CASE REPORT
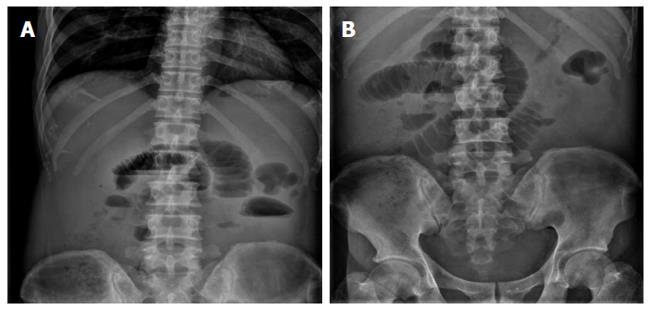
A 61-year-old man attended the Emergency Department of our hospital in October 2013 with complaints of significant weight loss, fatigue and anemia since May 2012. He underwent bone marrow biopsy on November 2012 and the results confirmed the diagnosis of PMF. In June 2013, the patient complained of vomiting, abdominal pain and abdominal distension with passage of flatus. He was then admitted to the Department of Hematology, where he underwent plain abdominal radiography which revealed incomplete small intestinal obstruction (Figure 1); computerized tomography (CT) scan and ultrasound B scan revealed hepatosplenomegaly and ascites, but no mass was found. Anal double-balloon enteroscopic examination was unremarkable, while peroral double-balloon enteroscopic examination was refused by the patient. After fasting for five days, the abdominal symptoms were relieved and the patient was discharged. The patient returned to our hospital with the chief complaints of vomiting, abdominal pain and abdominal distension, but with passage of flatus for a week. On physical examination, his vital signs were normal, however, pallor with ascites, hepatosplenomegaly and hyperactive bowel sounds with no palpable abdominal mass were observed.
Figure 1 Plain abdominal radiography revealed incomplete small intestinal obstruction.
A: The flat film of the abdomen in the standing position showed dilated small bowel loops with air-fluid levels; B: The flat film of the abdomen in the horizontal position showed dilated small bowel.
Laboratory blood examinations showed the following indices (normal range in parentheses): hemoglobin, 59 g/L (120-140 g/L); peripheral white cell count, 5.39 × 109/L (5 × 109/L-10 × 109/L); neutrophils, 72.2% (40%-60%); peripheral red cell count, 2.47 × 1012/L (4.0 × 1012/L-4.5 × 1012/L); platelet count, 210 × 109/L (100 × 109/L-300 × 109/L); peripheral eosinophil count, 0.02 × 109/L (0.02 × 109/L-0.52 × 109/L); C-reactive protein, 20.5 mg/L (0-6.0 mg/L); erythrocyte sedimentation rate, 31 mm/h (0-20 mm/h); albumin, 31.8 g/L (36-51 g/L); total immunoglobulin, 29.8 g/L (25-35 g/L); total bilirubin, 4.3 μmol/L (4-23.9 μmol/L); alkaline phosphatase, 57 U/L (35-125 U/L); c-glutamyl transpeptidase, 18 U/L (7-50 U/L); aspartate aminotransferase, 11 U/L (14-40 U/L); alanine aminotransferase, 5 U/L (5-35 U/L); creatinine, 192 μmol/L (31.8-91.0 μmol/L); blood urine nitrogen, 4.71 g/L (2.4-8.2 g/L); uric acid, 1108 μmol/L (90-420 μmol/L); prothrombin time, 14.3 s (11.0-14.5 s). Hepatitis B and C markers were negative. Serum tumor marker, cancer antigen 125 (CA125) was 321.2 U/mL (0-35 U/mL); alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) were not elevated. Tuberculosis (TB)-related antibodies were not found in the blood, and the TB-purified protein derivative (PPD) skin test was negative. Antinuclear antibodies (ANA), antineutrophil cytoplasmic antibody (ANCA) and rheumatoid factor (RF) were not found in the blood. Routine urine and stool tests did not reveal any RBCs or proteins on the first day of hospitalization. Routine ascites test: color, yellow; Rivalta test, negative; red cell count, 280 × 106/L; red cell count, 66 × 106/L; lymphocytes, 70%; granulocytes, 30%; and no obvious eosinophilic granulocytes. The total protein, albumin, glucose, lactic dehydrogenase (LDH) and adenosine deaminase (ADA) levels in the abdominal fluid were 30.9 g/L, 18 g/L, 4.87 mmol/L, 95 U/L and 3.0 U/L, respectively. The serum-ascites albumin gradient (SAAG) = 31.8 g/L - 18 g/L = 13.8 g/L >11 g/L. No tumor cells were found in the ascites fluid following two cytopathology tests.
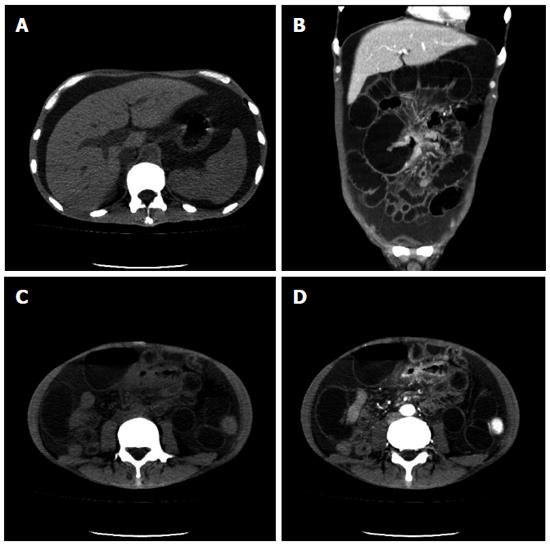
Abdominal CT scanning was repeated and revealed hepatosplenomegaly, huge ascites and thickened ileum wall with obvious enhancement in the arterial phase causing obstruction (Figure 2). However, it was difficult to determine whether the intestinal lesion was malignant or an inflammatory lesion, as there was an obvious enhancement in the arterial phase and ascites simultaneously.
Figure 2 Computerized tomography images indicated hepatosplenomegaly and ascites (A) and the presence of thickened intestinal wall with obvious enhancement in the arterial phase and dilated small bowel (B-D).
A: Plain computerized tomography (CT) scan; B: Coronal CT scan; C: Plain CT scan; D: The arterial phase.
During the first ten days of hospitalization, the patient received a nasogastric tube along with blood transfusion, albumin infusion, an intravenous proton pump inhibitor (pantoprazole 40 mg, twice daily), antibiotics and an intravenous diuretic (furosemide 20 mg, once or twice daily). Ascites reduced considerably, blood hemoglobin increased from 59 g/L to 89 g/L, blood creatinine decreased from 192 μmol/L to 116 μmol/L, and serum albumin increased from 31.8 g/L to 37 g/L, but there was no relief of abdominal symptoms.
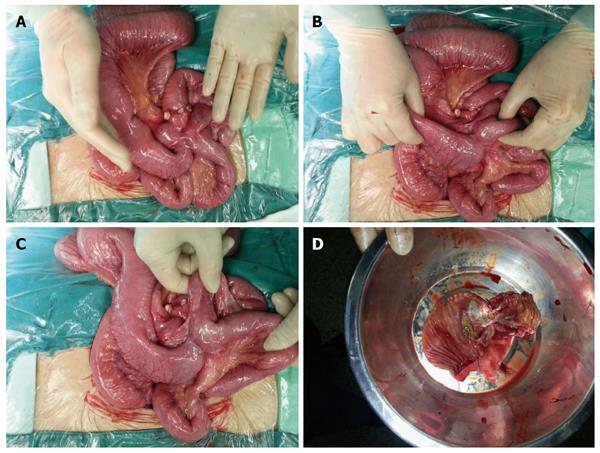
The patient was then referred to the Department of Gastrointestinal Surgery, where a laparotomy and partial enterectomy were performed. During surgery, an intestinal mass of approximately 5 cm × 3 cm × 3 cm and an obstruction in the ileum about 130 cm from the ileocecal valve were found, and intestinal adhesion forming a closed loop due to the mass was also observed. The resected ileum was 15 cm in length with a 5 cm × 3.5 cm yellow ulcerated mass in the center (Figure 3).
Figure 3 Exploratory laparotomy revealed intestinal obstruction caused by an intestinal mass approximately 5 cm × 3 cm × 3 cm and intestinal adhesions.
A: The intestinal mass; B-C: Intestinal adhesions; D: The brownish-yellow mass with an ulcer.
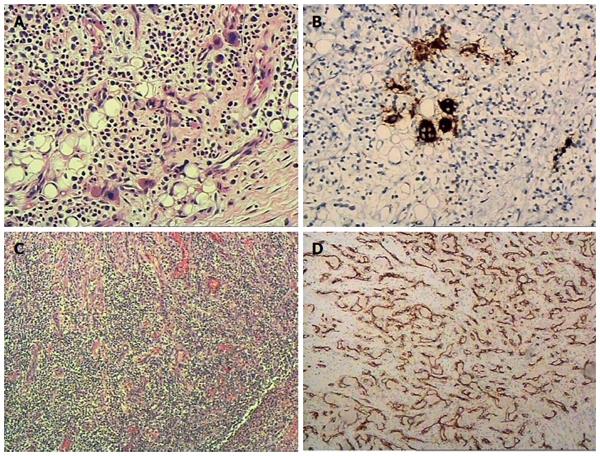
The pathological results were as follows: A gross view revealed a 5 cm × 3.5 cm brownish-yellow ulcerated mass in the 15 cm resected intestine. A microscopic view confirmed an ulcer in the resected specimen and there was significant hyperplasia of blood vessels in the deep layers of the intestine under the ulcer, along with hyperplasia of vascular endothelial cells. A large number of infiltrated inflammatory cells could be seen in the wall of the intestine; and a significant quantity of Megakaryocytes was observed around the serosal area along with an accumulation of immature myeloid cells and erythroid cells. Immunohistochemical (IHC) examination showed the following: CD61 (+), CD68 (-), MPO (+), CD34 (+), CD31 (+), CD11 (-), and Ki-67 (30%). An extramedullary hematopoietic mass of the small intestine with an ulcer and excessive vascular proliferation were confirmed pathologically (Figure 4).
Figure 4 Extramedullary hematopoietic mass of the small intestine with ulcer and excessive vascular proliferation was confirmed by histopathology.
A: A significant quantity of Megakaryocytes along with the accumulation of immature myeloid cells and erythroid cells were observed; B: Megakaryocytes were CD61 positive and CD68 negative (not shown); C: Significant hyperplasia of blood vessels and a large number of infiltrated inflammatory cells were seen; D: Blood vessels were confirmed by positive CD31 staining.
Following surgery, the patient’s abdominal symptoms and ascites completely resolved and he was discharged. Diuretics, testosterone undecanoate and thalidomide were prescribed in the outpatient department during a two-month follow-up and no abdominal symptoms were noted.
DISCUSSION
Extramedullary hematopoiesis occurs in conditions with an increased number of circulating myeloid progenitor cells, such as in PMF. The most common sites of extramedullary hematopoiesis are the spleen, liver, kidneys and the adrenal glands[9]. However, other organs are occasionally involved, such as the gastrointestinal tract[4-7], skin[10], joints[11,12], posterior mediastinum[13,14], the pericardium[15], and the brain[16-18]. A hematopoietic mass can cause symptoms resulting from stricture of hollow organs and compression of adjacent structures. In this patient, the hematopoietic mass was adherent to adjacent structures which also played an important role in causing symptoms. Intestinal obstruction, rectal stenosis, gastric outlet obstruction and bladder outlet obstruction due to extramedullary hematopoiesis have been reported[4-8], and progressive paraplegia may develop when extramedullary hematopoiesis occurs in the epidural space[17,18]. This patient suffered from a closed loop intestinal obstruction due to both intestinal stenosis and adhesion to the adjacent intestine caused by an extramedullary hematopoietic mass. As a closed loop was formed, it is reasonable that the ileac lesion could not be reached by a double-balloon enteroscopic examination. The lesion was identified on CT scan before surgery. In addition to gastrointestinal endoscopic examinations, CT scanning can also serve as an important tool in identifying gastrointestinal lesions. However, there was obvious enhancement in the arterial phase which was consistent with significant hyperplasia of blood vessels as confirmed by the pathological results in this patient, and it was difficult to determine whether the intestinal lesion was malignant on the CT scan.
Ascites are found in some PMF cases, the main cause of ascites is portal hypertension[2,3], peritoneal or other ectopic hematopoiesis can also be the main cause[19-22], and hypoalbuminemia may play a role, as seen in this patient. Portal hypertension is due to two main mechanisms: firstly, increased blood flow through the massively enlarged spleen; secondly, functional intrahepatic obstruction caused by extramedullary hematopoiesis or periportal fibrosis in the liver[2,3,23,24]. Portal hypertension was proved indirectly by the high SAAG level which was higher than 11 g/L in this patient. The ascites, due to typical liquid leakage, reduced significantly following albumin infusion and intravenous diuretic before surgery, and were completely resolved by oral diuretics after surgery. Unfortunately, some patients with ascites are refractory to a sodium-restricted diet and high-dose diuretic treatment, TIPS may be a rescue therapy for refractory ascites secondary to portal hypertension, however, caution is necessary with respect to the presence and/or development of peritoneal or other ectopic hematopoiesis[3,19-22]. Ascites caused by peritoneal hematopoiesis have been reported to respond well to chemotherapy[19].
Intestinal obstruction and ascites occurring simultaneously in a patient is not rare, however, intestinal obstruction caused by extramedullary hematopoiesis and ascites occurring at the same time in a patient with PMF has, to our knowledge, not been reported. A series of differential diagnoses should be considered. Firstly, tuberculous peritonitis causing intestinal obstruction or intestinal tuberculosis causing intestinal obstruction with tuberculous peritonitis have been reported previously, and it is possible that abdominal tuberculosis occurs in PMF; however, there were no symptoms of tuberculosis, tuberculosis (TB)-related antibodies were not found in the blood, the TB-purified protein derivative (PPD) skin test was negative, and ascites was not an inflammatory exudate; thus, tuberculous peritonitis and intestinal tuberculosis were not diagnosed. Secondly, gastrointestinal carcinoma with intestinal stenosis causing metastatic ascites[25] or malignant ascites, such as peritoneal mesothelioma, causing intestinal obstruction[26-28] should also be considered. Fortunately, in this patient, blood CEA was normal, the ascites was due to typical liquid leakage, and no tumor cells were found in the ascites. These results revealed that the patient did not have a tumor. Thirdly, some autoimmune diseases such as systemic lupus erythematosus (SLE) can cause both intestinal obstruction and ascites[29,30]; however, in this patient, ANA, RF or ANCA were not found in the blood and the ascites was not an inflammatory exudate; thus, the diagnosis of autoimmune diseases was not considered. Fourthly, eosinophilic gastroenteritis presenting with intestinal obstruction and ascites has been reported[31,32]. Talley et al[33] identified three main diagnostic criteria for eosinophilic gastroenteritis: (1) the presence of gastrointestinal symptoms; (2) biopsies demonstrating eosinophilic infiltration of one or more areas of the gastrointestinal tract; and (3) no evidence of parasitic or extraintestinal disease; taking the normal level of eosinophilic granulocytes in the blood and ascites and a normal double-balloon enteroscopic examination into consideration, eosinophilic gastroenteritis was not a reasonable diagnosis. Lastly, bloody ascites caused by strangulation obstruction is a common clinical emergency, and was easily excluded in this patient.
Clinical physicians should bear in mind that intestinal obstruction caused by extramedullary hematopoiesis and ascites due to portal hypertension can occur at the same time in PMF, although it is not very common. Ascites due to portal hypertension may be resolved by diuretics, as in this case. Intestinal obstruction caused by an extramedullary hematopoietic mass can be cured surgically by removing the mass.