Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.12993
Revised: February 28, 2014
Accepted: May 28, 2014
Published online: September 28, 2014
Processing time: 336 Days and 20.3 Hours
Many progresses have been done in the management of gastrointestinal (GI) lymphomas during last decades, especially after the discovery of Helicobacter pylori-dependent lymphoma development. The stepwise implementation of new endoscopic techniques, by means of echoendoscopy or double-balloon enteroscopy, enabled us to more precisely describe the endoscopic features of GI lymphomas with substantial contribution in patient management and in tailoring the treatment strategy with organ preserving approaches. In this review, we describe the recent progresses in GI lymphoma management from disease diagnosis to follow-up with a specific focus on the endoscopic presentation according to the involved site and the lymphoma subtype. Additionally, new or emerging endoscopic technologies that have an impact on the management of gastrointestinal lymphomas are reported. We here discuss the two most common subtypes of GI lymphomas: the mucosa-associated lymphoid tissue and the diffuse large B cell lymphoma. A general outline on the state-of-the-art of the disease and on the role of endoscopy in both diagnosis and follow-up will be performed.
Core tip: The understanding of gastro-intestinal lymphomas biology has led the scientific community to implement the diagnostic accuracy and the prognostic assessment of these patient. Such advances have recognized a pivotal role in more sophisticated endoscopic techniques and, in the advent of endoscopic ultrasonography, a clinical tool able to predict, with high levels of accuracy, the response to treatment. In this review we will focus on the application of endoscopy, and related techniques, in the management of gastrointestinal lymphomas. An overview of the literature will be performed on dependence of lymphoma subtype and disease location for both low-grade and high-grade gastro-intestinal lymphomas.
- Citation: Vetro C, Romano A, Amico I, Conticello C, Motta G, Figuera A, Chiarenza A, Raimondo CD, Giulietti G, Bonanno G, Palumbo GA, Raimondo FD. Endoscopic features of gastro-intestinal lymphomas: From diagnosis to follow-up. World J Gastroenterol 2014; 20(36): 12993-13005
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/12993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.12993
Non-Hodgkin lymphomas (NHL) represent the ninth and seventh most common cause of tumor-related mortality (for male and female gender respectively) in developed countries with an upward trend over years[1]. The main feature is nodal, but 40% of NHL cases will present a primary or secondary extra-nodal presentation[2]. The gastrointestinal (GI) tract represents the most involved extra-nodal site[3] and is involved in more than 20% of nodal NHL[4].
The definition of primary GI lymphomas has been given by Dawson et al[5], on the basis of 5 specific characteristics: (1) absence of peripheral lymphadenopathies at the time of presentation; (2) lack of enlarged mediastinal lymph nodes; (3) normal total and differential white blood cell count; (4) predominance of bowel lesion at laparotomy with only lymph nodes obviously affected in the immediate vicinity; and (5) no lymphomatous involvement of liver and spleen.
The stomach is the most common localization, accounting for 60%-70% of GI lymphomas, followed by small intestine lymphomas and colo-rectal lymphomas, accounting respectively for 20%-35% and 5%-10% of GI lymphomas[4,6,7]. Regarding the intestine, the ileum and the cecum are the most involved sites, probably because the abundant lymphoid tissue present[3,8]. In 5%-15% of cases there are multiple localizations[4,6]. It is noteworthy that lymphomas of the colon and the rectum are more frequent in patients affected by AIDS or Crohn disease[9,10], while gastric mucosa-associated lymphoid tissue (MALT) lymphomas and the immunoproliferative small intestinal disease (IPSID) recognize an etiological factor in Helicobacter pylori (H. pylori) and Campilobacter jejuni infection respectively and T-cell lymphoma are more frequent in celiac patients[7].
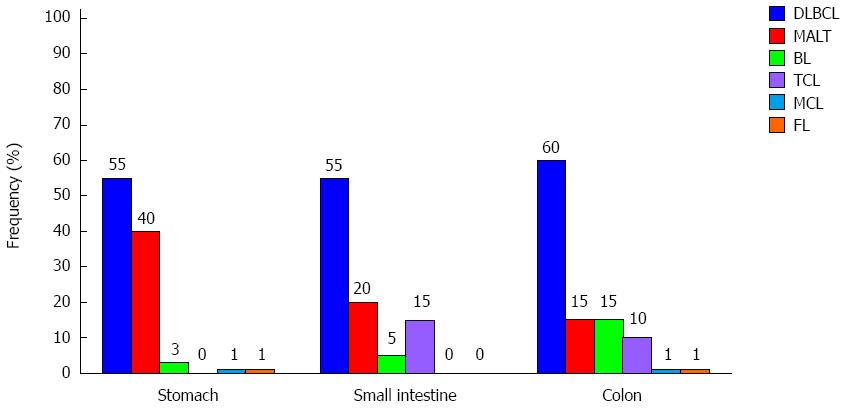
As regards histology, several types of lymphomas can arise and a proper characterization of the subtype is essential in driving the treatment. However, the histotype frequency can vary according to the site involved (Figure 1). Gastric lymphomas are mainly represented by MALT lymphoma, a form of indolent disease, followed by the diffuse large B cell lymphoma (DLBCL), a type of aggressive malignancy[4]. However, several groups[11,12] have recently registered an inverted trend in the distribution of PGL[7], being high grade lymphomas more frequent than low grade, probably cause the better characterization of PGL “Not Otherwise Specified” over time. Other subtypes of lymphomas are extremely rare in the stomach and are represented by sporadic cases of follicular lymphoma (FL), mantle cell lymphoma (MCL), small lymphocytic lymphoma (SLL), T-cell lymphoma (TCL) and plasmacytoma. Nevertheless, there are cases where different types of lymphoma can locate at different sites[13]. On the other hand, the intestine to court is primarily involved by aggressive lymphoma subtypes, in particular by DLBCL[6,14]. This subtype of lymphoma has been recently better characterized, taking into account molecular and genetic features able to influence the prognosis of the disease, representing a kaleidoscopic scenario[15]. In some cases, this aggressive type of lymphoma can arise from a progression of low-grade lymphomas (the so called “transformed lymphomas”)[14]. In addition, other lymphomas typically involving this site are Burkitt’s lymphoma (BL) and Burkitt’s like lymphoma (BLL), both showing a rapid and aggressive clinical course[2]. Rarely, the histotype is represented by FL, MCL, and TCL[2]. In some cases, the development of a lymphoma depends on autoimmune disorders, such as the celiac disease, configuring the enteropathy-associated T-cell lymphomas (EATL) framework. Interestingly, the gross intestine can be involved by post-transplantation lymphoproliferative disorder (PTLD) and also by Epstein-Barr virus (EBV)-associated lesions[16]. Finally, although a clear relationship between inflammatory bowel disease (IBD) and GI lymphomas has not been validated, patients with IBD have an augmented risk of develop NHL, especially if treated with immunosuppressant[17].
The clinical presentation of GI lymphomas depends principally on the involved site and can be lymphoma-related and site-related[18]. In most patients the finding of a GI lymphoma can be occasional[2]. Lymphoma-related symptoms encompass the “B-symptoms” and comprise fever, drenching night sweats and weight loss. Site-related symptoms are mainly dyspepsia, abdominal pain (principally epigastric), nausea or vomiting, and anorexia. Moreover, symptoms are divided into alarm (weight loss, vomiting, haematemesis/melena, anemia, perforation) and non-alarm (epigastric/abdominal pain, dyspepsia, and heartburn), being alarm symptoms more frequent in aggressive lymphomas[2,18-20]. It is noteworthy that the occurrence of alarm symptoms does not relate with endoscopic lesions[18]. In few patients with advanced disease, a palpable mass is present especially in lymphomas of the ileo-cecal area arising with bulky mass, obstruction, perforation, and bleeding[3,14,18,21,22].
The prognosis of the disease depends on several factors. First of all, the lymphoma subtype represents the main factor[6] together with the stage of the disease[4] and the clinical evaluation[14]. Usually, high-grade lymphomas (DLBCL, BL, BLL, PTLD) show a poor prognosis compared to low-grade lymphomas (MALT-lymphomas, FL, MCL)[21]. However, this is a general rule and more specific clinical and molecular tools are able to differentiate the outcome of the disease, especially in DLBCL[12]. Additionally, TCL and EATL have in general a prognosis poorer than B-cell lymphomas[23].
The stage of the disease is important especially in gastric MALT-lymphomas, where limited-diseases preserves a great chance to respond to H. pylori eradication, while more disseminated disease need a more aggressive approach[4]. However, classical staging systems, usually applied in nodal lymphomas, are not informative in GI lymphomas, where proper staging classifications have been developed[24]. Additionally, high grade lymphomas tend to be diagnosed at advanced stage more frequently than low-grade lymphomas[18].
The clinical evaluation is also essential in determining the prognosis. Poor performance status, high lactate dehydrogenase (LDH) levels and the surgical resection are the major prognostic indicator in B-cell and T-cell lymphomas[6]. On these basis, several prognostic scores have been developed over time[4,12]. However, their usage in GI lymphomas is limited since they have been developed for nodal lymphomas.
In conclusion, the prognostic features of GI lymphomas depends on biological and histological factors more than the sole clinical features.
The advent of endoscopy, together with the possibility to perform an endoscopic ultrasonography and a fine-needle aspiration, has made the study of GI lymphomas more and more sophisticated, making them a separate entity with respect to the nodal counterpart. Indeed a tailored approach has been also developed dealing with this disease[25]. However, GI lymphomas are still often diagnosed with considerable delay because of the aspecific endoscopic presentation[18,26] and the absence of symptoms in a considerable percentage of patients[18]. This atypical presentation is one of the major pitfalls in diagnosing the disease, since the median time from the onset of symptoms to diagnosis is 76 d[27]. Additionally, few data are present about the endoscopic behavior of these lymphomas in follow-up setting and are mainly extrapolated by limited series[25].
The purpose of this review is to analyze the clinical and instrumental characteristics of GI lymphomas (especially endoscopic and echo-endoscopic) in the different steps of patient management, from diagnosis to follow-up, highlighting their impact in clinical practice.
MALT lymphomas arise in the context of the lymphoid infiltrates of the mucosa layer of hollow organs and from glandular tissues[22,28]. The GI tract is the most frequently involved zone (almost 50% of cases)[29], particularly the stomach (85% of cases). The gross intestine represents only a 0.4% of cases. In one third of cases, there are multiple sites involved[28,29]. Additionally, patients with MALT lymphoma of the lungs, tend also to have a GI localization, so that, in this subset of patients, it is advisable to perform an endoscopic evaluation[28]. The simultaneous involvement of the bone marrow is uncommon (10% of cases)[30]. Rarely, there is an involvement of perigastric organs (pancreas and/or spleen) and this is a finding more frequent in H. pylori-negative MALT lymphoma compared to H. pylori-positive[31].
Kolve et al[32], found that secondary involvement of the stomach is characterized by multiple localizations with also an involvement of the duodenum, while in primary gastric lymphomas, there is usually a single unifocal lesion with bulky disease and an involvement of the duodenum in a minimal part of the patients.
The development of this lymphoma depends on precursor lesions characterized by a chronic immune stimulation, secondary to bacteria or autoimmune diseases. Indeed, gastric lymphomas recognizes as etiological factor the chronic infection of H. pylori, while the IPSID is secondary to Campylobacter jejuni[33].
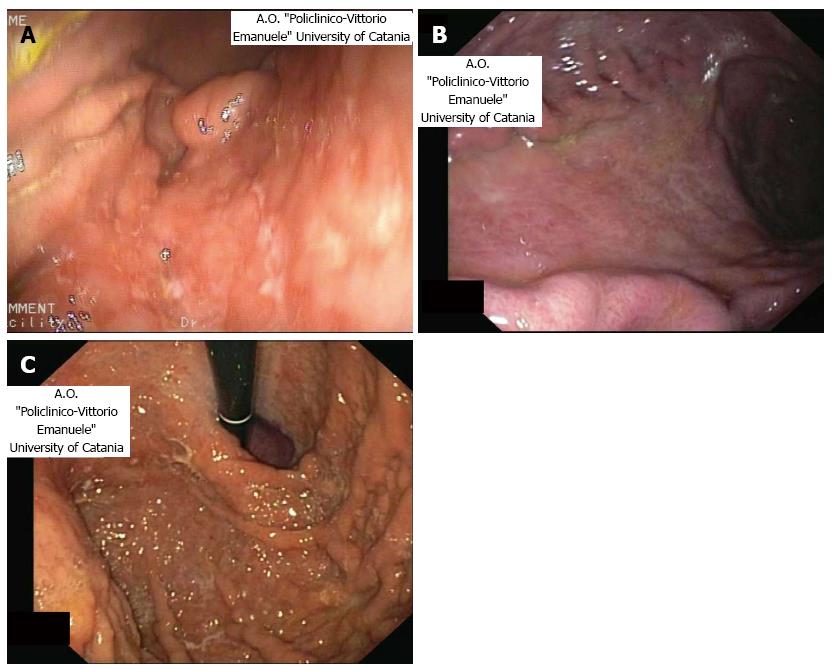
The endoscopic features of the disease is extremely variegated, often mimicking a benign disease, such as erosions, multifocal gastritis or other types of malignancies, such as gastric adenocarcinoma[34]. One of the most frequent and typical feature is the presence of gastric nodularity or enlarged folds located at the antrum or body of the stomach (Figure 2A, B)[22]. Another endoscopic feature is given by ulceration with elevated margins or a protrusion[13]. In the prospect multicenter study performed by Fischbach et al[14] in 2000, the most frequent lesion was the presence of an unifocal ulceration. However, polyps or normal patter can be present.
A delay in the diagnosis is frequent since in a large cohort of patients the typical symptoms are usually vague, there is not a suggestive endoscopic pattern , and the lesions are usually aspecific, resembling benign conditions[18,35]. However, the early diagnosis of the disease could be a critical point in order to avoid evolution to aggressive lymphoma[26]. Indeed the diagnostic accuracy of the technique is ranged between 11% and 22% when performed alone, rising to 50%-75% when biopsies are taken[14,36]. The greater accuracy has been related to removal of bioptic sample from ulcer margins (84%), surrounding wall (76%), erosive areas (57%), while low accuracy has been detected in bioptic samples of the base and of the protruding mass[37].
Certainly, the endoscopic biopsy (E-Bx) is a mandatory step in order to make a correct diagnosis. It should be emphasized that the sole lymphoid infiltrate is not a sufficient for the histological diagnosis. It is important to evaluate the distribution of the lymphoid infiltrate and also the relationship with the epithelium[38].
The fact that MALT lymphomas usually does not arise with alarm symptoms[19,21], augments the need to perform a gastroscopy (possibly with bioptic sampling) in patient older than 45 years with dyspepsia, in order to detect early lesions. Additionally, in about 10%-20% of cases, the gastric pattern is normal or nearly normal (gastritis-like flat lesions or petechial hemorrhages) at endoscopy[18,21] and this fact underlines the need to perform a bioptic sample during the procedure also in benign-like lesions (Figure 2C).
An interesting metanalysis by Zullo et al[20], evaluating 38 manuscripts and comprising 2000 patients, showed that MALT lymphomas were more frequently localized in the gastric antrum and body (60%-70% of cases) and presented the following endoscopic feature: 9.7% exophytic mass; 52.1% ulcerative pattern, 23.5% hypertrophic, 1% petechial hemorrhage, 12.7% normal/hyperemic mucosa.
In definitive, three patterns have been recognized at endoscopy[18,26]: (1) exophytic type; (2) ulcerative type; and (3) hypertrophic type with giant folds. However, recently, a new endoscopic 6-items classification has been proposed[19]: (1) Exophytic: a single mass with irregular or polypoid aspect; (2) Ulcerative: single to multiple ulcerations/erosions; (3) Hypertrophic: enlarged folds and nodular pattern; (4) Petechial hemorrhage: several petechiae at mucosal level; (5) Normal/hyperemic mucosa: normal to reddish mucosa; and (6) Mixed: more of the above-mentioned pattern present.
Interestingly, the endoscopic pattern differ statistically between high-grade and low-grade lymphomas since high grade lymphomas have a probability to present with an exophytic mass greater than low-grade lymphomas that conversely present more frequently with petechial hemorrhages and normal/hyperemic mucosa[20]. However, this has not a recognised prognostic value[39-41]. Nevertheless, some reports indicate that aggressive lymphomas tend to present with an ulcerative lesion more frequently than low-grade lymphomas whose privileged site of involvement is the antrum, where, in 20% of cases, does not give rise to a macroscopic lesion[18,37]. Contrarily, Takenaka et al[42] found a relationship between the presence of protruding appearance and a favorable outcome, even if in univariate analysis, probably because it is related to a minor wall involvement. Lee et al[41], found that protruding lesions are more likely to be refractor to H. pylori eradication. Additionally, the report of Inagaki et al[34] indicated that a superficial spread of the lymphoma at endoscopy had a better prognosis than the deep infiltration appearance and that the presence of cobblestone lesions is suggestive of the API2-MALT1 fusion gene. Another aspect is that multiple lesions confer a prognosis poorer than a single lesion, at least in H. pylori negative MALT lymphomas[43].
As suggested by Kim et al[39] and Steinbach et al[44], the distal stomach involvement could be related with a more favorable outcome because the proximal localization is likely at advanced stage and the remission rate is greater in lymphomas localized distally rather than proximally[45]. This fact can be explained considering that proximal gastritis is more likely autoimmune and H. pylori-independent. However, the report by Nakamura et al[46], and El-Zahabi et al[40] have not confirmed this finding. There are also indications that the presence of gastric mucosa atrophy in patients with H. pylori negative gastric MALT lymphoma is an hallmark of a previous H. pylori infection[43]. However, since the endoscopic features are not specific, the histological evaluation remains the golden standard in order to perform diagnosis[47].
A novel approach is given by the magnified endoscopy that can visualize also the microstructure of vessels and mucosa[48-50]. This technique is limited to specialized centers and was aimed to overpass the difficulties in distinguishing gastric cancers from gastric lymphomas at conventional endoscopy. Its clinical application is restricted to the detection of small cancer lesions[51]. In the report by Ono et al[48], even if there was not a significant change in the pattern at magnified endoscopy, the abnormality of vessel structures, that at diagnosis are larger and grosser than in gastritis, returned to normal in every responding patient. Additionally, some reports[51,52] indicate that magnified endoscopy would be useful in predicting the response to the treatment prior to the biopsy since, in their cohort, the irregularities of gastric pits returned to normal in responding patients, while persisted in non-responding patients. However, this technique is difficult to apply in GI lymphomas since they usually arise from the deep layer of the stomach and also because the presence of gastric mucus and H. pylori related inflammation can alter the results[48]. However, no consolidated evidences are present in literature regarding this technique.
Indeed, one of the major aspect regarding gastric MALT lymphomas is to establish the association with H. pylori infection. Biologically, H. pylori is able to promote a condition of persistent inflammation with the formation an “acquired” MALT of the stomach. Basically, tow steps of gastric MALT lymphoma development can be determined[53,54]. The first step is the development of monoclonal lymphocytes strongly related to H. pylori infection. The second step is the development of immortalized lymphocytes no longer dependent on the H. pylori infection. These biological considerations are fundamental in understanding why a limited disease will respond greatly to H. pylori eradication treatment, while in advanced stage disease, the usage of H. pylori eradication treatment alone would not be sufficient[53,54]. Indeed when the tumor is localized at the first or second layer of the gastric wall, it is still H. pylori-dependent, while the involvement of the third and fourth layer indicate an independence from H. pylori infection.
For these reasons, in the last years, endoscopic ultrasonography (EUS) has gained a great role in the management of extranodal-MALT lymphoma, especially in gastric MALT lymphomas[25]. This technique can be used at different steps of PGL management. The major role is the definition of the gastric wall involvement in the context of the diagnostic work-up with a levels of evidence of “A”[55]. In fact, contrarily to other images modalities, EUS is able to discriminate the five layers of the stomach defining the layer involvement with a sensitivity ranging from 80% to 99% and a specificity of 100%[56].
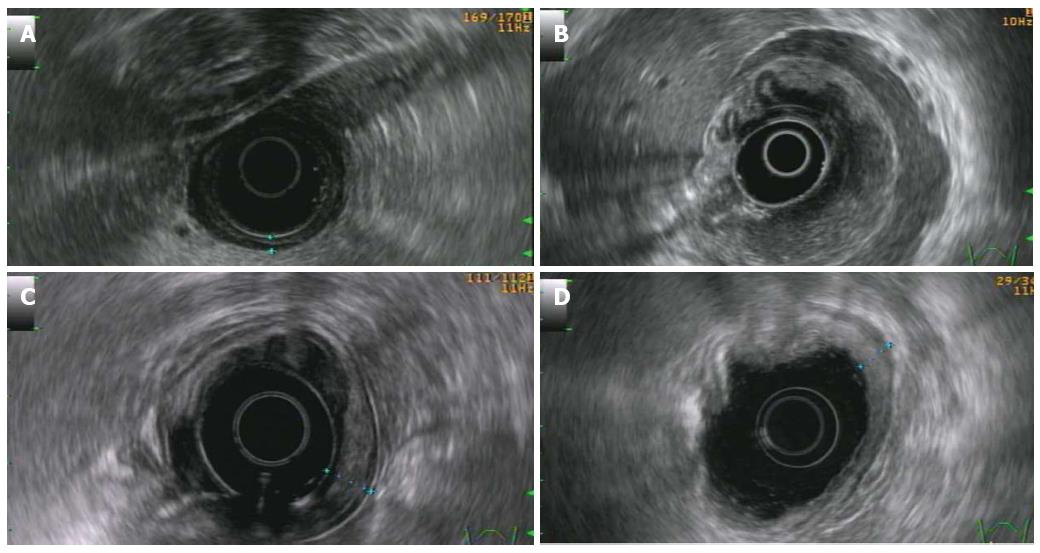
Basically, four patterns can be present[57]: (1) superficial: thickening of the second and third layer without involvement of the first and fourth layer (Figure 3A); (2) diffuse-infiltrative: diffuse trans-mural involvement of the gastric wall with irregular layer border and not uniform echogenicity (Figure 3B); (3) mass-forming: localized hypoechoic mass at the third-four layer well demarcated from the surrounding tissue (Figure 3C); and (4) mixed-type: combination of the previous three patterns.
When compared to the endoscopic pattern, it is possible to note a relationship between these two endoscopic techniques. Indeed, a nodular mucosa is present in patients with the pattern 1 and 2, while nodular hyper trophic pattern with ulcers are present in group 2. Polypoid elevation is exclusively present in group 3. The presence of deep mucosa ulcers relate with the finding of a mixed-type at EUS.
However, this is an operator-dependent technique and in some cases, especially in the definition of intermediate stage disease, the inter-operator variability tend to augment[58]. EUS usage is now widespread and the treatment of MALT gastric lymphomas depends on this valuation, since diseases limited to the I and II layer of the stomach preserve a greater possibility to respond to H. pylori eradication than more advanced disease. Additionally, with EUS is possible to detect peri-gastric nodal involvement.
Additionally, several Authors have investigated the usefulness of EUS also in follow-up settings[59-65]. It must considered that, in a long run, the endoscopic and echo-endoscopic features usually return to normal in every responding patients[25,41,66] but the EUS abnormalities in gastric MALT lymphomas return to normal appearance in much prolonged time compared to the histological evaluation[67]. On the other hand, the continuous persistence of EUS abnormalities over years could represent a sign of persistent, although inactive, lymphoma thus reserving a possible strict EUS follow-up to patients with EUS abnormalities[64].
Limited data are present in literature investigating the role of EUS during the follow-up[59-63,65]. So far, there is a lack of prospective trials and the retrospective evaluations have indicated that the value of EUS in follow-up setting is still limited and therefore it is not recommended[68]. However, in our opinion, the better and real value of the EUS is noticeable in a very long run, since in limited follow-up evaluation these abnormalities does not translate into a real risk of relapse while in a more prolonged follow-up the persistence of an EUS abnormality may be predictive of lymphoma relapse. Indeed, in our prior experience[67], where we had a median follow-up of 36.5 mo, the rate of relapse among patients with a negative endoscopic biopsy but persistence of EUS abnormalities was negligible and not statistically and clinically significant. In the subsequent follow-up study, in which we analyzed the patient status after a median follow-up of 86.5 mo, we noticed that patients with a constant EUS abnormality had a great probability to develop a relapse of the disease. Probably this is due to the biology of the disease since the very low growth rate is typical of low-grade lymphoma, especially if we consider that the continuous antigenic stimulation provided by H. pylori is removed in every case. Possibly, other antigenic stimulation can be present and can determine over time a clonal selection of persisting and dormant lymphocytes. However, to date, since GI MALT lymphomas preserve a great change to respond to the second line treatment (specially alkylating agents) the early recognition, or the recognition of risk factors, of relapse preserves a little importance in clinical practice, so that repeated EUS evaluations during the follow-up assessment of MALT GI lymphomas are not recommended[24].
Duodenal lymphomas are rare entities, encompassing 6%-8% of GI lymphomas. A variant of MALT lymphoma is given by the IPSID[38], more frequent in the Mediterranean area. Molecular and immunohistochemical studies have demonstrated an association with Campylobacter jejuni infection. This disease is typical of older children, there is often the secretion of alpha heavy chain and the clinical manifestation is abdominal pain associated with diarrhea and malabsorption[3,22].
While the endoscopic appearance of gastric MALT lymphomas has been widely studied over time, few reports describe the endoscopic appearance of duodenal MALT lymphomas and IPSID.
Similarly to gastric MALT lymphoma, duodenal MALT lymphomas have the appearance of multiple small erosions and nodular elevations with diffuse erythema in the duodenal bulb[69]. Specific reports describe the features as multiple active ulcer craters with nodular and erythematous margins without gastric involvement. However, also in these cases, the differential diagnosis is between benign (i.e., duodenal gastrinoma) and malignant conditions (i.e., duodenal carcinoma). Usually MALT lymphomas arising in the duodenum are considered independent from H. pylori infection. However, some cases indicate the regression of the lymphoma after H. pylori eradication[69]. Indeed, for early-stage patients a first-line treatment with antibiotics, including tetracycline and metronidazole, is recommended[70].
Lymphomas of the jejunum are extremely rare, representing almost one third of the jejunum tumors[71] and almost 13% of the small intestine lymphomas[72]. The endoscopic appearance is of ulcerating-scarring area at the double-balloon enteroscopy[71], however also multiple polypoid lesions[72] or a single stricture[73] have been described. The most common histotype is the DLBCL (with or without MALT component), however MALT lymphoma represents one third of small bowel GI lymphomas[72]. Ileocecal MALT lymphomas are also rare findings[74]. First reports[75] indicate that the endoscopic pattern is of multiple whitish nodules or reddish masses with smooth and polished mucosa aspect. The first classification of endoscopic features of ileal lymphomas[76] identified five patterns: mucosal fold thickening alone, nodular pattern, infiltrated pattern, ulcerative pattern, and mosaic pattern.
The detection of small intestine lymphomas have been ameliorated with the introduction of capsule endoscopy[77] and double ballon-technique of push-and-pull enteroscopy[3]. Usually the appearance is of polypoid or ulcerative lesions indistinguishable from lesions other than lymphomas[77]. Most often the portion of the small intestine interested by IPSID is proximal, while aggressive lymphomas have more tropism for distal sites[3]. Additionally, the Jejunum can be simultaneously involved in the context of gastric MALT lymphoma and can be a site of lymphoma relapse after treatment[77]. In this perspective, double balloon enteroscopy and capsule endoscopy could represent a valid tool to correctly stage the patient and for the follow-up.
Anecdotic is the finding of an ileal MALT-lymphoma and few cases have been reported in the literature[75,78-82]. Majority of cases indicate a negativity of H. pylori infection, so that the etiology of this disease remains unknown[81]. The gross appearances frequently is of a solitary mass resembling a carcinoma in almost half of the patients, while less frequently there are multiple masses or lymphomatoid polyposis[78,81].
Rarely, MALT lymphoma arises in the large intestine, representing 10%-20% of GI lymphomas[8]. Additionally, it represents less than 1% of malignancies affecting the gross intestine[8]. Its rarity and aspecific presentation makes this a challenge for endoscopists[74,83]. Some reports indicate a relationship with colon adenocarcinoma[83-85], even if there are few data to make a conclusion.
Usually the endoscopic appearance is of a whitish polypoid lesion usually not ulcerated 1-2 cm in diameter preserving a smooth aspect at endoscopy[86,87], while adenocarcinomas have an ulcerated feature and larger diameters, especially when in advanced stage. However, the occurrence of a tumor mass with adjacent enlarged satellite nodes should lead to lymphoma as differential diagnosis. Additionally, the presence of masses at the cecum, well demarcated from the peri-visceral fat without an invasion of the surrounding soft tissues in absence of desmographic reaction are typical of colon lymphomas and this must be taken into account in the differential diagnosis[87]. In any cases, the diagnosis of lymphoma remains histological and the fact that also in the colon the lymphoma can arise from sub-mucosal layers, can make the diagnosis difficult with a need to perform further endoscopic procedures[86].
Rare is the occurrence of MALT lymphoma in the rectum were it appears as a broad-based whitish/reddish protrusion with intact mucosal aspect or erosion[88,89]. Cases have been described on the development of MALT rectal lymphoma after gastric MALT lymphoma[89]. Interestingly, it has been reported that rectal lymphomas have a good chance to respond to antibiotic treatment[90-92], suggesting that other microorganisms can be involved in the development of rectal MALT lymphomas with a pathogenetic mechanism similar to H. pylori infection. As for gastric lymphoma, also in MALT rectal lymphoma, often the lesions arise from the sub-mucosa, posing also the problems related to the capability of taking a diagnostic sample. A proposed technique is the unroofing technique: namely, after the removal of the superficial mucosa layer with the usage of flex knife, the sub-epithelial lesion is exposed and a direct biopsy of the previously EUS detected sub-epithelial lesion can be performed[88].
High grade lymphomas can involve the stomach in almost 50%-60% of cases representing 20%-30% of GI NHL[14,93]. The etiology of the disease remains unknown, however H. pylori infection is present in 35% of cases and the usage of H. pylori eradication therapy can determine durable complete remission in 50%-60% of cases, especially in DLBCL with MALT lymphoma component[93]. Recent trials have also investigated the usefulness of H. pylori eradication in early-stage gastric DLBCL, indicating a percentage of complete remission of 60%-66%[94,95]. However, this kind of treatment is limited to clinical trials and contemplate a strict endoscopic follow-up in order to catch eventual lymphoma reappearance. Indeed, chemotherapy together with anti-CD20 antibodies immunotherapy plus/minus radiotherapy is the treatment of choice in these patients[96-98].
The clinical presentation recognizes a greater prevalence of alarm symptoms compared to low grade lymphomas[18]. The endoscopic pattern is, in the majority of cases, ulcerative (with single or multiple ulcerations), specially located at the gastric body or at the fundus[14]. In cases with c-Myc rearrangement (that confer a poor prognosis to the disease) more than one GI organ can be simultaneously involved and endoscopically appears with multiple dish-like lesions while the Narrow-Band Imaging shows honeycomb-like pattern and irregular microsurface pattern[99].
The diagnostic work-up of the disease includes the definition of the stage and the prognostic assessment of the disease with CT scan evaluations, bone marrow biopsy, physical examination (with evaluation of Waldeyer ring involvement) and the EUS. In early stage disease the diagnostic accuracy of endoscopic biopsy is high (70%-90%), even if the efficacy can be lower in cases of deep infiltration[93]. The stage of the disease is limited to the gastric wall in the majority of cases[100] and the definition of an involvement beyond the Muscularis propriae is of fundamental prognostic importance[94,95]. For prognostic purposes, the stage-modified IPI score for GI DLBCL has been validated, based on the Lugano staging system instead of the TNM and not taking into account the involvement of the GI wall layers[101].
Follow-up assessment requires periodic endoscopic evaluation with multiple biopsies. Generally, patients in complete remission show a resolution of endoscopic abnormalities[102]. However, the follow-up evaluation of PG DLBCL recognizes, as sole tool, the performance of endoscopic biopsies. This is due to the fact that a fibrotic scar can remain, thus rendering the morphological imaging, such as the EUS, an unreliable tool to assess the response to the treatment and for follow-up evaluation (Figure 3D)[64].
Indeed, some studies, including patients with GI DLBCL, have indicated that EUS is not a reliable technique for post-therapy assessment in this patient setting[67,103]. Our Group have also highlighted this aspect. We have observed that the presence of EUS abnormalities did not affect the progression-free survival of patients after a short[67] and long term[64] follow-up. In a similar report by Püspök et al[103], involving 33 patients, the concordance rate between the histological and the EUS evaluation was low (i.e., 64%) with high rates of false positive at EUS, that persisted positive notwithstanding a constant negative histology. In our study, the concordance rate between these too technique remained low and also the sensitivity of the EUS in follow-up evaluation was almost meaningless (i.e., 9%). Therefore, we do not recommend to perform EUS evaluations in the context of GI DLBCL follow-up.
DLBCL represent almost 58% of the small intestine lymphomas[21]. Endoscopic findings are characterized by a macroscopic feature of polypoid type in 25% of patients, ulcerative type in 54%, multiple polyposis type in 5%, diffuse-infiltrating type in 6% and mixed type in 10% of patients[72]. Additionally, this lymphoma can also be complicated by intestinal perforation in a greater frequency compared to other lymphomas especially if arise in the small intestine[104].
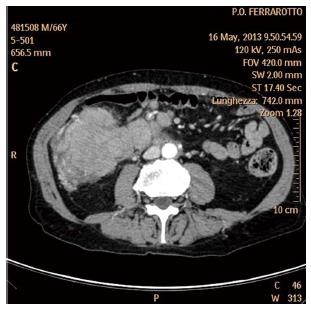
The gross intestine is mainly involved by high grade lymphomas, in particular DLBCL (about 50%-80% of cases)[8]. Most involved region is sigmoid tract, followed by rectum and ascending colon[21]. The etiology is unknown even if a state of immunodeficiency, such as HIV infection or organ transplantation, can lead to the development of the lymphoma as reported by Doolabh et al[105]. In more than half of patients there is a bulky mass that can be appreciated with the physical examination or the ultrasound of the abdomen[8]. Therefore, in many cases, the first approach is the CT scan analysis that poses the suspect of lymphoma when it detect the presence of bulky mass and satellite lymph nodes (Figure 4).
On the other hand, the differential diagnosis is more difficult when the lymphoma arises as a single bulky lesion[2,8]. In any case, due to the fact that colon lymphomas are rare entities and can mimic solid non-hematological malignancies, often the diagnosis is done on surgical approach[21]. Additionally, the fact that in the majority of cases the underlying disease is an high grade type of lymphoma, surgery is performed in urgent way due to alarm symptoms (sub-occlusion, body weight loss, hematochezia)[2,10]. This fact makes the colonscopy the less used approach to reach the diagnosis, even if it is considered the golden standard in the non-urgent setting[8]. The main endoscopic feature is a unique large ulcerated mass[21], even if also a polypoid aspects[21] and, very rarely, an annular napkin-ring lesion can be present[106]. Additionally, the macroscopic aspect can be a large pale mass[2] and this has been indicated as a sign of differentiation from low-grade lymphomas, especially if greater than 5 cm (bulky)[32].
Extension of the disease is different in the few series that have taken into account this aspect. Gonzalez et al[107] reported a case series of 15 patients, all of them at stage IE according to the Lugano classification and the German study Group, in the study GIT NHL 01/92, found similar results[27]. However DLBCL of the colon present sometimes in advanced stage with multiple nodes and bone marrow involvement and some reports indicated that more than half of patients have a stage III or IV disease[8].
Rarely, DLBCL arises in the rectum and few reports have described this disease indicating that the typical endoscopic presentation is nodularity[108].
GI lymphomas are rare diseases, but their prompt recognition, treatment and follow-up management is of crucial importance for patient safety. The current review was defined in order to give to the reader useful information about GI lymphoma presentation and management with particular emphasis to endoscopic and EUS features of the disease from diagnosis to follow-up according to the NHL histotype. The presented review emphasizes also the importance in diagnosis and prognostic implication of the endoscopic features of GI lymphomas and how the GI localization can affect patient survival. Indeed, despite nodal lymphomas represent the most frequent lymphoma presentation, GI NHL are increasing in incidence and prevalence and have been more and more studied over the years. In many cases, the diagnosis is posed on the basis of the endoscopic suspicion and also the performance of EUS would be of great help in detecting sub-mucosal lesions. Additionally, small series have investigated the role of EUS in the follow-up settings of MALT and DLBCL of the stomach but the results are not univocal and require further investigations.
P- Reviewer: Bass GA, Gold JS, Haque A, Takabe K, Zullo A S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Koniaris LG, Drugas G, Katzman PJ, Salloum R. Management of gastrointestinal lymphoma. J Am Coll Surg. 2003;197:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (3)] |
| 4. | Papaxoinis G, Papageorgiou S, Rontogianni D, Kaloutsi V, Fountzilas G, Pavlidis N, Dimopoulos M, Tsatalas C, Xiros N, Economopoulos T. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma. 2006;47:2140-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-89. [PubMed] |
| 6. | Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao XY, Li JJ, Zhang R. Primary intestinal non-Hodgkin’s lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol. 2011;17:4625-4631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Burke JS. Lymphoproliferative disorders of the gastrointestinal tract: a review and pragmatic guide to diagnosis. Arch Pathol Lab Med. 2011;135:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Stanojevic GZ, Nestorovic MD, Brankovic BR, Stojanovic MP, Jovanovic MM, Radojkovic MD. Primary colorectal lymphoma: An overview. World J Gastrointest Oncol. 2011;3:14-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 9. | Fan CW, Changchien CR, Wang JY, Chen JS, Hsu KC, Tang R, Chiang JM. Primary colorectal lymphoma. Dis Colon Rectum. 2000;43:1277-1282. [PubMed] |
| 10. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin‘s lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin‘s Lymphoma. J Clin Oncol. 2003;21:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Warrick J, Luo J, Robirds D, Branson J, Frater JL, Kreisel F, Hassan A, Nguyen TT. Gastrointestinal lymphomas in a North American population: clinicopathologic features from one major Central-Midwestern United States tertiary care medical center. Diagn Pathol. 2012;7:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Huang J, Jiang W, Xu R, Huang H, Lv Y, Xia Z, Sun X, Guan Z, Lin T, Li Z. Primary gastric non-Hodgkin’s lymphoma in Chinese patients: clinical characteristics and prognostic factors. BMC Cancer. 2010;10:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Terada T. One patient with double lymphomas: simultaneous gastric MALT lymphoma and ileal diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2012;5:260-263. [PubMed] |
| 14. | Fischbach W, Dragosics B, Kolve-Goebeler ME, Ohmann C, Greiner A, Yang Q, Böhm S, Verreet P, Horstmann O, Busch M. Primary gastric B-cell lymphoma: results of a prospective multicenter study. The German-Austrian Gastrointestinal Lymphoma Study Group. Gastroenterology. 2000;119:1191-1202. [PubMed] |
| 15. | Hwang JH, Rulyak SD, Kimmey MB; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Cho YU, Chi HS, Jang S, Park SH, Park CJ. Pattern analysis of Epstein-Barr virus viremia and its significance in the evaluation of organ transplant patients suspected of having posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2014;141:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Andriani A, Zullo A, Di Raimondo F, Patti C, Tedeschi L, Recine U, Caruso L, Bonanno G, Chiarenza A, Lizzani G. Clinical and endoscopic presentation of primary gastric lymphoma: a multicentre study. Aliment Pharmacol Ther. 2006;23:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Zullo A, Hassan C, Cristofari F, Perri F, Morini S. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: Helicobacter pylori and beyond. World J Gastrointest Oncol. 2010;2:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zullo A, Hassan C, Andriani A, Cristofari F, Cardinale V, Spinelli GP, Tomao S, Morini S. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Terada T. Gastrointestinal malignant lymphoma: a pathologic study of 37 cases in a single Japanese institution. Am J Blood Res. 2012;2:194-200. [PubMed] |
| 22. | Zinzani PL. The many faces of marginal zone lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 23. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 24. | Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi144-vi148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Vetro C, Romano A, Palumbo GA, Bonanno G, Di Raimando F. Role of the Endoscopic Ultrasonography in the Management of Gastric Lymphomas: Our Experience and Review of Literature. Ultrasound Imaging - Medical Applications: InTech 2011; 235-258 Available from: http://www.docin.com/p-414577141.html. |
| 26. | Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol. 2003;98:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3861-3873. [PubMed] |
| 28. | Ishii Y, Tomita N, Takasaki H, Ogusa E, Hattori Y, Matsuura S, Matsumoto C, Takemura S, Kuwabara H, Ishigatsubo Y. Clinical features of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. Hematol Oncol. 2012;30:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Oh SY, Kim WS, Kim JS, Kim SJ, Lee S, Lee DH, Won JH, Hwang IG, Kim MK, Lee SI. Multiple mucosa-associated lymphoid tissue organs involving marginal zone B cell lymphoma: organ-specific relationships and the prognostic factors. Consortium for improving survival of lymphoma study. Int J Hematol. 2010;92:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | d‘Amore F, Brincker H, Grønbaek K, Thorling K, Pedersen M, Jensen MK, Andersen E, Pedersen NT, Mortensen LS. Non-Hodgkin‘s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 1994;12:1673-1684. [PubMed] |
| 31. | Shinagare AB, Ramaiya NH, O’Regan K, Jagannathan JP, Hornick JL, LaCasce AS. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. J Clin Oncol. 2011;29:e297-e300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-Hodgkin’s lymphoma. German Gastrointestinal Lymphoma Study Group. Gastrointest Endosc. 1999;49:307-315. [PubMed] |
| 33. | Ruskoné-Fourmestraux A, Audouin J. Primary gastrointestinal tract mantle cell lymphoma as multiple lymphomatous polyposis. Best Pract Res Clin Gastroenterol. 2010;24:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Inagaki H, Nakamura T, Li C, Sugiyama T, Asaka M, Kodaira J, Iwano M, Chiba T, Okazaki K, Kato A. Gastric MALT lymphomas are divided into three groups based on responsiveness to Helicobacter Pylori eradication and detection of API2-MALT1 fusion. Am J Surg Pathol. 2004;28:1560-1567. [PubMed] |
| 35. | Chen CH, Pai R. Unusual presentation of MALT lymphoma as diffuse gastric erythema. Gastrointest Endosc. 2012;75:422-43; discussion 423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Nonaka K, Ishikawa K, Arai S, Nakao M, Shimizu M, Sakurai T, Nagata K, Nishimura M, Togawa O, Ochiai Y. A case of gastric mucosa-associated lymphoid tissue lymphoma in which magnified endoscopy with narrow band imaging was useful in the diagnosis. World J Gastrointest Endosc. 2012;4:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 37. | Takeshita K, Ashikawa T, Watanuki S, Tani M, Saito N, Sunagawa M, Habu H, Endo M. Endoscopic and clinicopathological features of primary gastric lymphoma. Hepatogastroenterology. 1993;40:485-490. [PubMed] |
| 38. | Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer 2008; . |
| 39. | Kim JS, Chung SJ, Choi YS, Cheon JH, Kim CW, Kim SG, Jung HC, Song IS. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br J Cancer. 2007;96:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | El-Zahabi LM, Jamali FR, El-Hajj II, Naja M, Salem Z, Shamseddine A, El-Saghir NS, Zaatari G, Geara F, Soweid AM. The value of EUS in predicting the response of gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication. Gastrointest Endosc. 2007;65:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Lee SK, Lee YC, Chung JB, Chon CY, Moon YM, Kang JK, Park IS, Suh CO, Yang WI. Low grade gastric mucosa associated lymphoid tissue lymphoma: treatment strategies based on 10 year follow-up. World J Gastroenterol. 2004;10:223-226. [PubMed] |
| 42. | Takenaka R, Yokota K, Mizuno M, Okada H, Toyokawa T, Yamasaki R, Yoshino T, Sugiyama T, Asaka M, Shiratori Y. Serum antibodies to Helicobacter pylori and its heat-shock protein 60 correlate with the response of gastric mucosa-associated lymphoid tissue lymphoma to eradication of H. pylori. Helicobacter. 2004;9:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Asano N, Iijima K, Terai S, Jin X, Ara N, Chiba T, Fushiya J, Koike T, Imatani A, Shimosegawa T. Eradication therapy is effective for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. Tohoku J Exp Med. 2012;228:223-227. [PubMed] |
| 44. | Steinbach G, Ford R, Glober G, Sample D, Hagemeister FB, Lynch PM, McLaughlin PW, Rodriguez MA, Romaguera JE, Sarris AH. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88-95. [PubMed] |
| 45. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 46. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [PubMed] |
| 47. | Fischbach W. Gastric mucosa-associated lymphoid tissue lymphoma: a challenge for endoscopy. Gastrointest Endosc. 2008;68:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Ono S, Kato M, Ono Y, Itoh T, Kubota K, Nakagawa M, Shimizu Y, Asaka M. Characteristics of magnified endoscopic images of gastric extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue, including changes after treatment. Gastrointest Endosc. 2008;68:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Norimura D, Fukuda E, Yamao T, Niino D, Haraguchi M, Ozawa E, Sawayama Y, Moriuchi Y, Ohnita K, Isomoto H. Education and Imaging. Gastrointestinal: gastric mucosa-associated lymphoid tissue (MALT) lymphoma observed by magnifying endoscopy with narrow band imaging. J Gastroenterol Hepatol. 2012;27:987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Isomoto H, Ishii H, Matsushima K, Ohnita K, Nakayama T, Hayashi T, Nakao K. Gastrointestinal: Novel endocytoscopic findings of gastric low-grade mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2012;27:1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Chou YP, Wang MC, Chuah SK. Sa1646 Magnifying Endoscopic Narrow-Band Imaging of Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma: A Useful Modality for Discriminating Treatment Response. Gastrointest endosc. 2011;73:AB233. |
| 52. | Isomoto H, Shikuwa S, Yamaguchi N, Miyazato K, Ohnita K, Hayashi T, Mizuta Y, Ito M, Kohno S. Magnified endoscopic findings of gastric low-grade mucosa-associated lymphoid tissue lymphoma. Endoscopy. 2008;40:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 54. | Bertoni F, Coiffier B, Salles G, Stathis A, Traverse-Glehen A, Thieblemont C, Zucca E. MALT lymphomas: pathogenesis can drive treatment. Oncology (Williston Park). 2011;25:1134-142, 1147. [PubMed] |
| 55. | Fusaroli P, Caletti G. Endoscopic ultrasonography: current clinical role. Eur J Gastroenterol Hepatol. 2005;17:293-301. [PubMed] |
| 56. | Kusakari J. [A suggestion to promotion of regional nursing plans]. Kango Tenbo. 1977;2:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Suekane H, Iida M, Yao T, Matsumoto T, Masuda Y, Fujishima M. Endoscopic ultrasonography in primary gastric lymphoma: correlation with endoscopic and histologic findings. Gastrointest Endosc. 1993;39:139-145. [PubMed] |
| 58. | Fusaroli P, Buscarini E, Peyre S, Federici T, Parente F, De Angelis C, Bonanno G, Meroni E, Napolitano V, Pisani A. Interobserver agreement in staging gastric malt lymphoma by EUS. Gastrointest Endosc. 2002;55:662-668. [PubMed] |
| 59. | Lévy M, Hammel P, Lamarque D, Marty O, Chaumette MT, Haioun C, Blazquez M, Delchier JC. Endoscopic ultrasonography for the initial staging and follow-up in patients with low-grade gastric lymphoma of mucosa-associated lymphoid tissue treated medically. Gastrointest Endosc. 1997;46:328-333. [PubMed] |
| 60. | Lügering N, Menzel J, Kucharzik T, Koch P, Herbst H, Tiemann M, Domschke W. Impact of miniprobes compared to conventional endosonography in the staging of low-grade gastric malt lymphoma. Endoscopy. 2001;33:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Hoepffner N, Lahme T, Gilly J, Koch P, Foerster EC, Menzel J. [Endoscopic ultrasound in the long-term follow-up of primary lymphomas of the stomach under conservative therapy]. Z Gastroenterol. 2003;41:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 62. | Yeh HZ, Chen GH, Chang WD, Poon SK, Yang SS, Lien HC, Chang CS, Chou G. Long-term follow up of gastric low-grade mucosa-associated lymphoid tissue lymphoma by endosonography emphasizing the application of a miniature ultrasound probe. J Gastroenterol Hepatol. 2003;18:162-167. [PubMed] |
| 63. | Pavlović AR, Krstić M, Tomić D, Bjelović M, Jesić R, Suvajdzić N. [Endoscopic ultrasound (EUS) in initial assessment and follow-up of patients with MALT lymphoma treated drug therapy]. Acta Chir Iugosl. 2005;52:83-89. [PubMed] |
| 64. | Vetro C, Romano A, Chiarenza A, Conticello C, Donnarumma D, Gorgone A, Coppolino F, Palumbo GA, Bonanno G, Di Raimondo F. Endoscopic ultrasonography in gastric lymphomas: appraisal on reliability in long-term follow-up. Hematol Oncol. 2012;30:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Pavlick AC, Gerdes H, Portlock CS. Endoscopic ultrasound in the evaluation of gastric small lymphocytic mucosa-associated lymphoid tumors. J Clin Oncol. 1997;15:1761-1766. [PubMed] |
| 66. | Ohashi S, Segawa K, Okamura S, Urano F, Kanamori S, Hosoi T, Ishikawa H, Kanamori A, Kitabatake S, Sano H. Gastrin and Helicobacter pylori in low-grade MALT lymphoma patients. Scand J Gastroenterol. 2002;37:279-286. [PubMed] |
| 67. | Di Raimondo F, Caruso L, Bonanno G, Naso P, Chiarenza A, Fiumara P, Bari A, Palumbo GA, Russo A, Giustolisi R. Is endoscopic ultrasound clinically useful for follow-up of gastric lymphoma? Ann Oncol. 2007;18:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Zucca E, Dreyling M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v175-v176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Nagashima R, Takeda H, Maeda K, Ohno S, Takahashi T. Regression of duodenal mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori. Gastroenterology. 1996;111:1674-1678. [PubMed] |
| 70. | Rambaud JC, Halphen M, Galian A, Tsapis A. Immunoproliferative small intestinal disease (IPSID): relationships with alpha-chain disease and “Mediterranean” lymphomas. Springer Semin Immunopathol. 1990;12:239-250. [PubMed] |
| 71. | Chen CT, Yen HH, Wu L. Primary jejunal mucosa-associated lymphoid tissue lymphoma. QJM. 2013;106:195-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 72. | Nakamura S, Matsumoto T, Takeshita M, Kurahara K, Yao T, Tsuneyoshi M, Iida M, Fujishima M. A clinicopathologic study of primary small intestine lymphoma: prognostic significance of mucosa-associated lymphoid tissue-derived lymphoma. Cancer. 2000;88:286-294. [PubMed] |
| 73. | Yoneda K, Takahashi H, Abe Y, Inamori M, Kato S, Uchiyama T, Iida H, Mawatari H, Hosono K, Endo H. A mucosa-associated lymphoid tissue (MALT) lymphoma of the small intestine that was difficult to diagnose endoscopically. Endoscopy. 2010;42 Suppl 2:E175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Di Cataldo A, Lanteri R, Rapisarda C, Di Raimondo F, Licata A. Lymphoma of the cecum: a case report. Int Surg. 2002;87:12-14. [PubMed] |
| 75. | Hasegawa N, Tsuboi Y, Kato K, Yamada K, Morita K, Kuroiwa M, Ito H, Matsushima T, Ono K, Oshiro M. Endoscopic diagnosis of ileocecal mucosa-associated lymphoid tissue lymphoma. Gastrointest Endosc. 1999;50:115-117. [PubMed] |
| 76. | Halphen M, Najjar T, Jaafoura H, Cammoun M, Tufrali G. Diagnostic value of upper intestinal fiber endoscopy in primary small intestinal lymphoma. A prospective study by the Tunisian-French Intestinal Lymphoma Group. Cancer. 1986;58:2140-2145. [PubMed] |
| 77. | Flieger D, Keller R, May A, Ell C, Fischbach W. Capsule endoscopy in gastrointestinal lymphomas. Endoscopy. 2005;37:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Terada T. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) of the ileum in a 35-year-old Japanese woman. Int J Clin Exp Pathol. 2013;6:951-956. [PubMed] |
| 79. | Makino Y, Suzuki H, Nishizawa T, Kameyama K, Hisamatsu T, Imaeda H, Mukai M, Hibi T. Ileal Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma with a Large-Cell Component That Regressed Spontaneously. Gut Liver. 2010;4:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Ohashi S, Yazumi S, Watanabe N, Matsumoto S, Fukui T, Nishio A, Chiba T. Education and imaging. Gastrointestinal: MALT lymphoma of the terminal ileum. J Gastroenterol Hepatol. 2006;21:1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Saito T, Toyoda H, Yamaguchi M, Nakamura T, Nakamura S, Mukai K, Fuke H, Wakita Y, Iwata M, Adachi Y. Ileocolonic lymphomas: a series of 16 cases. Endoscopy. 2005;37:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Matsumoto Y, Matsumoto T, Nakamura S, Kawasaki A, Aso M, Aoyagi K, Sadoshima S, Onoyama K, Fujishima M. Primary ileal plasmacytoma arising in mixed low- and high-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Abdom Imaging. 2000;25:139-141. [PubMed] |
| 83. | Shaheen S, Guddati AK. Secondary mucosa-associated lymphoid tissue (MALT) lymphoma of the colon. Med Oncol. 2013;30:502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Argyropoulos T, Foukas P, Kefala M, Xylardistos P, Papageorgiou S, Machairas N, Boltetsou E, Machairas A, Panayiotides IG. Simultaneous occurrence of colonic adenocarcinoma and MALT lymphoma: A series of three cases. World J Gastrointest Oncol. 2012;4:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Redman R, Chesney J. Interesting Collision between an Indolent B-Cell Lymphoma and a Microsatellite Unstable Adenocarcinoma of the Colon. J Hematol Thromb Dis. 2013;1:2. |
| 86. | Ikuta K, Fujiya M, Ueno N, Hosoki T, Moriichi K, Honda M, Torimoto Y, Yamochi T, Ota H, Kohgo Y. Atypical mucosa-associated lymphoid tissue lymphoma in the transverse colon associated with macroglobulinemia. Intern Med. 2010;49:677-682. [PubMed] |
| 87. | Konstantinidis IT, Probstfeld MR. Lymphoma presenting as a necrotic colonic mass. World J Gastrointest Surg. 2012;4:102-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Park H, Chung JW, Kim AJ, Park SY, Rim MY, Jang YR, Lee JH, Park S. A case of rectal mucosa-associated lymphoid tissue lymphoma diagnosed by endoscopic unroofing technique. Korean J Gastroenterol. 2012;59:428-432. [PubMed] |
| 89. | Watanabe T, Suda T, Hirono H, Hasegawa K, Soga K, Shibasaki K, Umezu H. Successful treatment of mucosa-associated lymphoid tissue lymphoma in a patient with gastric and rectal lesions with metachronous and ectopic development. Rare Tumors. 2011;3:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Nakase H, Okazaki K, Ohana M, Ikeda K, Uchida K, Uose S, Itoh T, Iwano M, Watanabe N, Yazumi S. The possible involvement of micro-organisms other than Helicobacter pylori in the development of rectal MALT lymphoma in H. pylori-negative patients. Endoscopy. 2002;34:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Matsumoto T, Iida M, Shimizu M. Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter pylori. Lancet. 1997;350:115-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Inoue F, Chiba T. Regression of MALT lymphoma of the rectum after anti-H. pylori therapy in a patient negative for H. pylori. Gastroenterology. 1999;117:514-515. [PubMed] |
| 93. | Ferreri AJ, Montalbán C. Primary diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol Hematol. 2007;63:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Ferreri AJ, Govi S, Raderer M, Mulè A, Andriani A, Caracciolo D, Devizzi L, Ilariucci F, Luminari S, Viale E. Helicobacter pylori eradication as exclusive treatment for limited-stage gastric diffuse large B-cell lymphoma: results of a multicenter phase 2 trial. Blood. 2012;120:3858-3860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN, Wang HP, Chen LT, Cheng AL. Helicobacter pylori eradication therapy is effective in the treatment of early-stage H pylori-positive gastric diffuse large B-cell lymphomas. Blood. 2012;119:4838-444; quiz 5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Leopardo D, Di Lorenzo G, De Renzo A, Federico P, Luponio S, Buonerba C, Matano E, Merola G, Imbimbo M, Montesarchio E. Efficacy of rituximab in gastric diffuse large B cell lymphoma patients. World J Gastroenterol. 2010;16:2526-2530. [PubMed] |
| 97. | Tanaka T, Shimada K, Yamamoto K, Hirooka Y, Niwa Y, Sugiura I, Kitamura K, Kosugi H, Kinoshita T, Goto H. Retrospective analysis of primary gastric diffuse large B cell lymphoma in the rituximab era: a multicenter study of 95 patients in Japan. Ann Hematol. 2012;91:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170-4176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 99. | Tanaka S, Nagata N, Mine S, Igari T, Kobayashi T, Sugihara J, Honda H, Teruya K, Kikuchi Y, Oka S. Endoscopic appearance of AIDS-related gastrointestinal lymphoma with c-MYC rearrangements: case report and literature review. World J Gastroenterol. 2013;19:4827-4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Li X, Xia B, Guo S, Zhan Z, Zhang L, Zhao D, Wu X, Zhang Y. A retrospective analysis of primary gastric diffuse large B-cell lymphoma with or without concomitant mucosa-associated lymphoid tissue (MALT) lymphoma components. Ann Hematol. 2013;92:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Cortelazzo S, Rossi A, Roggero F, Oldani E, Zucca E, Tondini C, Ambrosetti A, Pasini F, Pinotti G, Bertini M. Stage-modified international prognostic index effectively predicts clinical outcome of localized primary gastric diffuse large B-cell lymphoma. International Extranodal Lymphoma Study Group (IELSG). Ann Oncol. 1999;10:1433-1440. [PubMed] |
| 102. | Zepeda-Gomez S, Camacho J, Oviedo-Cardenas E, Lome-Maldonado C. Gastric infiltration of diffuse large B-cell lymphoma: endoscopic diagnosis and improvement of lesions after chemotherapy. World J Gastroenterol. 2008;14:4407-4409. [PubMed] |
| 103. | Püspök A, Raderer M, Chott A, Dragosics B, Gangl A, Schöfl R. Endoscopic ultrasound in the follow up and response assessment of patients with primary gastric lymphoma. Gut. 2002;51:691-694. [PubMed] |
| 104. | Vaidya R, Habermann TM, Donohue JH, Ristow KM, Maurer MJ, Macon WR, Colgan JP, Inwards DJ, Ansell SM, Porrata LF. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | Doolabh N, Anthony T, Simmang C, Bieligk S, Lee E, Huber P, Hughes R, Turnage R. Primary colonic lymphoma. J Surg Oncol. 2000;74:257-262. [PubMed] |
| 106. | Montgomery M, Chew FS. Primary lymphoma of the colon. AJR Am J Roentgenol. 1997;168:688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Gonzalez QH, Heslin MJ, Dávila-Cervantes A, Alvarez-Tostado J, de los Monteros AE, Shore G, Vickers SM. Primary colonic lymphoma. Am Surg. 2008;74:214-216. [PubMed] |
| 108. | Van Hauwaert V, Meers S, Verhoef G, Tousseyn T, Sagaert X, Vermeire S, Rutgeerts P, Van Assche G. Rectal non-Hodgkin‘s lymphoma in an infliximab treated patient with ulcerative colitis and primary sclerosing cholangitis. J Crohns Colitis. 2010;4:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |