ, Cirino G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci USA. 2009;106:4513-4518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 421] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 11. | Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, Chunyu Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun. 2004;317:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Poliakova VS, Shakhlamov VA, Stadnikov AA, Solnyshkova TG. [Structural-biochemical reorganization of rat liver caused by hydrogen sulfide-containing gas mixture]. Morfologiia. 2003;124:84-87. [PubMed] |
| 13. | Ebrahimkhani MR, Mani AR, Moore K. Hydrogen sulphide and the hyperdynamic circulation in cirrhosis: a hypothesis. Gut. 2005;54:1668-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Köhn C, Dubrovska G, Huang Y, Gollasch M. Hydrogen sulfide: potent regulator of vascular tone and stimulator of angiogenesis. Int J Biomed Sci. 2012;8:81-86. [PubMed] |
| 15. | Schleifenbaum J, Köhn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Zhu YZ. A Surgical Illustration of Portal Hypertension. Shenyang: Liaoning Science and Technology Press 2006; 68-126. |
| 17. | Chunyu Z, Junbao D, Dingfang B, Hui Y, Xiuying T, Chaoshu T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267-277. [PubMed] |
| 19. | Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: a regulator of hepatobiliary function. Hepatology. 2000;31:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Liu H, Zheng Y, Chen W, Zhao J, Li R, Zhang N, Liu F, Yan J. Effect of endogenous hydrogen sulfide on apoptosis of cirrhosis rat liver cells. Shijie Huaren Xiaohua Zazhi. 2012;20:670-674. |
| 21. | Liu Y, Li Y, Yang W, Cao G. H2 S inhibits the activation of hepatic stellate cells and downregulates the expression of urotensin II. Hepatol Res. 2013;43:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 23. | Kiliçarslan A, Kahraman A, Akkiz H, Yildiz Menziletoğlu S, Fingas CD, Gerken G, Canbay A. Apoptosis in selected liver diseases. Turk J Gastroenterol. 2009;20:171-179. [PubMed] |
| 24. | Fan HN, Wang HJ, Yang-Dan CR, Ren L, Wang C, Li YF, Deng Y. Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep. 2013;7:247-253. [PubMed] |
| 25. | Lu F, Xing J, Zhang X, Dong S, Zhao Y, Wang L, Li H, Yang F, Xu C, Zhang W. Exogenous hydrogen sulfide prevents cardiomyocyte apoptosis from cardiac hypertrophy induced by isoproterenol. Mol Cell Biochem. 2013;381:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |