Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.12713
Revised: April 28, 2014
Accepted: May 23, 2014
Published online: September 28, 2014
Processing time: 215 Days and 18.9 Hours
In the intestine a balance between proinflammatory and repair signals of the immune system is essential for the maintenance of intestinal homeostasis. The innate immunity ensures a primary host response to microbial invasion, which induces an inflammatory process to localize the infection and prevent systemic dissemination of pathogens. The key elements of this process are the germline encoded pattern recognition receptors including Toll-like receptors (TLRs). If pathogens cannot be eliminated, they may elicit chronic inflammation, which may be partly mediated via TLRs. Additionally, chronic inflammation has long been suggested to trigger tissue tumorous transformation. Inflammation, the seventh hallmark of cancer, may affect all phases of tumor development, and evade the immune system. Inflammation acts as a cellular stressor and may trigger DNA damage or genetic instability. Furthermore, chronic inflammation can provoke genetic mutations and epigenetic mechanisms that promote malignant cell transformation. Colorectal cancers in inflammatory bowel disease patients are considered typical examples of inflammation-related cancers. Although data regarding the role of TLRs in the pathomechanism of cancer-associated colitis are rather conflicting, functionally these molecules can be classified as ”largely antitumorigenic” and ”largely pro-tumorigenic” with the caveat that the underlying signaling pathways are mainly context (i.e., organ-, tissue-, cell-) and ligand-dependent.
Core tip: Colorectal cancers arising in inflammatory bowel disease patients are considered typical examples of inflammation-associated cancers. The exact role of Toll-like receptor (TLR)-signaling in colitis-associated cancer initiation and development is conflicting. Here we aimed to summarize recent data on the contribution of TLR-mediated immune responses to inflamation-related colonic carcinogenesis.
- Citation: Sipos F, Fűri I, Constantinovits M, Tulassay Z, Műzes G. Contribution of TLR signaling to the pathogenesis of colitis-associated cancer in inflammatory bowel disease. World J Gastroenterol 2014; 20(36): 12713-12721
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/12713.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.12713
Crohn’s disease (CD) and ulcerative colitis (UC), the main clinical phenotypes of idiopathic, relapsing-remitting inflammatory bowel disease (IBD) are systemic disorders affecting the GI-tract with frequent extraintestinal manifestations and other associated autoimmune conditions. IBD is considered a polygenic autoimmune disorder with a complex multifactor etiology. Generally, IBD arises in susceptible individuals in whom upon an environmental trigger a sustained disturbed, deleterious mucosal immune reaction is provoked towards commensal microbiota[1].
A balance between proinflammatory and repair signals of the immune system is essential for the maintenance of intestinal homeostasis. The interplay of genes regulating immune functions is strongly affected by the environment, especially gut resident microbiota. On the basis of genetic alterations in CD, impaired sensing and handling of intracellular bacteria by the innate immunity seem to be one of the most relevant pathophysiologic features[1].
The innate immunity ensures a primary host response to microbial invasion, which induces an inflammatory process to localize the infection and prevent systemic dissemination of pathogens. The key elements of this process are the germline encoded pattern recognition receptors (PRRs) including Toll-like receptors (TLRs), NOD-like receptors (NLRs), ribonucleic-acid (RNA) helicases, C-type lectin receptors, and cytosolic deoxyribonucleic-acid (DNA) sensors, which sense evolutionarily conserved pathogen-associated molecular patterns (PAMPs) of microbiota. The detection of PAMPs by PRRs triggers sequential activation of intracellular signaling pathways resulting in induction of a wide range of cytokines and chemokines that unite the early host response to infection[2]. If pathogens cannot be eliminated, they may elicit chronic inflammation, which may be partly mediated via TLRs. Additionally, chronic inflammation has long been suggested to trigger tissue tumorous transformation. Indeed, a higher incidence of intestinal cancers has been observed in IBD patients. However, the exact role of TLR-signaling in colitis-associated cancer (CAC) initiation and development is still unknown, therefore, we aimed to summarize the currently available information on the contribution of TLR-mediated immune responses to inflammation-related colonic carcinogenesis.
The highly conserved TLRs represent sentinels of the innate immune system. TLRs belong to the type 1 transmembrane glycoproteins, which contain extracellular leucin-rich repeated sequences and Toll/interleukin-1 receptor (TIR) signaling domains. TLRs have five TIR-containing adaptor proteins, Myeloid differentiation factor 88 (MyD88), MyD88 adaptor-like (TIRAP), TIR domain containing adaptor inducing interferon-β (TRIF), TRIF-related adaptor molecule (TRAM)[3], and sterile α and heat-armadillo motifs[4]. TLR4 was the first receptor to be identified, and currently 10 TLRs have been identified in humans, and 13 in mice[5]. TLRs are mainly expressed in the cells of the innate and adaptive immune system [i.e., monocytes, macrophages, lymphocytes, mast cells, dendritic cells (DCs)], however, all TLR1-9 have also been identified as being expressed in human intestinal epithelial cells (IECs)[6-8].
TLRs usually recognize microbial wall components, as well as DNA and RNA fragments. TLRs bind specific motifs appearing in bacteria, fungi, protozoa, and viruses[9,10]. These motifs are mainly lipids and lipopeptides (TLR-1, -2, -4, -6), bacterial flagellin (TLR5), and nucleic acid fragments (TLR-3,-7, -8, -9). TLR3 binds double-stranded RNA from viruses, while TLR7 and -8 can recognize single-stranded RNAs. Moreover, TLR7 recognizes immunoglobulin/self-RNA complexes within autoimmune disease conditions. Imiquimod is a specific ligand for TLR7. TLR9 is activated by bacterial and viral DNA, immunoglobulin-DNA complexes, and synthetic oligodeoxynucleotides (ODNs), which contain unmethylated CpG sequences[9,10].
In vitro data have demonstrated hyporesponsiveness of IECs to TLR ligands[7,8]. Antigen-presenting cells (APCs) in the lamina propria (LP) also seem to be unresponsive to TLR ligands[11]. Under physiologic conditions, TLR3, -7, -8, and -9 are expressed in endosomes, or basolateral membrane (TLR5), where these TLRs are not exposed to pathogens unless microbiota get into the cells or invade mucosa[2]. Apical epithelial TLR9 activation by bacterial DNA fragments has been reported to take part in colonic homeostasis[12]. These findings underline a unique feature of TLRs (and other PRRs) in IECs that establishes immune tolerance to the commensal flora of the colonic mucosal interface.
In addition, epithelial TLRs contribute to balancing the composition of luminal microorganisms by regulating the secretion of different antimicrobial peptides and mucosal IgA. TLR9-/- mice have impaired expression of cryptidin (α-defensin) compared to wild-type mice[12]. Signaling through TLR-2, -3, and -4 have all been implicated with the expression of β-defensins in IECs[13,14]. Several TLR signals in IECs induce B cell-activating factors leading to immunoglobulin class switch recombination in B cells of the LP without T cell activation, resulting in IgA secretion[15]. Moreover, activation of TLR-3 and -4 has been found to induce epithelial expression of an epithelial immunoglobulin transporter (polymeric immunoglobulin receptor) that enhances luminal IgA secretion[16,17].
To date, TLR signaling can be classified into classical/canonical and alternative/noncanonical pathways[18]. All TLRs, except TLR3, utilize the MyD88-dependent signaling pathway to induce the expression of proinflammatory cytokine genes[19]. TLR3 exclusively uses the TRIF pathway[19]. The classical inflammatory signaling pathway is mainly activated through MyD88, which, in turn, recruits IRAKs and TRAF6[20]. TRAF6 activates transforming growth factor-β activated kinase 1 which phosphorylates and activates the inhibitor of kappa light polypeptide gene enhancer in B-cells kinase complex, finally resulting in the release and translocation of NF-κB into the nucleus, thereby inducing the production of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6, the key mediators of (intestinal) proinflammatory responses[21-23]. However, TLR3 and some of the TLR4 signals utilize the TRIF adaptor molecule signaling independently of MyD88. This alternative pathway culminates in the activation of TRAF3 and IRF3, resulting in the secretion of type I interferons (IFNs), even in the gut[24]. TLR4 is unique among the TLRs as it can activate two distinct signaling pathways: the classical pathway (through TIRAP and MyD88) and the alternative pathway (via TRIF and TRAM)[18].
As early as ancient times, Hippocrates and Galenus realized the similarity between inflammation and cancer, and hypothesized that cancer evolved from inflammatory lesions[25]. In 1863, Rudolf Virchow observed a close etiologic relation between chronic inflammation and carcinogenesis, realizing that tumors possess a typical “lymphoreticular infiltrate”[26,27]. The first evidence of the antitumoral effects of microbial products dates to the beginning of the 18th century, when Deider reported that infection in patients with cancer could be accompanied by the remission of malignancies[28]. In the 1890s, William B. Coley, a surgeon from New York, observed that repeated injections of a mixture of bacterial toxins served as an efficient antitumoral therapeutic agent[29]. Later, in 1943, lipopolysaccharide (LPS) was discovered as the “hemorrhage-producing fraction” of Coley’s lysate, which accounted for its antitumoral effects[28]. After the discovery of TLRs, their ligands and signaling pathways, it was found that microbe-derived factors act by stimulating TLR signaling and activating both the innate and adaptive immune responses to enhance anti-tumor immunity[30].
In 2000, Hanahan and Weinberg[31] proposed a model to define the six hallmarks of carcinogenesis. Generally, inflammation is required to fight microbial infections, heal wounds, and maintain tissue homeostasis, however, it can lead to cancer. Inflammation, the seventh hallmark of cancer may affect all phases of tumor development, including tumor initiation, promotion, invasion and metastatic dissemination, and can evade the immune system. Inflammation acts as a cellular stressor and may trigger DNA damage or genetic instability. Furthermore, chronic inflammation can provoke genetic mutations and epigenetic mechanisms which promote malignant cell transformation. Based on these results, nowadays increasing evidence suggests that inflammation should also be included in this list[18,32]. In inflammation, a peculiar tissue microenvironment is induced with the capacity to tolerate tumor cell growth and metastasis by altering the immunoregulatory mechanism, and thus making the immune system incapable of destroying tumor cells[18]. Moreover, the expression of TLRs in tumor cells may directly or indirectly contribute to tumorigenesis in several tissues and organs. Activation of TLR signaling pathways may promote tumor invasion, apoptosis resistance, chemoresistance, tumor progression and metastasis development[18].
In chronic inflammatory conditions, when organs with large epithelial surfaces are affected, as in IBD, the epithelial barrier function is critical for disease onset. As the epithelium is densely inhabited by resident microbial flora, the role of native immunity is particularly important in recognising and distinguishing commensal enteric bacteria from invading bacteria, and thus, in maintaining tolerance and homeostasis. Subsequently, the chronic unrestrained inflammatory response which occurs in IBD is mainly driven by a disintegrated host immune regulatory network, and is further responsible for the increased susceptibility to colorectal cancer (CRC).
TLRs are involved in the maintenance and functioning of the epithelial barrier integrity in the gut and regulating the MyD88 adaptor protein. Therefore, TLRs may display a protective function in the control of intestinal inflammation and inflammation-associated cancer[33]. Colorectal cancers in IBD patients are considered typical examples of inflammation-related cancers. However, tumors usually appear after several years of active disesase, with a cumulative lifetime risk of 18%-20% in UC, and up to 8% in CD[34-36]. Indeed, recent epidemiological data indicate that over 25% of all cancers are related to chronic infection and other unresolved inflammation[37]. Current results indicate that TLRs have a potential role in microbiota-associated gastrointestinal cancer metastasis through the recognition of microbiota ligands, initiating inflammation, and promoting tumorigenesis[38].
Most colorectal cancers are sporadic without any obvious connection to intestinal inflammation. Interestingly, IBD patients also have an increased susceptibility to other malignancies, such as lymphomas/leukemias and hepatocellular carcinoma suggesting that local inflammation could not only have intestinal, but also systemic tumor-promoting effects, or the genetic alterations that affect inflammatory and immune homeostasis in IBD also predispose patients to cancer in other tissues[39,40]. In IBD, the increased susceptibility to extraintestinal tumors could also be related to immunosuppressive treatment. However, the types of tumors increasingly found in IBD patients are different from those observed in transplant patients under immunosuppression[41,42].
Both intrinsic and extrinsic inflammatory pathways are linked to carcinogenesis. Intrinsic inflammation is mainly initiated by mutations leading to oncogene activation as well as to inactivation of tumor suppressors. The extrinsic pathway in terms of infection or inflammation increases cancer risk. Although in IBD patients inflamed intestinal cells already have CRC-related genetic abnormalities before developing dysplasia, in CAC, genetic alterations seem only to be a secondary cause rather than a primary cause of carcinogenesis[43]. It is likely that abnormalities in PRR signaling lead to dysregulated expression of genes and enzymes involved in cell proliferation, apoptosis, and DNA repair prior to gene alterations. Frequent alternative cycles of mucosal injury and repair in the presence of tumorigenic cytokines, chemokines, and prostaglandins may also predispose to genetic mutations, which increase cancer risk[44,45].
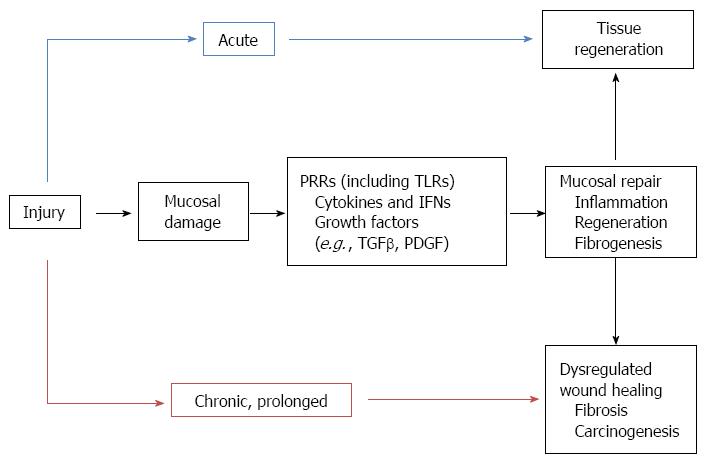
The definite similarities between tumor stroma and chronic wounds have led to the suggestion that cancers are wounds that do not heal, leading finally to uncontrolled tissue repair processes[46] (Figure 1). CRC arises from the intestinal epithelium, a highly proliferative tissue which renews itself every several days under steady-state conditions. Repeated or prolonged wound repair responses to tissue injury may provoke malignant cell transformation[47]. Epithelial regeneration and myofibroblast activation, two major events in wound-healing, are strongly influenced by TLR signaling. The contribution of TLR signals to regeneration can be found in the intestine, where a TLR2/TLR4/MyD88 cascade mediates mucosal healing in the regenerative phase of DSS-colitis[48,49]. It has also been reported that TLR-mediated MyD88 signaling in macrophages of the LP regulate crypt stem cell differentiation and epithelial proliferation through cyclooxygenase-2 and prostaglandin (PG)E2 expression[49,50]. TLR4 activation has also been shown to induce IEC proliferation via induction of EGFR ligands[51,52]. Moreover, in inflammatory circumstances the surface expression of TLR-2 and -4 may be enhanced leading to IECs responsiveness to their ligands[53,54]. Based on these results, it seems that abnormal TLR signaling may induce enhanced epithelial proliferation, and thus may contribute to colitis-associated carcinogenesis.
Previous studies have shown that certain TLRs are expressed in colon cancers and colon cancer cell lines[18]. In colorectal cancers, TLR3, -4, -5, -7, and -8 have been found to be expressed[18], while several TLRs (including TLR7-9) are also expressed in the human colon carcinoma cell lines, HCT15, SW620 or HT29[55-57]. TLR expression in tumor cells appears to promote tumorigenesis by facilitating survival and migration in a tumorous microenvironment that is characterized by chronic inflammation and PAMPs[58]. On the other hand, the complicated interactions among tumor cells, immune cells, and PAMPs/DAMPs in the tumorous microenvironment may support an inappropriate immune response or antitumor immune tolerance through TLR signaling. With regard to tumorigenesis, a typical dual role of TLR signaling pathways has been proposed, as they may be critical for cancer cell survival and progression, however they may also elicit tumor death signaling. TLR-mediated signaling is directed toward cytoprotection or tumor cell suppression, thus the pro-survival or pro-death function is context-dependent, and influenced by many intra- and extracellular factors, such as the involved tissues, surrounding microenvironment, genetic background, and stage of tumor development, nevertheless its precise relation to cancer networks has not yet been fully elucidated[18].
During the past two decades, studies have established that boosting TLRs and downstream mediators such as type I IFNs, can be used therapeutically to shift the balance from immunotolerance to antitumoral effects[59].
The antigen-presenting capacity of tumor cells is poor, therefore, antitumoral immune responses usually depend on professional APCs like DCs[60]. DCs have been a focus of cancer research due to their ability to initiate potent antitumoral immune responses. A lack of DC activation, often resulting from inhibitory signals from cancer cells, may also induce immune tolerance via T cell deletion or regulatory T cells (Tregs)[60], which favors tumor progression. TLR-activated DCs can mediate antitumoral responses through antigen presentation, T cell activation, and direct cytotoxic effects on tumor cells[61,62]. TLR5 activation on DCs as well as TLR9-stimulated plasmocytoid DCs promote antitumoral immunity[63,64]. It is hypothesized that DC-mediated tumor cell killing triggers a more efficient antigen presentation to cytotoxic T cells, thus amplifying antitumoral responses.
Activation of TLRs on DCs regulates T cell activation not only via the class II major histocompatibility complex and co-stimulatory molecules, but also through TLR-induced signals in DCs that block the suppressive effect of regulatory T cells in an IL-6-dependent manner[65]. Moreover, TLR8 activation can directly inhibit Treg function, hence support antitumoral immunity[66]. TLR9 agonists as well as TLR-induced IFNα also have an important role since both are known to reduce tumor growth by blocking angiogenesis[67,68].
In a recent study, the colonic tumor development modulatory effect of TLR5-dependent signaling was assayed in a mouse xenograft model of human colon cancer[69]. The lack of MyD88 or TLR5 expression was found to enhance tumor growth and inhibit tumor necrosis. In contrast, TLR5 activation by peritumoral flagellin treatment substantially increased tumor necrosis, leading to significant tumor regression.
Within the TLR family, TLR9 is specifically stimulated upon sequence- and methylation-dependent DNA signaling. Self-DNA and oligonucleotides containing unmethylated CpG motifs are also sensed by and activate TLR9. Modifications in the structure of nucleic acids influence their immunomodulatory, i.e., agonistic or suppressive, as well as pro- or anti-tumorigenic capacity[57,70]. TLR9 activation by synthetic oligodeoxynucleotide agonists (CpG-ODN) has also demonstrated antitumor activity in xenograft models of murine colon cancer[71]. Moreover, TLR9 agonists induce type I IFN secretion in DCs finally resulting in cytotoxic DCs, activated NK cells and cytotoxic T cells, all of which possess a remarkable antitumor immune response[72,73].
TLRs may act as tumor promoting factors by transmitting proinflammatory, anti-apoptotic, proliferative or profibrogenic signals in either the tumor cells or the tumorous microenvironment.
TLRs are key elements of inflammatory signaling which can be mediated by MyD88-dependent and MyD88-independent pathways. Enhancement of the signaling pathway of transcription factor nuclear factor (NF)-κB is one of the major tumor-promoting effects of TLRs. TLR activation upregulates several tumorigenic inflammatory cytokines (e.g., IL-1β, TNFα, IL-6) in a NF-κB-dependent manner[59,74,75]. which transcriptionally controls a large set of target genes that play important roles in cell survival, inflammation, and immune responses[76].
TLR signaling is also involved in the inhibition of apoptosis. NF-κB is considered an important anti-apoptotic pathway controlling the expression of anti-apoptotic genes and restricting the activation of proapoptotic pathways[77,78]. TLR signaling can activate NF-κB through MyD88-dependent and MyD88-independent pathways, moreover, the TLR-mediated release of IL-1β and TNFα promotes NF-κB activation. In colorectal cancer, TLR-induced NF-κB activation has been found to facilitate tumor cell survival[48]. Furthermore, in the MC26 mouse colon cancer cell line TLR4 activation was found to mediate resistance of tumor cells to cytotoxic T cell-mediated cell death and favor tumor growth[55].
The TLR-mediated promotion of wound healing may also lead to cancer development. After DSS-mediated injury, TLR2 and TLR4 activation facilitates epithelial repair via the MyD88-dependent pathway[48], and TLR-MyD88 signaling also regulates the expression of epiregulin, which may contribute to colon cancer development[78].
In mice, Faubion et al[79] demonstrated that chronic inflammation arising from the bowel may induce thymic involution and Treg cell suppression. These events are suggested to lead to the enhancement of inflammation-mediated processes, and worsen IBD. Restoration of homeostasis through suppression of TNFα production and fortification of Treg cells were proposed for the treatment of human IBD[80]. In the existing connection between TLR-signaling and Treg cells[65,81], these data on IBD may support the concept that uncontrolled inflammation weakens the Treg-mediated inhibition and increases the risk for inflammation-associated carcinogenesis.
Controversial data exist regarding the role of TLR2 in CAC. In one study, increased tumor development and higher IL-6, IL-17A and phospho-STAT3 levels were reported in a TLR2-deficient azoxymethane (AOM)-dextran sodium sulfate (DSS) murine model[82], while there were no differences in CAC between wild-type and TLR2-deficient AOM-DSS colitic animals[83].
The pro-tumorigenic role of TLR4 in CAC is well established. The intestinal microbiota, which normally colonize mucosal surfaces in symbiotic mutualism with the host is unique and quite stable over time[84]. The basic challenge for intestinal immune recognition is the requirement of a simultaneous delicate balance between tolerance and responsiveness towards microbes[85]. Several data suggest the existence of immune tolerance to antigens in an individual’s bacterial flora, whereas its breakdown definitely contributes to IBD pathogenesis[86]. In the colon, where there is a constant interaction between microbiota and IECs, TLR4 deletion significantly reduces inflammation and tumor size in the AOM-DSS induced CAC-model in mice[87]. Additionally, overexpression of the constitutively actived TLR4 exhibits a higher sensitivity to CAC in a transgenic mouse model[88]. Other studies[89,90] also support the results that both the deletion of the TLR4 adaptor MyD88 molecule and the depletion of TLR4 activating gut microbiome reduce colon cancer development.
Inflammation affects many aspects of tumorigenesis. One of the important discoveries in the field of TLR signaling and inflammation-associated cancer biology is the realization that TLRs may promote or suppress tumor formation in certain organs, including the intestine.
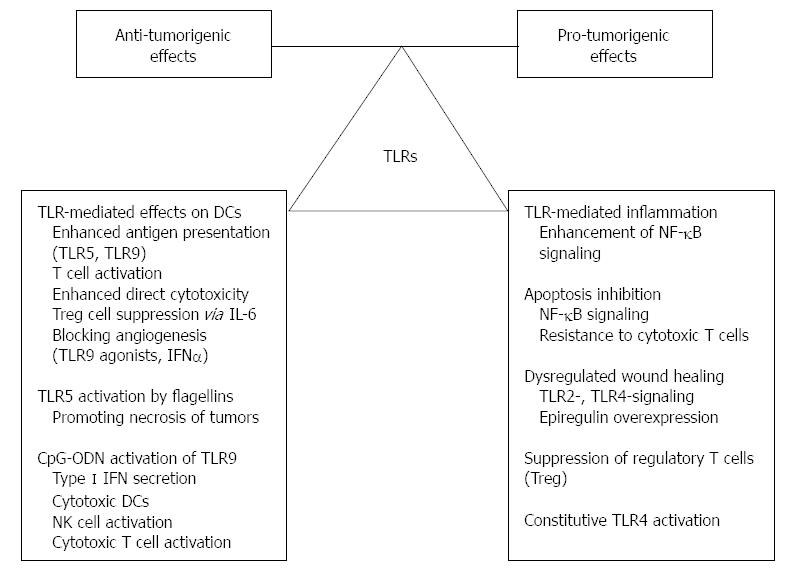
Although recent data regarding the role of TLRs in the pathomechanism of CAC are rather conflicting, functionally they can be classified as “largely antitumoral” and “largely tumorigenic” molecules with the caveat that the underlying signaling pathways are mainly context, (i.e., organ-, tissue-, cell-) and ligand-dependent (Figure 2). Advanced exploration and better understanding of the relationship amongst TLR-signaling, inflammatory microenvironment and colitis-associated carcinogenesis should provide further insight into cancer development in IBD patients.
Further detailed functional analyses of TLRs in inflammation-related tumor biology are needed to explore and define more precisely the subcellular and molecular mechanisms, hopefully allowing the introduction of selective new therapeutic approaches into daily practice.
P- Reviewer: Rao VS S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1041] [Article Influence: 94.6] [Reference Citation Analysis (18)] |
| 2. | Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Jenkins KA, Mansell A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine. 2010;49:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 6. | Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054-1070. [PubMed] |
| 8. | Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406-1415. [PubMed] |
| 9. | Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 827] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 10. | Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 678] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 11. | Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66-75. [PubMed] |
| 12. | Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 457] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 13. | Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100-3111. [PubMed] |
| 14. | Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398-5405. [PubMed] |
| 15. | Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175:376-384. [PubMed] |
| 17. | Bruno ME, Rogier EW, Frantz AL, Stefka AT, Thompson SN, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor in intestinal epithelial cells by Enterobacteriaceae: implications for mucosal homeostasis. Immunol Invest. 2010;39:356-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res. 2012;35:1297-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253-258. [PubMed] |
| 20. | Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3-9. [PubMed] |
| 21. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [PubMed] |
| 22. | Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338-344. [PubMed] |
| 23. | O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3-9. [PubMed] |
| 24. | Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006;84:333-341. [PubMed] |
| 25. | Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 365] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 26. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] |
| 27. | Kim EH, Hong KS, Hong H, Hahm KB. Detouring the Undesired Route of Helicobacter pylori-Induced Gastric Carcinogenesis. Cancers (Basel). 2011;3:3018-3028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Garay RP, Viens P, Bauer J, Normier G, Bardou M, Jeannin JF, Chiavaroli C. Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol. 2007;563:1-17. [PubMed] |
| 29. | Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 306] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | de Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front Immunol. 2014;5:60. [PubMed] |
| 32. | Lazebnik Y. What are the hallmarks of cancer? Nat Rev Cancer. 2010;10:232-233. [PubMed] |
| 33. | Aviello G, Corr SC, Johnston DG, O’Neill LA, Fallon PG. MyD88 adaptor-like (Mal) regulates intestinal homeostasis and colitis-associated colorectal cancer in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G769-G778. [PubMed] |
| 34. | Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] |
| 36. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [PubMed] |
| 37. | Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831-3852. [PubMed] |
| 38. | Lu Q, Ding H, Li W. Role of Toll-like receptors in microbiota-associated gastrointestinal cancer metastasis. J Cancer Res Ther. 2013;9 Suppl:S142-S149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Farrell RJ, Ang Y, Kileen P, O’Briain DS, Kelleher D, Keeling PW, Weir DG. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514-519. [PubMed] |
| 40. | Castro FA, Liu X, Försti A, Ji J, Sundquist J, Sundquist K, Koshiol J, Hemminki K. Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin Gastroenterol Hepatol. 2014;12:1038-45.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Sodemann U, Bistrup C, Marckmann P. Cancer rates after kidney transplantation. Dan Med Bull. 2011;58:A4342. [PubMed] |
| 42. | AlBugami M, Kiberd B. Malignancies: pre and post transplantation strategies. Transplant Rev (Orlando). 2014;28:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 44. | Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 576] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 45. | Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 46. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. [PubMed] |
| 47. | Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230:676-678. [PubMed] |
| 48. | Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862-877. [PubMed] |
| 49. | Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99-104. [PubMed] |
| 50. | Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258-269. [PubMed] |
| 51. | Brandl K, Sun L, Neppl C, Siggs OM, Le Gall SM, Tomisato W, Li X, Du X, Maennel DN, Blobel CP. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA. 2010;107:19967-19972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 52. | Hsu D, Fukata M, Hernandez YG, Sotolongo JP, Goo T, Maki J, Hayes LA, Ungaro RC, Chen A, Breglio KJ. Toll-like receptor 4 differentially regulates epidermal growth factor-related growth factors in response to intestinal mucosal injury. Lab Invest. 2010;90:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255-24263. [PubMed] |
| 54. | Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773-9781. [PubMed] |
| 55. | Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009-5014. [PubMed] |
| 56. | Fűri I, Sipos F, Germann TM, Kalmár A, Tulassay Z, Molnár B, Műzes G. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects. World J Gastroenterol. 2013;19:4119-4126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Fűri I, Sipos F, Spisák S, Kiszner G, Wichmann B, Schöller A, Tulassay Z, Műzes G, Molnár B. Association of self-DNA mediated TLR9-related gene, DNA methyltransferase, and cytokeratin protein expression alterations in HT29-cells to DNA fragment length and methylation status. ScientificWorldJournal. 2013;2013:293296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [PubMed] |
| 59. | Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 60. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1555] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 61. | Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4:120ra16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 63. | Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 64. | Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG, Schoenberger SP. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428-6437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033-1036. [PubMed] |
| 66. | Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380-1384. [PubMed] |
| 67. | Damiano V, Garofalo S, Rosa R, Bianco R, Caputo R, Gelardi T, Merola G, Racioppi L, Garbi C, Kandimalla ER. A novel toll-like receptor 9 agonist cooperates with trastuzumab in trastuzumab-resistant breast tumors through multiple mechanisms of action. Clin Cancer Res. 2009;15:6921-6930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Pfeffer LM. Biologic activities of natural and synthetic type I interferons. Semin Oncol. 1997;24:S9-63-S9-S9-63-69. [PubMed] |
| 69. | Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008;135:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Sipos F, Műzes G, Fűri I, Spisák S, Wichmann B, Germann TM, Constantinovits M, Krenács T, Tulassay Z, Molnár B. Intravenous Administration of a Single-Dose Free-Circulating DNA of Colitic Origin Improves Severe Murine DSS-Colitis. Pathol Oncol Res. 2014;Epub ahead of print. [PubMed] |
| 71. | Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdörfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892-3899. [PubMed] |
| 72. | Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184-1194. [PubMed] |
| 73. | Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291-2295. [PubMed] |
| 74. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1770] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 75. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 76. | Tukhvatulin AI, Gitlin II, Shcheblyakov DV, Artemicheva NM, Burdelya LG, Shmarov MM, Naroditsky BS, Gudkov AV, Gintsburg AL, Logunov DY. Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2013;81:3855-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800-6816. [PubMed] |
| 78. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [PubMed] |
| 79. | Faubion WA, De Jong YP, Molina AA, Ji H, Clarke K, Wang B, Mizoguchi E, Simpson SJ, Bhan AK, Terhorst C. Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery. Gastroenterology. 2004;126:1759-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 81. | Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, Wang JY, Jiang W. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat. 2014;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, Shimada K, Wong MH, Michelsen KS, Arditi M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS One. 2010;5:e13027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 83. | Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’hUigin C. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 84. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [PubMed] |
| 85. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [PubMed] |
| 86. | Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 636] [Cited by in RCA: 571] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 87. | Fukata M, Hernandez Y, Conduah D, Cohen J, Chen A, Breglio K, Goo T, Hsu D, Xu R, Abreu MT. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Fukata M, Shang L, Santaolalla R, Sotolongo J, Pastorini C, España C, Ungaro R, Harpaz N, Cooper HS, Elson G. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2011;17:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 89. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [PubMed] |
| 90. | Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Kärre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |