Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13159
Revised: May 12, 2014
Accepted: June 26, 2014
Published online: September 28, 2014
Processing time: 251 Days and 5.5 Hours
AIM: To investigate the incidence of de novo hepatitis B virus (HBV) infection after pediatric living donor liver transplantation (LDLT) and to analyze the risk factors associated with this de novo HBV infection.
METHODS: The clinical and laboratory data of children who underwent LDLT from June 2010 to September 2012 in First Center Hospital in Tianjin, China, were retrospectively included in the study. Intrahepatic HBV DNA in donors and recipients was quantified by real-time polymerase chain reaction using DNA extracted from formalin-fixed, paraffin-embedded tissues.
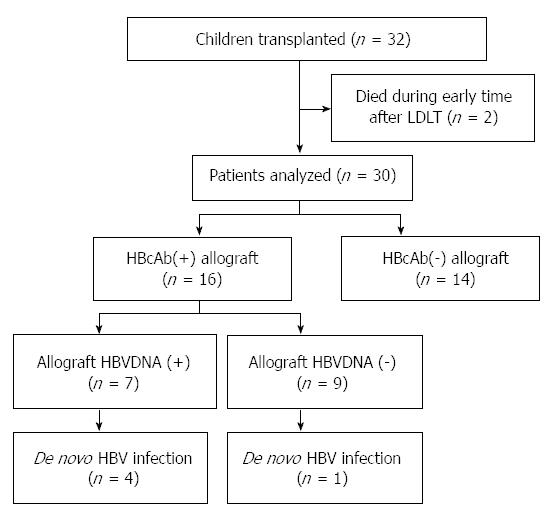
RESULTS: Between June 2010 to September 2012, 32 consecutive pediatric patients underwent LDLT in our institute. Thirty LDLT patients (13 girls and 17 boys) were followed up for a median of 15 mo, of whom 53.3% (16/30) were hepatitis B core antibody (HBcAb) positive and 36.7% (11/30) were hepatitis B surface antibody (HBsAb)/HBcAb positive before transplantation. Sixteen of the children received HBcAb-positive allografts, and 43.7% (7/16) of the grafts were found to be intrahepatic HBV DNA positive. De novo HBV infection developed in 16.1% (5/30) of the children within a median of 11 mo after transplantation. All five of the HBV-infected children had received HBcAb-positive allografts, four of which were intrahepatic HBV DNA positive. Two of the children developed de novo HBV infection despite the preoperative presence of both HBsAb and HBcAb
CONCLUSION: In pediatric recipients, positive intrahepatic HBV DNA in allografts could be a risk factor for de novo HBV infection from HBcAb-positive allografts. HBsAb/HBcAb positivity in pediatric LDLT patients before transplantation exhibited only weak effectiveness in protecting them against de novo HBV infection from HBcAb-positive allografts.
Core tip: We reported our experience of de novo hepatitis B virus (HBV) infection after pediatric living donor liver transplantation (LDLT), which showed that the incidence of de novo HBV infection in pediatric LDLT was 16.7% in our center. Among hepatitis B core antibody (HBcAb)-positive allografts, 43.7% were intrahepatic HBV DNA positive. Positive intrahepatic HBV DNA in allografts was predictive of de novo HBV infection after LDLT in children who were given HBcAb-positive allografts. Hepatitis B surface antibody/HBcAb positivity exhibited weak effectiveness in preventing children receiving HBcAb-positive allografts from de novo HBV infection. Lamivudine prophylaxis therapy is essential to prevent de novo HBV infection in pediatric patients from HBcAb-positive allografts with positive intrahepatic HBV DNA.
-
Citation: Rao W, Xie M, Yang T, Zhang JJ, Gao W, Deng YL, Zheng H, Pan C, Liu YH, Shen ZY. Risk factors for
de novo hepatitis B infection in pediatric living donor liver transplantation. World J Gastroenterol 2014; 20(36): 13159-13166 - URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13159
It is well known that the use of hepatitis B core antibody (HBcAb)-positive liver allografts carries a 48%-58% risk of de novo hepatitis B virus (HBV) infection in HBV-naïve patients without a prophylaxis strategy[1,2]. Hence, many authors have suggested prohibiting or restricting the use of HBcAb-positive allografts in certain groups of recipients. However, this approach is not practical in HBV-endemic areas, such as China, where almost 10% of the general population is hepatitis B surface antigen (HBsAg) positive and approximately 50% of the adults are HBcAb positive[3-5]. In China, HBcAb-positive allografts have not been a remarkable problem in adults because HBV-related disease is a dominant indication for liver transplantation (LT) for adults, and it has been reported that the HBV serological status of the donors did not affect the graft survival rate if the recipients had a similar HBV status[6]. However, HBcAb-positive donors pose a difficult problem in pediatric liver transplants because non-HBV-related liver disease is the main indication for pediatric LT in China[7,8]. Although there have been great achievements in adult and pediatric living donor liver transplantation in recently years all over the world[6,7,9], little data are available on de novo HBV infection in pediatric patients who receive allografts from HBcAb-positive donors, and pertinent risk factors analysis has rarely been performed. The aim of our study is to investigate the prevalence of de novo HBV infection in pediatric liver transplant recipients and to analyze the risk factors associated with the development of de novo HBV infection in these patients.
Between June 2010 to September 2012, 32 consecutive pediatric patients who underwent living donor liver transplantation (LDLT) in the organ transplantation department of the First Center Hospital in Tianjin, China, were recruited retrospectively. Exclusion criteria included lost to follow-up, incomplete data or death within 3 mo of LDLT.
The medical records of all enrolled patients were reviewed, including the following: age, gender, date of liver transplant, and vaccination status; HBV serological markers in recipients and donors before and after transplantation, including HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antigen antibody (anti-HBe), and HBcAb; serum HBV DNA before and after transplantation (a value less than 100 IU/mL was considered negative); pediatric model of end-stage liver disease (PELD) score before transplantation; blood transfusion, level of immunosuppressants, dosage of steroids, biochemical levels; and follow-up time. All clinical data were followed up as of October 2013.
The immunosuppressive regimen in these patients were maintained as tacrolimus (TAC) combined with corticosteroids in the first 6 mo. Intravenous basiliximab (10 mg) was used intraoperatively and on the fourth postoperative day. The blood trough level of TAC after transplantation was kept in the range of 10-15 ng/mL in the first month, then 5-8 ng/mL. Corticosteroid was withdrawn gradually within 6 mo after liver transplantation in all patients.
Samples of donor and recipient liver tissues were tested for occult HBV DNA according to previously reported techniques[9,10]. Briefly, DNA was extracted from freshly cut sections of formalin-fixed, paraffin-embedded liver tissues, with a thickness of 5 μm and an area of almost 20 mm2, using the QIAamp DNA FFPE Tissue kit (56404, Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. The storage time of the selected liver tissues varied from 1 year up to 3 years. DNA was eluted in 100 μL of Buffer ATE and was stored at -70 °C until the time of analysis. A 50-μL aliquot was put aside for the quantification of total HBV DNA and β-actin DNA. The remaining 50 μL was treated with 40 units of Plasmid-Safe adenosine triphosphate-dependent DNase (Epicentre Technologies, Madison, WI, United States) for 30 min at 37 °C to digest relaxed circular and single-stranded forms of HBV DNA. The DNase enzyme was inactivated by incubation at 70 °C for 30 min.
Ten liver samples from HBsAg-positive recipients were collected as positive controls. Ten liver specimens from HBV-uninfected patients [HBsAg and HBcAb negative with undetectable HBV DNA in serum by polymerase chain reaction (PCR)] were analyzed as negative controls.
An external standard curve was constructed by using 10-fold serial dilutions of a plasmid of known concentration carrying the entire HBV genome (genotype B) for real-time PCR analysis. A linear relationship was observed over the plasmid concentration range of 102-108 copies/mL. The standard curve was included in each real-time PCR run for total HBV DNA and HBV covalently closed circular DNA (cccDNA), and the inter-run correlation coefficient of the standard curve was routinely greater than 0.990. All real-time PCR analysis was performed in an ABI PRISM 7500 sequence detector system (Applied Biosystems, Foster City, CA, United States).
Total HBV DNA was measured using a primer set located within the S gene. The primer and probe sequences are as follows: forward primer (nt 361-381), 5’-CACATCAGGATTCCTAGGACC-3’; reverse primer (nt 419-398), 5’ GCAGCAGGATGCATAGGAAGAT-3’; and TaqMan probe (nt 382-396), 5’-TCTGCGGCGTTTTAT-3’. HBV cccDNA was measured using a pair of cccDNA-selective primers and a probe that targets the gap region between the two direct repeat regions of the viral genome. The primer and probe sequences are as follows: forward primer (nt 1596-1612), 5’-TCTGCACGTCGCATGGA-3’; reverse primer (nt1678-1652), 5’-CAGAGAGTCCAAGAGTCCTCTTATGT-3’; and TaqMan probe (nt1624-1638), 5’-CCGCCCACCAGTTCT-3’. A reporter dye (6-carboxy-fluorescein, FAM) was attached to the 5’ end and a quencher dye (non-fluorescent quencher, NFQ) was incorporated at the 3’ end of the probe. Briefly, real-time PCR was performed in a 20-μL reaction mixture containing 10 μL of TaqMan Universal PCR Master Mix (ABI; Roche, Branchburg, NJ, United States), 1.5 mmol/L MgCl2, 0.6 pmol of each primer, 0.3 pmol of the probe, and 5 μL of extracted DNA. Real-time PCR was performed with an initial 2-min incubation at 50 °C for uracil-N-glycosylase activity, which was followed by a 10-min incubation at 95 °C. Subsequently, 40 cycles of 95 °C for 15 s and 60 °C for 60 s were performed. All specimens were run in duplicate, and negative and positive controls were included in each run.
Quantification of β-actin was used to estimate the amount of genomic DNA in each liver sample. β-Actin amplification was performed by real-time PCR using the TaqMan β-actin control kit (PE Applied Biosystems, Foster City, CA, United States). Serial dilutions of human genomic DNA served as standards for the β-actin quantification. The conversion factor of 6.667 pg of human genomic DNA per cell was used. The amount of HBV DNA in the liver tissues was expressed as copies per cell.
After liver transplantation, HBV serological and virological markers were routinely monitored every 3 mo in the first year and every 6 mo thereafter and observed for abnormal liver function. De novo HBV infection was defined as detectable serum HBsAg (with or without biochemical features of hepatitis) and histologic and immunohistochemical confirmation of HBV by liver biopsy after liver transplantation in those who were negative for serum HBsAg before liver transplantation.
The performance of this study was approved by the Ethical Affairs Committee of Tianjin First Central Hospital and it adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patients’ parents or guardians.
Data are presented as mean ± SD or as median and range as appropriate. Independent t tests or Mann-Whitney rank sum tests were used for continuous variables. Categorical variables were compared with the χ2 test. All statistical analyses were performed by SPSS (Version 17.0, SPSS, Inc., Chicago, IL, United States). A P-value less than 0.05 was considered statistically significant.
Between June 2010 to September 2012, 32 patients were admitted to our center for LDLT diagnosed as biliary atresia. Two patients were excluded from the study because of death from multiple organ failure (unrelated to HBV) within 3 mo after LT, leaving 30 eligible patients finally. A flow-chart summary of the patients enrolled is presented in Figure 1.
The median age of the patients was 12 mo (range: 6-57 mo), including 13 girls and 17 boys. The average PELD score before LT was 17 ± 4. All 30 children received one dose of HBV vaccine at birth, but none of them received the other two doses of HBV vaccine owing to hyperbilirubinemia or malnutrition.
Among the 30 pediatric patients, the median follow-up period was 15 mo (range: 4-40 mo), and all patients survived through the last follow-up except one who died of fulminant hepatic failure caused by HBV and Epstein-Barr virus co-infection, even though they were receiving active antivirus therapy with acyclovir and entecavir for 4 mo after LDLT, 2 wk after de novo HBV infection was diagnosed.
All transplants involved living donors, and the average age of the donors (17 female and 13 male patients) was 32 years. Before transplantation, the rates of HBcAb, HBsAb, and HBsAb/HBcAb positivities in the pediatric patients were 53.3% (16/30), 66.7% (20/30), and 36.7% (11/30), respectively, whereas in the donors, the rates were 53.3% (16/30), 66.7% (20/30), and 46.7% (14/30), respectively. Both serum HBsAg and HBV DNA before transplantation were negative in all of these recipients and donors.
During the follow-up period, 16.1% (5/30) of children experienced de novo HBV infection after a median of 11 mo (range: 3-25 mo) after LDLT, with both serum HBsAg and HBV DNA being positive. The median age of the five children at the time of their de novo HBV infection diagnosis was 25 mo. Among the five children, two were HBV naive, one was HBsAb positive, and the remaining two were HBsAb/HBcAb positive before LT. Abnormal liver function was observed in two of the five children when de novo HBV infection was diagnosed, one of whom died of fulminant hepatic failure, as mentioned above, and the other survived with normal liver function at the last follow-up. The other three children had normal liver function through the last follow-up (Table 1). None of the children in this group received antiviral treatment because of young age and the risk of virus mutation.
| Pre-operation data (serum) | Allograft | De novo HBV infection | HBV status follow-up | |||||||||||
| No. | HBsAb (IU/mL) | HBcAb (IU/mL) | HBVDNA (IU/mL) | HBsAb (IU/mL) | HBcAb (IU/mL) | HBVDNA/cccDNA (copies/cell) | Time after LT (mo) | HBsAg (IU/mL) | Serum HBVDNA (IU/mL) | Liver enzymes | HBsAb (IU/mL) | Serum HBVDNA (IU/mL) | Liver enzymes | Follow-up (mo) |
| 1 | < 2 | 1.04 | < 100 | 251.8 | 0.010 | 2.57/0.08 | 25.4 | 1348 | 1.62 × E8 | N | 4745 | 1.62 × E8 | N | 35.3 |
| 2 | < 2 | 1.36 | < 100 | 462.4 | 0.007 | Neg/neg | 10.6 | 164.9 | 1.12 × E2 | AN | 5791 | 1.62 × E8 | N | 17.0 |
| 3 | < 2 | 0.98 | < 100 | 262.5 | 0.339 | 1.50/0.09 | 12 | 3608 | 2.90 × E9 | N | 3608 | 2.9 × E9 | N | 16.8 |
| 4 | 164.4 | 0.03 | < 100 | < 2 | 0.007 | 12.4/0.15 | 3.4 | 746.1 | 7.14 × E8 | AN | 1866 | 7.14 × E8 | AN | 4.0 |
| 5 | 31.4 | 0.23 | < 100 | 210.0 | 0.009 | 3.61/0.09 | 8.7 | 2345 | 1.46 × E8 | N | 3213 | 1.46 × E8 | N | 15.2 |
The children were divided into two groups according to the HBcAb status of the donors: the HBcAb(+) group (n = 16) and the HBcAb(-) group (n = 14). The de novo HBV infection rates of the two groups were analyzed, which showed that HBcAb positivity in allografts was a risk factor for de novo HBV infection after LT (P = 0.02). Other donor and patient factors analyzed showed no significant differences between the two groups. Of the donors, age, gender, and preoperative HBsAb positive rate were similar between the two groups. Of the pediatric recipients, no significant differences were observed in age, gender, pre-LT PELD score, and pre-LT HBV serum markers (HBcAb/HBsAb, HBcAb, and HBsAb statuses), amount of blood transfusion, dosage of corticosteroids, trough level of TAC, and follow-up time between the two groups (Table 2).
| Characteristic | HBcAb(+) donor(n = 16) | HBcAb(-) donor(n = 14) | P value |
| Donors | |||
| Gender (n, M/F) | 7/9 | 9/5 | 0.261 |
| Age (yr) | 35 (20-55) | 32 (22-54) | 0.884 |
| HBsAb (+/-) | 14/2 | 9/5 | 0.134 |
| Recipients | |||
| Age (mo) | 11 (7-43) | 12 (6-57) | 0.787 |
| Gender (n, M/F) | 9/7 | 8/6 | 0.981 |
| PELD score | 16 ± 3 | 17 ± 4 | 0.378 |
| Preoperative HBcAb (n, +/-) | 11/5 | 5/9 | 0.070 |
| Preoperative HBsAb (n, +/-) | 11/5 | 7/7 | 0.300 |
| Preoperative HBsAb/HBcAb (n, +/-) | 9/7 | 3/11 | 0.052 |
| Blood transfusion | |||
| RBC (mL) | 502 ± 284 | 534 ± 224 | 0.379 |
| FFP (mL) | 404 ± 177 | 424 ± 210 | 0.753 |
| Dose of corticosteroid/body weight (mg/kg) | 44 ± 7 | 42 ± 8 | 0.359 |
| Trough value of TAC | |||
| First 3 mo (ng/mL) | 8.1 ± 5.6 | 7.8 ± 4.6 | 0.789 |
| 3 mo later (ng/mL) | 6.7 ± 3.2 | 6.4 ± 2.3 | 0.661 |
| Follow-up (mo) | 15 (4-40) | 16 (14-30) | 0.228 |
| De novo HBV infection (n, +/-) | 5/11 | 0/14 | 0.022 |
To analyze the effect of HBcAb-positive allografts on the occurrence of de novo HBV infection after LT, the 16 children who received HBcAb-positive allografts were classified as a de novo HBV infection (+) group (n = 5) and a de novo HBV infection (-) group (n = 11). The incidence of de novo HBV infection in children with HBcAb-positive allografts was 31.3% (5/16).
Factors that might affect the incidence of de novo HBV infection from HBcAb-positive allografts were analyzed. Of the donors, the basic characteristics of age, gender, and HBsAb status were similar between the two groups (P > 0.05, Table 3). Of the pediatric recipients, no significant differences were observed in terms of their age, gender, PELD score before LT, HBV serology (HBcAb/HBsAb, HBcAb, and HBsAb status before LT), blood transfusion, dosage of corticosteroids, trough level of TAC, and follow-up time between the two groups (P > 0.05, Table 3). However, a higher rate of positive intrahepatic HBV DNA in grafts was observed in children who developed de novo HBV infection than in children who did not (4/1 vs 3/8, P = 0.04).
| Characteristics | De novo HBV infection (n = 5) | De novo HBV infection-free (n = 11) | P value |
| Donors | |||
| Age (yr) | 38 (22-55) | 30 (27-50) | 0.223 |
| Gender (n, M/F) | 3/2 | 5/6 | 0.590 |
| HBsAb (n, +/-) | 4/1 | 10/1 | 0.541 |
| Intrahepatic HBV DNA (n, +/-) | 4/1 | 3/8 | 0.043 |
| Recipients | |||
| Age (mo) | 14 (10-18) | 10 (8-43) | 0.113 |
| Gender (n, M/F) | 2/3 | 6/5 | 0.590 |
| PELD score | 17 ± 3 | 17 ± 4 | 0.469 |
| Preoperative HBcAb (n, +/-) | 3/2 | 3/8 | 0.610 |
| Preoperative HBsAb (n, +/-) | 2/3 | 2/9 | 0.350 |
| Preoperative ant-HBs/HBcAb (n, +/-) | 2/3 | 7/4 | 0.377 |
| Blood transfusion | |||
| RBC (mL) | 420 ± 220 | 540 ± 172 | 0.216 |
| FFP (mL) | 416 ± 184 | 400 ± 183 | 0.908 |
| Dose of prednisone/bodyweight (mg/kg) | 46 ± 6 | 43 ± 8 | 0.318 |
| Follow-up (mo) | 17 (4-35) | 14 (12-40) | 0.356 |
Intrahepatic HBV DNA and cccDNA were detectable in 43.7% (7/16) of the HBcAb-positive allografts. The median values of intrahepatic HBV DNA and HBV cccDNA were 3.87 copies per cell (range: 0.5-12.4 copies per cell) and 0.09 copies per cell (range: 0.07-0.15 copies per cell), respectively. The median intrahepatic HBV DNA level in the samples of the positive control group was 172.9 copies per cell (range: 20.0-1444.64 copies per cell), which was significantly higher than that in HBcAb-positive allografts (P < 0.01). None of the patient samples in the negative control group were intrahepatic HBV DNA positive: all of the 16 naive livers were intrahepatic HBV DNA and cccDNA negative.
Without any prophylaxis treatments, the incidence of de novo HBV infection in the pediatric patients in our study was 16.7% (5/30), whereas the incidence was 15.3%-23.9% in previous studies[7,12,13]. In our study, 53.3% (16/30) of the donors were positive for HBcAb, which is consistent with the epidemiologic data for HBV in China[5]; and 43.7% (7/16) of the HBcAb-positive allografts were intrahepatic HBV DNA positive, which is similar to the findings of previous studies carried out in Italy (62.5%)[14] and Japan (72.7%)[15]. Our study is the first to demonstrate that positive intrahepatic HBV DNA in allografts can predict de novo HBV infection in children who receive HBcAb-positive allografts. Furthermore, HBsAb/HBcAb positivity in the pediatric recipients before LT exhibited weak effectiveness in protecting the children who received HBcAb-positive allografts from de novo HBV infection, which contradicts previous findings in adults[1,2].
In our cohort, 60% (16/30) of the children had been exposed to HBV before LT, which is a significantly higher rate than the rate observed in previous studies[7,16].The higher HBV exposure rate before LT is not unexpected, because those children were diagnosed as having biliary atresia early after birth, and the majority of them were hospitalized repeatedly before LT; besides, the execution of the immunization protocol tended to be far from satisfactory for this particular group of children in China because of severe jaundice or malnutrition. All 30 children in our study received one dose of recombinant HBV vaccine (10 μg) within 24 h of birth, but none of them ever received the other two doses of the vaccine. In addition, a number of studies have found that a high titer of circulating HBsAb from active immunization before LT can prevent de novo HBV infection in pediatric LT recipients[12,17,18]. Since Sep. 2012, the pediatric candidates for LT had been enrolled in a program of hepatitis B vaccination in our center, while the clinical effects remained to be confirmed in the further study. In our opinion, an uncompleted immunization prophylaxis protocol could lead to a higher risk of HBV infection, and strict execution of the HBV immunization protocol should be advocated among infants with biliary atresia, especially in a highly HBV-endemic area. Despite a high HBV exposure rate before LT, all of the 16 naive livers in the HBcAb(+) allograft group were negative for intrahepatic HBV DNA, consistent with a previous study’s finding that intrahepatic HBV DNA was detectable in only a small proportion of HBcAb-positive recipients (5/30, 16.7%)[14].
There are several possible transmitted ways for de novo HBV infection after LT, including an HBcAb-positive allograft[19], reactivation of intrahepatic HBV in HBcAb-positive recipients[20], transfusion, and contact with hospital personnel[21]. Similarly to previous studies[7,12], an HBcAb-positive allograft was a risk factor for de novo HBV infection in the pediatric patients in our center (P = 0.02, Table 1). However, it is clear that the presence of circulating HBcAb does not always reflect the presence of HBV genomes in the liver tissue of HBcAb-positive patients. Without any prophylaxis, 31.2% (5/16) of the children receiving HBcAb-positive allografts experienced de novo HBV infection, which is also in agreement with previous studies carried out with pediatric patients (25%-52.4%)[7,12]. To our knowledge, our study is the first to investigate the correlation between de novo HBV infection in pediatric LT patients and the presence of intrahepatic HBV DNA in allografts, and confirms that intrahepatic HBV DNA positivity is a risk factor associated with de novo HBV infection in pediatric LT patients with HBcAb-positive allografts (P = 0.04, Table 2). Intrahepatic HBV DNA and HBV cccDNA have been reported to persist in HBcAb-positive patients after acute, self-limited infections[22,23] or after HBsAg clearance in chronic infections[24], and these viral DNAs could be a potential reservoir for viral reactivation after LT. It has been reported that as many as 58% of HBV-naïve patients were HBsAg positive after accepting HBcAb-positive liver grafts, and this finding is the basis for the general agreement on performing antiviral prophylaxis in HBV-naïve patients to prevent de novo HBV infection if they received HBcAb-positive allografts[1,2,25]. In China, HBcAb-positive allografts will be a difficult problem to avoid for a long time to come, particularly in pediatric patients. Our study suggests that the presence of intrahepatic HBV DNA in allografts can be a potential criterion for predicting de novo HBV infection in pediatric LT patients receiving HBcAb-positive allografts, which can increase the use of this type of allografts in clinical applications and reduce the risk of virus mutation from the use of antiviral drugs.
It is interesting that in our study preoperative HBcAb/HBsAb positivity in children exhibited only weak effectiveness in preventing de novo HBV infection acquired from HBcAb-positive allografts (P = 0.337), which is inconsistent with the results from studies in adults[1,2]. A considerable number of studies carried out in adults found that HBcAb/HBsAb-positive transplant recipients had the lowest risk of developing de novo HBV infection from HBcAb-positive allografts (< 4%), and that such HBcAb/HBsAb-positive recipients may need no prophylaxis at all[1,2]. Recently, Loggi et al[26] found that patients who successfully controlled their HBV infection before LT were able to re-evoke an effective antiviral response when they received an HBsAb-positive allograft, and that the majority of them showed robust HBsAb production and/or virus-specific T-cell responses. Regarding children, there is very little information about the protective effect of pre-transplant HBcAb/HBsAb positivity in preventing de novo HBV infection. In our opinion, differences in immune status between adults and children could be the main cause of the discrepancy between our and other findings. Considering that the sample size of our cohort is a little small, in the future more studies are needed to identify our view.
For adults, it has become clear that lamivudine (LAM) monotherapy is essential to prevent de novo HBV infection from HBcAb-positive allografts in HBV-naïve recipients[27]; no prophylaxis therapy is needed for HBcAb/HBsAb-positive recipients; prophylaxis antiviral therapy is still controversial in recipient single-positive for HBcAb or HBsAb[1,2,25]. In contrast, limited data are available on pediatric LT recipients. In addition, long-term antiviral prophylaxis in children will significantly increase disease burden, induce resistance mutations to antiviral drugs, and reduce compliance, which may affect the patients’ quality of life and prognosis. Some authors explored the use of HBV vaccine and demonstrated that a high titer of HBsAb yielded good protection against de novo HBV infection[12,17,18]. However, those studies had short follow-up periods and small sample sizes. In our study, four of the seven children who received HBcAb-positive allografts with positive intrahepatic HBV DNA experienced de novo HBV infection. Because the treatment of de novo HBV infection after pediatric LT has not been well documented yet, prophylaxis therapy after LT is essential to prevent de novo HBV infection in those children who received intrahepatic HBV DNA-positive allografts. Considering that LAM can be safely used in children older than 2 years[28], we recommend that LAM be used to prevent de novo HBV infection from HBcAb-positive allografts with positive intrahepatic HBV DNA. At the same time, all the pediatric patients in a highly HBV-endemic area are recommended to receive active immunization for achieving high HBsAb titers before LT.
Our study has several limitations. First, in our study, formalin-fixed, paraffin-embedded liver tissues were used to detect HBV DNA, not frozen liver tissues, which may cause a lower sensitivity of HBV DNA detection[29], and a higher rate of intrahepatic HBV DNA positivity may be expected in this study. However, it has been confirmed in other studies that HBV DNA levels are comparable between fresh frozen tissues and formalin-fixed, paraffin-embedded tissues[30]. Second, this was a retrospective cohort study with a relatively limited sample size. Despite these limitations, our study is the first to have investigated the prevalence of intrahepatic HBV DNA positivity in HBcAb-positive allografts and analyzed its correlation with de novo HBV infection in pediatric LT patients, and our findings have provided preliminary data for further clinical research in this area.
In conclusion, the incidence of de novo HBV infection in pediatric LDLT was 16.7% in our center. Among HBcAb-positive allografts, 43.7% were intrahepatic HBV DNA positive. Our results suggest that positive intrahepatic HBV DNA in allografts was predictive of de novo HBV infection after LDLT in children who received HBcAb-positive allografts. HBsAb/HBcAb positivity exhibited weak effectiveness in preventing children who received HBcAb-positive allografts from de novo HBV infection. Besides, LAM prophylaxis therapy is apparently essential to prevent de novo HBV infection in pediatric patients from HBcAb-positive allografts with positive intrahepatic HBV DNA.
The conduct of this study was approved by the Ethical Affairs Committee of Tianjin First Central Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patients’ parents or guardians.
We thank the staff at the Organ Transplantation Center, First Center Hospital, for patient management.
It is well known that the use of hepatitis B core antibody (HBcAb)-positive liver allografts carries a 48%-58% risk of de novo hepatitis B virus (HBV) infection in HBV-naïve recipients without a prophylaxis strategy. However, this approach is not practical in HBV-endemic areas, such as China, where almost 10% of the general population is hepatitis B surface antigen (HBsAg) positive and approximately 50% of the adults are HBcAb positive. In China, HBcAb-positive donors pose a difficult problem in pediatric liver transplants because non-HBV-related liver disease is the main indication for pediatric liver transplantation (LT). Little data are available on de novo HBV infection in pediatric patients who receive grafts from HBcAb-positive donors, and pertinent risk factors analysis has rarely been performed.
There are several possible routes for de novo HBV infection after LT, including an HBcAb-positive allograft, reactivation of intrahepatic HBV in HBcAb-positive recipients, transfusion, and contact with hospital personnel. In addition, a number of studies have found that a high titer of circulating HBsAb from active immunization before LT can prevent de novo HBV infection in pediatric LT recipients. Besides, intrahepatic HBV DNA and HBV covalently closed circular DNA (cccDNA) have been reported to persist in HBcAb-positive patients after acute, self-limited infections or after HBsAg clearance in chronic infections, and these viral DNAs could be a potential reservoir for viral reactivation after LT.
To author’s knowledge, the study is the first to investigate the correlation between de novo HBV infection in pediatric LT patients and the presence of intrahepatic HBV DNA in allografts, and confirms that intrahepatic HBV DNA positivity is a risk factor associated with de novo HBV infection in pediatric LT patients with HBcAb-positive allografts. Besides, an uncompleted immunization prophylaxis protocol could lead to a high risk of HBV infection, and strict execution of the HBV immunization protocol should be advocated among infants with biliary atresia, especially in a highly HBV-endemic area.
The study is a retrospective cohort study which suggests that positive intrahepatic HBV DNA in allografts is predictive of de novo HBV infection after LDLT in children who receive HBcAb-positive allografts and provides preliminary data for further clinical research in this area, although with a relatively limited sample size.
De novo HBV infection: detectable serum HBsAg (with or without biochemical features of hepatitis) and histologic and immunohistochemical confirmation of HBV by liver biopsy in those who were negative for serum HBsAg before liver transplantation. cccDNA: a stable chromatinized episome form of HBV, which is long-lasting persistent in the nucleus of the infected hepatic cells.
This is an excellent manuscript that, presents suitable method and obtains results that can aid in clinical practice. The results are interesting and suggest that intrahepatic HBV DNA positivity is a risk factor associated with de novo HBV infection in pediatric LT patients with HBcAb-positive allografts.
P- Reviewer: Abdala E, Gruttadauria S, Ohkohchi N S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts - a systematic analysis. Clin Transplant. 2011;25:E243-E249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, Ng IO, Wong J. Safety and outcome of hepatitis B core antibody-positive donors in right-lobe living donor liver transplantation. Liver Transpl. 2003;9:827-832. [PubMed] |
| 5. | Chen YS, Wang CC, de Villa VH, Wang SH, Cheng YF, Huang TL, Jawan B, Chiu KW, Chen CL. Prevention of de novo hepatitis B virus infection in living donor liver transplantation using hepatitis B core antibody positive donors. Clin Transplant. 2002;16:405-409. [PubMed] |
| 6. | Angelico M, Nardi A, Marianelli T, Caccamo L, Romagnoli R, Tisone G, Pinna AD, Avolio AW, Fagiuoli S, Burra P. Hepatitis B-core antibody positive donors in liver transplantation and their impact on graft survival: evidence from the Liver Match cohort study. J Hepatol. 2013;58:715-723. [PubMed] |
| 7. | Xi ZF, Xia Q, Zhang JJ, Chen XS, Han LZ, Zhu JJ, Wang SY, Qiu de K. De novo hepatitis B virus infection from anti-HBc-positive donors in pediatric living donor liver transplantation. J Dig Dis. 2013;14:439-445. [PubMed] |
| 8. | Sun LY, Yang YS, Zhu ZJ, Gao W, Wei L, Sun XY, Qu W, Rao W, Zeng ZG, Dong C. Outcomes in children with biliary atresia following liver transplantation. Hepatobiliary Pancreat Dis Int. 2013;12:143-148. [PubMed] |
| 9. | Gruttadauria S, Pagano D, Cintorino D, Arcadipane A, Traina M, Volpes R, Luca A, Vizzini G, Gridelli B, Spada M. Right hepatic lobe living donation: a 12 years single Italian center experience. World J Gastroenterol. 2013;19:6353-6359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hussain M, Soldevila-Pico C, Emre S, Luketic V, Lok AS. Presence of intrahepatic (total and ccc) HBV DNA is not predictive of HBV recurrence after liver transplantation. Liver Transpl. 2007;13:1137-1144. [PubMed] |
| 11. | Cheung CK, Lo CM, Man K, Lau GK. Occult hepatitis B virus infection of donor and recipient origin after liver transplantation despite nucleoside analogue prophylaxis. Liver Transpl. 2010;16:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, Chang MH, Lee PH. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr. 2009;48:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Su WJ, Ho MC, Ni YH, Wu JF, Jeng YM, Chen HL, Wu YM, Hu RH, Chang MH, Lee PH. Clinical course of de novo hepatitis B infection after pediatric liver transplantation. Liver Transpl. 2010;16:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Raimondo G, Navarra G, Mondello S, Costantino L, Colloredo G, Cucinotta E, Di Vita G, Scisca C, Squadrito G, Pollicino T. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol. 2008;48:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Katsurada A, Marusawa H, Uemoto S, Kaburagi A, Tanaka K, Chiba T. Circulating antibody to hepatitis B core antigen does NOT always reflect the latent hepatitis B virus infection in the liver tissue. Hepatol Res. 2003;25:105-114. [PubMed] |
| 16. | Lin CC, Chen CL, Concejero A, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am J Transplant. 2007;7:195-200. [PubMed] |
| 17. | Chang SH, Suh KS, Yi NJ, Choi SH, Lee HJ, Seo JK, Lee KU. Active immunization against de novo hepatitis B virus infection in pediatric patients after liver transplantation. Hepatology. 2003;37:1329-1334. [PubMed] |
| 18. | Park JB, Kwon CH, Lee KW, Choi GS, Kim DJ, Seo JM, Kim SJ, Joh JW, Lee SK. Hepatitis B virus vaccine switch program for prevention of de novo hepatitis B virus infection in pediatric patients. Transpl Int. 2008;21:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Dodson SF, Bonham CA, Geller DA, Cacciarelli TV, Rakela J, Fung JJ. Prevention of de novo hepatitis B infection in recipients of hepatic allografts from anti-HBc positive donors. Transplantation. 1999;68:1058-1061. [PubMed] |
| 20. | Chazouillères O, Mamish D, Kim M, Carey K, Ferrell L, Roberts JP, Ascher NL, Wright TL. “Occult” hepatitis B virus as source of infection in liver transplant recipients. Lancet. 1994;343:142-146. [PubMed] |
| 21. | Liu CJ, Lo SC, Kao JH, Tseng PT, Lai MY, Ni YH, Yeh SH, Chen PJ, Chen DS. Transmission of occult hepatitis B virus by transfusion to adult and pediatric recipients in Taiwan. J Hepatol. 2006;44:39-46. [PubMed] |
| 22. | Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, Omura M, Hikiji K, Kato M. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology. 2003;37:1172-1179. [PubMed] |
| 23. | Bläckberg J, Kidd-Ljunggren K. Occult hepatitis B virus after acute self-limited infection persisting for 30 years without sequence variation. J Hepatol. 2000;33:992-997. [PubMed] |
| 24. | Komori M, Yuki N, Nagaoka T, Yamashiro M, Mochizuki K, Kaneko A, Yamamoto K, Hikiji K, Kato M. Long-term clinical impact of occult hepatitis B virus infection in chronic hepatitis B patients. J Hepatol. 2001;35:798-804. [PubMed] |
| 25. | Saab S, Waterman B, Chi AC, Tong MJ. Comparison of different immunoprophylaxis regimens after liver transplantation with hepatitis B core antibody-positive donors: a systematic review. Liver Transpl. 2010;16:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Loggi E, Micco L, Ercolani G, Cucchetti A, Bihl FK, Grazi GL, Gitto S, Bontadini A, Bernardi M, Grossi P. Liver transplantation from hepatitis B surface antigen positive donors: a safe way to expand the donor pool. J Hepatol. 2012;56:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Vizzini G, Gruttadauria S, Volpes R, D’Antoni A, Pietrosi G, Filì D, Petridis I, Pagano D, Tuzzolino F, Santonocito MM. Lamivudine monoprophylaxis for de novo HBV infection in HBsAg-negative recipients with HBcAb-positive liver grafts. Clin Transplant. 2011;25:E77-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Jonas MM, Block JM, Haber BA, Karpen SJ, London WT, Murray KF, Narkewicz MR, Rosenthal P, Schwarz KB, McMahon BJ. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology. 2010;52:2192-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194-203. [PubMed] |
| 30. | Zhong Y, Han J, Zou Z, Liu S, Tang B, Ren X, Li X, Zhao Y, Liu Y, Zhou D. Quantitation of HBV covalently closed circular DNA in micro formalin fixed paraffin-embedded liver tissue using rolling circle amplification in combination with real-time PCR. Clin Chim Acta. 2011;412:1905-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |