Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11560
Revised: January 10, 2014
Accepted: April 1, 2014
Published online: September 7, 2014
Processing time: 314 Days and 5 Hours
Recent studies show that ion channels/transporters play important roles in fundamental cellular functions that would be involved in the cancer process. We review the evidence for their expression and functioning in human gastric cancer (GC), and evaluate the potential of cellular physiological approach in clinical management. Various types of ion channels, such as voltage-gated K+ channels, intracellular Cl- channels and transient receptor potential channels have been found to express in GC cells and tissues, and to control cell cycles. With regard to water channels, aquaporin 3 and 5 play an important role in the progression of GC. Regulators of intracellular pH, such as anion exchanger, sodium-hydrogen exchanger, vacuolar H+-ATPases and carbonic anhydrases are also involved in tumorigenesis of GC. Their pharmacological manipulation and gene silencing affect cellular behaviours, suggesting their potential as therapeutic targets for GC. Our studies indicate the intracellular Cl- concentration could act as a mediator of cellular signaling and control cell cycle progression in GC cells. Further, we demonstrate the cytocidal effects of hypotonic shock on GC cells, and indicate that the blockade of Cl- channels/transporters enhances these effects by inhibiting regulatory volume decrease. A deeper understanding of molecular mechanisms may lead to the discovery of these cellular physiological approaches as a novel therapeutic strategy for GC.
Core tip: This article aims to systematically review the current knowledge on expression and functioning of ion transporters in gastric cancer (GC). Various types of ion channels, water channels and regulators of intracellular pH have been found to express in GC, and to control tumorigenesis. Our studies indicate the intracellular Cl- concentration could control cell cycle progression in GC cells. Further, we demonstrate the cytocidal effects of hypotonic shock, and indicate that regulation of ion transport enhances these effects. A deeper understanding of molecular mechanisms may lead to the discovery of these cellular physiological approaches as a novel therapeutic strategy for GC.
- Citation: Shiozaki A, Ichikawa D, Otsuji E, Marunaka Y. Cellular physiological approach for treatment of gastric cancer. World J Gastroenterol 2014; 20(33): 11560-11566
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11560
Gastric cancer (GC) represents the second most common cause of cancer-related deaths in the world[1]. Recently, the prognosis of GC has been improved with advances in surgical techniques, adjuvant therapy, chemoradiotherapy and molecular targeted therapy[2]. However, long-term outcomes of patients with GC remain dismal, especially for advanced disease. An improvement in the treatment of recurrent or metastatic GC depends on understanding of the molecular mechanisms regulating the tumorigenesis and the progression of the disease.
Over the past few decades, many reports have revealed that ion channels and water transporters play important roles in fundamental cellular functions. Particularly, their physiological roles in cell proliferation have been considered since cell volume changes, which require the participation of ion and water movement across the cell membrane, are indispensable in cell cycle progression. Recently, the roles of ion and water channels/transporters have been studied in cancer cells[3-7] and various types of transporters have been found in cancers of digestive organs.
This article aims to systematically review the current knowledge on expression and functioning of ion and water channels/transporters in GC cells and tissues. The ultimate objective is to evaluate the potential of cellular physiological approaches, such as regulation of ion channels, water channels, intracellular pH, intracellular ion concentration and osmolality, in clinical management of GC.
Recent studies have demonstrated that several subtypes of K+ channels are expressed in human GC cells, and are associated with cell proliferation. Altered expression of several voltage-gated K+ channels (Kv) has been observed in GC. Lan et al[8] have demonstrated that Kv1.5 protein is frequently detected in GC tissues and down-regulation of the expression of Kv1.5 in SGC7901 cells inhibits their proliferation and tumorigenicity. Further, Han et al[9] have shown that up-regulation of Kv1.5 increases the K+ current density and sensitivity of SGC7901 cells to multiple chemotherapeutic drugs, such as adriamycin or 5-fluorouracil. Expression of Kv4.1 has been found in human GC cell lines, and its down-regulation inhibited cell proliferation via the blockage of G1-S transition[10]. Eag1 (Kv10.1) was aberrantly expressed in GC tissues and associated with cancer lymph node metastasis and stage[11]. Human ether-a-go-go-related gene (HERG) encodes one of the components of delayed rectifier K+ currents. In GC, HERG channel has cancer-limited expression and its blocker diminishes the G1-S transition[12,13]. Ding et al[14] have shown that the survival rates for the hERG1-positive expression group are significantly lower than the negative group, and hERG1 expression is found to be an independent prognostic factor. Further, HERG expression has been reported to be essential for cisplatin to induce apoptosis in human GC[15]. Disruption of K+ channel protein, voltage-gated K+ channel subfamily E member 2 (KCNE2), has been shown as a possible risk factor for gastric neoplasia[16]. Kuwahara et al[17] have analyzed the expression of KCNE2 in surgically excised tissue from human GC associated with gastritis cystica profunda and confirmed that reduced KCNE2 expression correlates with disease formation. It has been proposed that atrial natriuretic peptide modulates the proliferation of human GC cells via voltage-gated K+ channels, KQT-like subfamily member 1 (KCNQ1)[18]. Inwardly rectifying K+ channels (Kir) have been also implicated in GC. Lee et al[19] have demonstrated that knockdown of Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence.
There is evidence also for Cl- channels involvement in GC. Overexpression of chloride intracellular channel 1 (CLIC1) is shown to be a potential prognostic marker for GC[20]. Elevated CLIC1 expression is strongly correlated with lymph node metastasis, lymphatic invasion, perineural invasion and pathological staging, suggesting that it is a potential prognostic marker[20]. Zheng et al[21] have shown that PA28β regulates cell invasion of GC by modulating the expression of CLIC1. On the other hand, Ma et al[22] have shown that high CLIC1 expression inhibits proliferation and enhances apoptosis, migration and invasion of GC cells.
The transient receptor potential (TRP) superfamily consists of a highly diverse group of ion channels that are mostly permeable to monovalent and divalent cations. TRP channels may be divided into seven subfamilies, including the classical (TRPC), the vanilloid receptor related (TRPV) and the melastatin related (TRPM) channels. In GC, Cai et al[23] have shown that Ca2+ elevation regulated by TRPC6 channels is essential for G2/M phase transition and suppresses growth in human GC cells. TRPV6 has been implicated in capsaicin-induced apoptosis in GC cells[24]. Further, several reports have shown important roles of TRPM7 in apoptosis and cell viability of GC cells[25-28].
There is significant evidence for involvement of these ion channels in GC cell proliferation and disease progression. Hence, their clinical potential would be worth investigating further.
Aquaporins (AQPs) are transmembrane proteins that facilitate transport of water and, in some cases, small solutes across membranes; charged species are not permeated. Thus, AQPs are important for cell volume regulation and electrolyte balance under both physiological and pathophysiological conditions. To date, 13 AQP subtypes and their pathophysiologic roles have been characterized in humans. In GC, several reports indicated the role of AQP3 in signal pathway. Huang et al[29] have shown that AQP3 plays a critical role in human epidermal growth factor (hEGF) -induced cancer cell migration and proliferation and that hEGF induced AQP3 expression via ERK signal transduction pathways. Wang et al[30] have demonstrated that c-Met regulates the expression of AQP3 via the ERK signalling pathway in GC. Xu et al[31] have shown that AQP3 positively regulates matrix metalloproteinases (MMPs) proteins expression through the PI3K/AKT signal pathway in human GC cells. Recently, the microRNA-mediated gene repression mechanism involved in AQP3’s role has been investigated in GC[32]. AQP5 also plays an important role in the tumorigenesis, progression and differentiation of human GC cells[33,34]. Shen et al[35] have reported expression profiles of multiple AQPs in human GC and their clinical significance. AQP3 and AQP5 are detected remarkably more strongly in carcinoma tissues than in normal mucosa by immunofluorescence. They have shown that both AQP3 and AQP5 expression are associated with lymph node metastasis and lymphovascular invasion in patients.
These results indicate that AQPs play important roles in the tumorigenesis and progression of human GC and suggest that, especially, AQP3 and 5 can become potential therapeutic targets against GC.
Anion exchanger (AE) proteins facilitate the electroneutral exchange of Cl- for HCO3- across the plasma membrane of mammalian cells and thus contribute to regulation of intracellular pH. The AE family is now comprised of three members, AE1, AE2 and AE3. AE1 is frequently expressed in GC, where it fails to traffic to the plasma membrane, but interacts with the tumor suppressor p16 in the cytoplasm. Down-regulation of AE1 in gastric cancer SGC7901 cells is shown to inhibit cell growth and clinical analyses have indicated that AE1 expression is associated with a low survival rate of GC patients. Suppression of AE1 induces cell death in human GC cells[36-39]. Expression of AE2 in human GC has been also investigated, and AE2 is associated with gastric carcinogenesis and achlorhydria[40]. Wang et al[41] have shown that early growth response protein 1 is critical for gastrin-dependent up-regulation of AE2 in GC cells.
The sodium-hydrogen exchanger (NHE) mediates a coupled counter-transport of one H+ ion in exchange for one Na+ ion. The basic role is to maintain intracellular pH, but NHE proteins are also important for regulation of cell volume and growth. Liu et al[42] have shown that the NHE1 antisense gene significantly suppresses cell growth and induced cell apoptosis in SGC7901 cells. Nagata et al[43] have shown that rapid and extensive decrease of intracellular pH caused by NHE1 inhibitors leads MKN45 and MKN74 cells to apoptotic and cytotoxic events.
Vacuolar H+-ATPases (V-H+-ATPases), as the specific proton pump of the cell, play an important role in maintaining intracellular pH. Proton pump inhibitors (PPI), mainly treating acid-related diseases, inhibit the expression of V-H+-ATPases. Chen et al[44] have shown that PPIs decreased the intracellular pH of SGC7901 cells, by inhibiting V-H+-ATPases, and enhanced the cytotoxic effects of antitumor drugs.
The carbonic anhydrases (CAs) are a family of zinc metalloenzymes that have an important role in cellular pH regulation through reversible hydration of carbon dioxide to carbonic acid. To date, 16 isozymes have been identified, which differ in tissue distribution, subcellular localization, and catalytic activity. Expression of CA IX has been found at the invasion front of gastric cancers[45]. Kato et al[46] have shown that the CA IX expression level is significantly high in cases of type 4 GC and diffuse type GC, and significantly correlates with the invasion depth in lymph node metastasis. The prognosis for CA IX-positive patients is significantly poorer than that of CA IX-negative patients.
These results suggest that pH regulators, such as AEs, NHEs, V-H+-ATPases and CAs are potentially key therapeutic targets and the silencing of their expression could provide a new therapeutic approach for treating GC.
Several reports indicating the important roles of Cl- channels/ transporters on cell proliferation suggest that the intracellular chloride concentration ([Cl-]i) regulated by them would be one of the critical messengers. We have investigated roles of the [Cl-]i in cell cycle progression of human GC cells[47]. We have found that furosemide, a blocker of Na+/K+/2Cl- cotransporter (NKCC), diminished cell growth by delaying the G1-S phase progression in GC cells with high expression and activity of NKCC[48]. NKCC is one of the important transporters controlling the [Cl-]ivia uptake of Cl- into the intracellular space and, therefore, furosemide decreases the [Cl-]i[49]. Cl- channels also contribute to the regulation of [Cl-]i which is related to cell volume. When cell shrinkage occurs isosmotically, [Cl-]i decreases because the major membrane-permeable anion is Cl-[50]. Furthermore, we have found that the decrease of the [Cl-]i inhibits cell growth of GC cells and that this inhibition of cell growth is due to cell cycle arrest at the G0/G1 phase caused by diminution of CDK2 and phosphorylated Rb[51]. The decrease of the [Cl-]i significantly increased expressions of p21 mRNA and protein[51]. In addition, we revealed that the [Cl-]i affects cell proliferation via activation of MAPKs through up-regulation of p21 in GC cells[52]. Similar phenomena are also observed in GC cells with low [Cl-]i caused by inhibition of NHE[53]. These findings suggest that the [Cl-]i regulates important cellular functions in GC cells, leading to the development of novel therapeutic strategies.
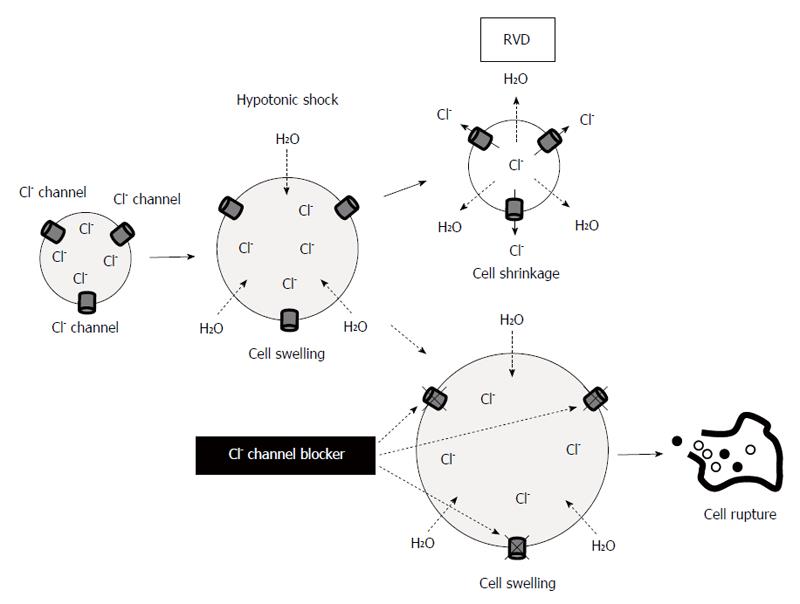
Several previous studies have indicated the cytocidal effects of hypotonic stress on cancer cells. Lin et al[54] have reported that peritoneal lavage with distilled water improves the survival rate in patients with spontaneously ruptured hepatocellular carcinoma. Huguet et al[55] have discussed the optimal method for peritoneal lavage with distilled water during colorectal cancer surgery. In GC, Mercill et al[56] have reported that exposure to distilled water reduces the number of surviving gastric cells. Tsujitani et al[57] have shown that hypotonic intraperitoneal cisplatin treatment with distilled water at the time of a gastric resection is well tolerated for patents with GC. Recently, we have analyzed the changes in the cellular morphology and volume of GC cells subjected to hypotonic stress using several unique methods and apparatus, such as a differential interference contrast microscope connected to a highspeed digital video camera and a high-resolution flow cytometer[58]. Video recordings by high-speed digital camera have demonstrated that hypotonic shock with distilled water induces cell swelling followed by cell rupture. Measurements of cell volume changes using a high-resolution flow cytometer indicate that severe hypotonicity with distilled water increases broken fragments of GC cells within 5 min. In addition, we treated the GC cells with 5-nitro-2-3-phenylpropylamino)-benzoic acid (NPPB), a Cl- channel blocker, to enhance the cytocidal effects of the lavage by increasing their cell volume during hypotonic stress via the inhibition of regulatory volume decrease (RVD)[59,60]. RVD occurs after hypotonicity-caused cell swelling. RVD is caused by activation of ion channels and transporters, which cause effluxes of K+, Cl-, and H2O, leading to cell shrinkage. NPPB is the broad spectrum Cl- channel blocker which is fat-soluble and inhibits both Cl- channels in cell membrane and CLIC. In MKN45 and Kato-III cells, treatment with NPPB increases cell volume by inhibiting RVD and enhances the cytocidal effects of the hypotonic solution (Figure 1). We have found similar phenomena in esophageal[61] and pancreatic cancer cells[62]. AQPs also contribute to RVD[63]. On the other hand, NKCC plays some roles in regulatory volume increase (RVI)[64].
These findings demonstrate the cytocidal effects of hypotonic shock on GC cells, and suggest that the regulation of ion transport enhances these effects. A deeper understanding of ion transport mechanisms in gastric cancer cells during hypotonic shock could lead us to the development of novel therapeutic strategies.
This review shows a variety of ion channels, AQPs and pH regulators are expressed in human GC cells and tissues. Their expression relates to the pathological character of the GC tissues. Pharmacological manipulation and gene silencing affect their activities and fundamental cellular functions that would be involved in the GC process. Overall, we can suggest that ion, water channels and pH regulators are functional biomarkers and therapeutic targets in GC. A deeper understanding of molecular mechanisms may lead us to the discovery of these cellular physiological approaches, such as regulation of ion channels, water channels, intracellular pH, intracellular ion concentration and osmolality, as a novel therapeutic strategy for GC.
P- Reviewer: Chen CC S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 2. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 3. | Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Fraser SP, Pardo LA. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on Ion Channels and Cancer. EMBO Rep. 2008;9:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205:159-173. [PubMed] |
| 7. | Schönherr R. Clinical relevance of ion channels for diagnosis and therapy of cancer. J Membr Biol. 2005;205:175-184. [PubMed] |
| 8. | Lan M, Shi Y, Han Z, Hao Z, Pan Y, Liu N, Guo C, Hong L, Wang J, Qiao T. Expression of delayed rectifier potassium channels and their possible roles in proliferation of human gastric cancer cells. Cancer Biol Ther. 2005;4:1342-1347. [PubMed] |
| 9. | Han Y, Shi Y, Han Z, Sun L, Fan D. Detection of potassium currents and regulation of multidrug resistance by potassium channels in human gastric cancer cells. Cell Biol Int. 2007;31:741-747. [PubMed] |
| 10. | Kim HJ, Jang SH, Jeong YA, Ryu PD, Kim DY, Lee SY. Involvement of Kv4.1 K(+) channels in gastric cancer cell proliferation. Biol Pharm Bull. 2010;33:1754-1757. [PubMed] |
| 11. | Ding XW, Luo HS, Jin X, Yan JJ, Ai YW. Aberrant expression of Eag1 potassium channels in gastric cancer patients and cell lines. Med Oncol. 2007;24:345-350. [PubMed] |
| 12. | Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther. 2005;4:295-301. [PubMed] |
| 13. | Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008;7:45-50. [PubMed] |
| 14. | Ding XW, Yang WB, Gao S, Wang W, Li Z, Hu WM, Li JJ, Luo HS. Prognostic significance of hERG1 expression in gastric cancer. Dig Dis Sci. 2010;55:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Zhang R, Tian P, Chi Q, Wang J, Wang Y, Sun L, Liu Y, Tian S, Zhang Q. Human ether-à-go-go-related gene expression is essential for cisplatin to induce apoptosis in human gastric cancer. Oncol Rep. 2012;27:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (105)] |
| 17. | Kuwahara N, Kitazawa R, Fujiishi K, Nagai Y, Haraguchi R, Kitazawa S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J Gastroenterol. 2013;19:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (111)] |
| 18. | Zhang J, Zhao Z, Zu C, Hu H, Shen H, Zhang M, Wang J. Atrial natriuretic peptide modulates the proliferation of human gastric cancer cells via KCNQ1 expression. Oncol Lett. 2013;6:407-414. [PubMed] |
| 19. | Lee I, Park C, Kang WK. Knockdown of inwardly rectifying potassium channel Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence. Mol Cancer Ther. 2010;9:2951-2959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ, Lin KH. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007;7:155-167. [PubMed] |
| 21. | Zheng DL, Huang QL, Zhou F, Huang QJ, Lin JY, Lin X. PA28β regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem. 2012;113:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ma PF, Chen JQ, Wang Z, Liu JL, Li BP. Function of chloride intracellular channel 1 in gastric cancer cells. World J Gastroenterol. 2012;18:3070-3080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, Jin Y, Wang Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009;125:2281-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Chow J, Norng M, Zhang J, Chai J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment. Biochim Biophys Acta. 2007;1773:565-576. [PubMed] |
| 25. | Kim BJ. Involvement of melastatin type transient receptor potential 7 channels in ginsenoside Rd-induced apoptosis in gastric and breast cancer cells. J Ginseng Res. 2013;37:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Kim BJ, Nah SY, Jeon JH, So I, Kim SJ. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99:2502-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Kim BJ, Kim SY, Lee S, Jeon JH, Matsui H, Kwon YK, Kim SJ, So I. The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can J Physiol Pharmacol. 2012;90:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Huang Y, Zhu Z, Sun M, Wang J, Guo R, Shen L, Wu W. Critical role of aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biol Ther. 2010;9:1000-1007. [PubMed] |
| 30. | Wang J, Gui Z, Deng L, Sun M, Guo R, Zhang W, Shen L. c-Met upregulates aquaporin 3 expression in human gastric carcinoma cells via the ERK signalling pathway. Cancer Lett. 2012;319:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Xu H, Xu Y, Zhang W, Shen L, Yang L, Xu Z. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J Exp Clin Cancer Res. 2011;30:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Jiang B, Li Z, Zhang W, Wang H, Zhi X, Feng J, Chen Z, Zhu Y, Yang L, Xu H. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol. 2014;49:1011-1025. [PubMed] |
| 33. | Huang YH, Zhou XY, Wang HM, Xu H, Chen J, Lv NH. Aquaporin 5 promotes the proliferation and migration of human gastric carcinoma cells. Tumour Biol. 2013;34:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Watanabe T, Fujii T, Oya T, Horikawa N, Tabuchi Y, Takahashi Y, Morii M, Takeguchi N, Tsukada K, Sakai H. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 35. | Shen L, Zhu Z, Huang Y, Shu Y, Sun M, Xu H, Zhang G, Guo R, Wei W, Wu W. Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother. 2010;64:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Wu J, Zhang YC, Suo WH, Liu XB, Shen WW, Tian H, Fu GH. Induction of anion exchanger-1 translation and its opposite roles in the carcinogenesis of gastric cancer cells and differentiation of K562 cells. Oncogene. 2010;29:1987-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Tian H, Zhang N, Suo WH, Wang T, Song LJ, Wu J, Liu Q, Shen WW, Fu GH. Gastrin suppresses the interdependent expression of p16 and anion exchanger 1 favoring growth inhibition of gastric cancer cells. Int J Cancer. 2010;127:1462-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Xu WQ, Song LJ, Liu Q, Zhao L, Zheng L, Yan ZW, Fu GH. Expression of anion exchanger 1 is associated with tumor progress in human gastric cancer. J Cancer Res Clin Oncol. 2009;135:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Shen WW, Wu J, Cai L, Liu BY, Gao Y, Chen GQ, Fu GH. Expression of anion exchanger 1 sequestrates p16 in the cytoplasm in gastric and colonic adenocarcinoma. Neoplasia. 2007;9:812-819. [PubMed] |
| 40. | Yang Y, Wu PP, Wu J, Shen WW, Wu YL, Fu AF, Zheng L, Jin XL, Fu GH. Expression of anion exchanger 2 in human gastric cancer. Exp Oncol. 2008;30:81-87. [PubMed] |
| 41. | Wang T, Zhao L, Yang Y, Tian H, Suo WH, Yan M, Fu GH. EGR1 is critical for gastrin-dependent upregulation of anion exchanger 2 in gastric cancer cells. FEBS J. 2013;280:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Liu HF, Teng XC, Zheng JC, Chen G, Wang XW. Effect of NHE1 antisense gene transfection on the biological behavior of SGC-7901 human gastric carcinoma cells. World J Gastroenterol. 2008;14:2162-2167. [PubMed] |
| 43. | Nagata H, Che XF, Miyazawa K, Tomoda A, Konishi M, Ubukata H, Tabuchi T. Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK45 and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncol Rep. 2011;25:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Chen M, Zou X, Luo H, Cao J, Zhang X, Zhang B, Liu W. Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901) cells. Cell Biol Int. 2009;33:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Chen J, Röcken C, Hoffmann J, Krüger S, Lendeckel U, Rocco A, Pastorekova S, Malfertheiner P, Ebert MP. Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut. 2005;54:920-927. [PubMed] |
| 46. | Kato Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Fuyuhiro Y, Doi Y, Hirakawa K. Expression of a hypoxia-associated protein, carbonic anhydrase-9, correlates with malignant phenotypes of gastric carcinoma. Digestion. 2010;82:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Shiozaki A, Otsuji E, Marunaka Y. Intracellular chloride regulates the G(1)/S cell cycle progression in gastric cancer cells. World J Gastrointest Oncol. 2011;3:119-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 48. | Shiozaki A, Miyazaki H, Niisato N, Nakahari T, Iwasaki Y, Itoi H, Ueda Y, Yamagishi H, Marunaka Y. Furosemide, a blocker of Na+/K+/2Cl- cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J Physiol Sci. 2006;56:401-406. [PubMed] |
| 49. | Hiraoka K, Miyazaki H, Niisato N, Iwasaki Y, Kawauchi A, Miki T, Marunaka Y. Chloride ion modulates cell proliferation of human androgen-independent prostatic cancer cell. Cell Physiol Biochem. 2010;25:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Marunaka Y. Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Jpn J Physiol. 1997;47:499-511. [PubMed] |
| 51. | Miyazaki H, Shiozaki A, Niisato N, Ohsawa R, Itoi H, Ueda Y, Otsuji E, Yamagishi H, Iwasaki Y, Nakano T. Chloride ions control the G1/S cell-cycle checkpoint by regulating the expression of p21 through a p53-independent pathway in human gastric cancer cells. Biochem Biophys Res Commun. 2008;366:506-512. [PubMed] |
| 52. | Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E, Marunaka Y. Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol. 2010;223:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Hosogi S, Miyazaki H, Nakajima K, Ashihara E, Niisato N, Kusuzaki K, Marunaka Y. An inhibitor of Na(+)/H(+) exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl(-) concentration via DIDS-sensitive pathways. Cell Physiol Biochem. 2012;30:1241-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Lin CH, Hsieh HF, Yu JC, Chen TW, Yu CY, Hsieh CB. Peritoneal lavage with distilled water during liver resection in patients with spontaneously ruptured hepatocellular carcinomas. J Surg Oncol. 2006;94:255-256. [PubMed] |
| 55. | Huguet EL, Keeling NJ. Distilled water peritoneal lavage after colorectal cancer surgery. Dis Colon Rectum. 2004;47:2114-2119. [PubMed] |
| 56. | Mercill DB, Jones NR, Harbell JW. Human tumor cell destruction by distilled water. An in vitro evaluation. Cancer. 1985;55:2779-2782. [PubMed] |
| 57. | Tsujitani S, Fukuda K, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The administration of hypotonic intraperitoneal cisplatin during operation as a treatment for the peritoneal dissemination of gastric cancer. Surgery. 2002;131:S98-104. [PubMed] |
| 58. | Iitaka D, Shiozaki A, Ichikawa D, Kosuga T, Komatsu S, Okamoto K, Fujiwara H, Ishii H, Nakahari T, Marunaka Y. Blockade of chloride ion transport enhances the cytocidal effect of hypotonic solution in gastric cancer cells. J Surg Res. 2012;176:524-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Caplanusi A, Kim KJ, Lariviere E, Van Driessche W, Jans D. Swelling-activated K+ efflux and regulatory volume decrease efficiency in human bronchial epithelial cells. J Membr Biol. 2006;214:33-41. [PubMed] |
| 60. | Miyazaki H, Shiozaki A, Niisato N, Marunaka Y. Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl- concentration acting as an intracellular signaling. Am J Physiol Renal Physiol. 2007;292:F1411-F1417. [PubMed] |
| 61. | Kosuga T, Shiozaki A, Ichikawa D, Fujiwara H, Komatsu S, Iitaka D, Tsujiura M, Morimura R, Takeshita H, Nagata H. Pleural lavage with distilled water during surgery for esophageal squamous cell carcinoma. Oncol Rep. 2011;26:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Nako Y, Shiozaki A, Ichikawa D, Komatsu S, Konishi H, Iitaka D, Ishii H, Ikoma H, Kubota T, Fujiwara H. Enhancement of the cytocidal effects of hypotonic solution using a chloride channel blocker in pancreatic cancer cells. Pancreatology. 2012;12:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Kida H, Miyoshi T, Manabe K, Takahashi N, Konno T, Ueda S, Chiba T, Shimizu T, Okada Y, Morishima S. Roles of aquaporin-3 water channels in volume-regulatory water flow in a human epithelial cell line. J Membr Biol. 2005;208:55-64. [PubMed] |
| 64. | Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys. 2004;41:233-258. [PubMed] |