Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11422
Revised: April 3, 2014
Accepted: May 28, 2014
Published online: August 28, 2014
Processing time: 250 Days and 8.5 Hours
AIM: To investigate the efficacy of tandospirone in patients with irritable bowel syndrome-diarrhea (IBS-D) and anxiety in a prospective, randomized, controlled study.
METHODS: Two hundred patients with IBS-D and moderate anxiety were randomized to receive pinaverium and tandospirone (arm A) or pinaverium and placebo (arm B). Tandospirone or placebo was given thrice daily at a fixed dose of 10 mg and pinaverium was given thrice daily at a fixed dose of 50 mg. The duration of treatment was 8 wk. Patients were assessed for abdominal pain and diarrhea. Anxiety was evaluated using the Hamilton Rating Scale for Anxiety (HAM-A). The primary study endpoints were response rates for abdominal pain and diarrhea. The secondary study endpoints were response rates for anxiety. Adverse events were also evaluated.
RESULTS: One hundred and seventy of 200 patients (82 patients in arm A and 88 patients in arm B) completed the study. Demographic and baseline characteristics of the 200 participants were comparable in the two arms. At week 8, the overall response rate for abdominal pain and diarrhea was 52.0% for arm A and 37.0% for arm B (P < 0.05). The HAM-A score showed that the response rate was 61.0% for arm A and 21.0% for arm B (P < 0.01). The treatments were well tolerated and no significant adverse events were reported.
CONCLUSION: Tandospirone is effective and can be combined with pinaverium in IBS-D patients with anxiety.
Core tip: Irritable bowel syndrome (IBS) is associated with psychological stress, anxiety and depression, which may contribute to perpetuating the condition. IBS-diarrhea (IBS-D), an isotype of IBS, is often accompanied by anxiety, and conventional therapy is unfavorable. IBS-D may respond positively to anti-anxiety/-depression therapies. However, existing medications are not sufficiently effective for patients with IBS-D. To our knowledge, few randomized, controlled and multi-center studies have focused on the efficacy of anti-anxiety agents in IBS-D patients. This is a prospective, randomized, controlled study to evaluate the efficacy of tandospirone in patients with combined IBS-D and anxiety.
- Citation: Lan L, Chen YL, Zhang H, Jia BL, Chu YJ, Wang J, Tang SX, Xia GD. Efficacy of tandospirone in patients with irritable bowel syndrome-diarrhea and anxiety. World J Gastroenterol 2014; 20(32): 11422-11428
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11422
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders (GIDs) with major symptoms such as abdominal discomfort and pain accompanied by constipation or diarrhea. The syndrome has complex mechanisms and is usually refractory to treatment[1]. IBS is associated with psychological stress, anxiety and depression, which may contribute to perpetuating the condition[2-4], and may respond positively to anti-anxiety/-depression therapies[5-7]. However, existing medications are not sufficiently effective for patients with IBS.
IBS-diarrhea (IBS-D) is the most frequent subtype of IBS[8]. IBS-D patients usually suffer from anxiety[9]. Anti-anxiety agents should be effective in relieving anxiety and symptoms of IBS-D. However, there have been few randomized and controlled trials of anti-anxiety agents in IBS-D patients.
Tandospirone citrate is a partial agonist of the 5-hydroxytryptamine 1A (5-HT1A) receptor and has also demonstrated neuropharmacological properties that may contribute to its efficacy in the treatment of anxiety[10,11]. However, there have been no studies on the efficacy and safety of tandospirone in IBS-D patients with anxiety. We conducted a prospective, multicenter, single-blind, randomized, controlled study to evaluate the efficacy (whether abdominal pain, diarrhea and anxiety could be improved) of tandospirone in patients with IBS-D and anxiety.
This study enrolled patients from three tertiary care centers in China: The People’s Hospital and the First Affiliated Hospital of Zhengzhou University, Henan, China and the First Affiliated Hospital of Luzhou Medical College, Sichuan, China. This study was conducted from March 2011 to May 2013. IBS-D was diagnosed according to the Rome III criteria (mainly including abdominal pain, diarrhea and without any organic alteration)[12], and anxiety was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders-Fourth edition (DSM-IV) criteria[13].
Patients were eligible for enrollment if they (1) were aged between 18 and 65 years; (2) had a Hamilton Rating Scale for Anxiety (HAM-A) score between 14 and 24 (moderate anxiety)[14]; (3) had negative routine fecal and occult blood test within 3 mo prior to the study; (4) had no organic diseases by enteroscopy within three months prior to study, or no hepatobiliary and pancreatic diseases by laboratory studies and ultrasonographic evaluations; and (5) received no agents that influence motility of the gastrointestinal tract and digestion and/or anti-anxiety/depressive drugs within 4 wk prior to study entry. Major exclusion criteria were (1) allergy to tandospirone and/or pinaverium bromide; (2) the presence of functional dyspepsia (FD); (3) breast-feeding or pregnancy or be going to be pregnant in the period of the study; (4) clinically significant diseases or any psychiatric disorder other than anxiety; (5) previous abdominal surgery; and (6) daily alcohol consumption > 40 g and/or history of drug abuse. Patients were also excluded if they used other psychotropic medications in the previous week (14 d for monoamine oxidase inhibitors and 28 d for fluoxetine), or required ongoing use of psychotropic medications.
All participating institutions had implemented good clinical practice and were eligible for conduction of clinical trials. The study was compliant with the Declaration of Helsinki, and approved by local ethical committees and institutional review boards at the participating institutions. All study participants or their legal surrogates provided written informed consent.
Eligible subjects were blinded to this study and randomized at an allocation ratio of 1:1 to receive pinaverium (a calcium channel blocker for helping to restore the normal contraction process of the bowel) and tandospirone (arm A) or pinaverium and placebo (arm B). Tandospirone or placebo was given thrice daily at a fixed dose of 10 mg and pinaverium was given thrice daily at a fixed dose of 50 mg. The duration of treatment was 8 wk. Patients who missed more than five consecutive days of treatment in 8 wk of the study (non-compliant) were withdrawn from the trial.
Patients were assessed for eligibility at a screening visit, with eligible patients returning for a baseline assessment approximately one week, and then evaluated at week 8 for a total of four visits. The primary study endpoints were response rates for abdominal pain and diarrhea. The secondary study endpoints were the response rates for anxiety.
Abdominal pain was assessed using a 10-point abdominal pain numeric rating scale (NRS) from 0 (none) to 10 (worst possible pain), and mild (NRS score, 1-3), moderate (NRS score, 4-6) and severe pain (NRS score, 7-10) were then assigned a score of 1, 2 or 3, respectively. In addition, the frequency of abdominal pain was assigned a score of 0, 1, 2 and 3, respectively, if the pain occurred 0, 1-2, 3-4 and ≥ 5 times per week. The abdominal pain score represented the sum of the severity and frequency of pain in a patient. Diarrhea was assessed by stool consistency, frequency and urgency. Normal/hard feces, roughly normal feces, soft feces, loose feces and watery feces were assigned a score of 0, 1, 2, 3 or 4, respectively. Defecations were assigned a score of 0, 1, 2, 3 or 4 if they occurred ≤ 1-2, 3-4, 5-6 and ≥ 7 times daily. The absence or presence of urgency was assigned a score of 0 or 1. The diarrhea score represented the combination of scores for stool consistency, frequency and urgency of a patient. Furthermore, anxiety was evaluated using the HAM-A scale.
For abdominal pain and diarrhea, clinical response was evaluated based on the treatment associated-reduction rate (TARR), which was defined as the post-treatment scores minus the pretreatment scores and then divided by the pretreatment scores and multiplied by 100%. Complete response (CR) had a TARR ≥ 75%, partial response (PR) had a TARR ≥ 50% but <75%, slight response (SR) had a TARR ≥ 25% but < 50% and non-response (NR) had a TARR < 25%. The response rate = CRAbdominal pain or diarrhea + PRAbdominal pain or diarrhea. The overall response rate = CR (abdominal pain plus diarrhea) + PR (abdominal pain plus diarrhea). Clinical response in anxiety was evaluated using the same methods as those for abdominal pain and diarrhea.
Adverse events were monitored at baseline and week 2 and 8 using the Treatment Emergent Symptom Scale (TESS) (NIMH, 1973). Safety assessments were based mainly on the occurrence, frequency, and severity of adverse events and were also based on laboratory parameters including hematology, hepatorenal function, electrolytes, urinalysis, fecal tests and electrocardiography, and treatment-emergent adverse events were recorded. For all adverse events, where necessary, patients were withdrawn from the study.
Sample size calculation was based on the assumption of a 40% response in the arm A vs 20% in arm B using the Z statistic to compare dichotomous variables with α = 0.05 (two-tailed) and β = 0.20. The estimated sample size was 81 patients per arm.
Randomization procedures were performed using a computer code generated by a study statistician who did not have contact with the study subjects. Trial name and all involved parameters were set. Participating centers were pre-added in the system. Common users of the system at each center were granted corresponding permission and user-names in advance. They accessed the system, input patient demographic data, and selected items according to the inclusion/exclusion criteria. The system then automatically decided whether a patient was eligible to participate in the study.
The statistical analysis was carried out using SPSS14.0 software. The statistical analyses were pre-specified and performed on an intention-to-treat basis with the inclusion of all patients who underwent randomization. Both full and per-protocol analyses were used. The full analysis sets included all patients who were randomized to treatment and had a baseline assessment and at least one post-baseline assessment. The per-protocol sets included all evaluable patients who completed at least three weeks of active treatment and were not excluded as protocol violators. Unless otherwise specified, all efficacy results reported herein are based on the full analysis, whereas, for patients who withdrew or were lost to follow-up, we used the last observation carried forward approach. Descriptive statistics were used to summarize some safety measures. The χ2 test was used in the statistical analysis and P < 0.05 was considered statistically significant. The homogeneity of the HAM-A score obtained from different investigators was analyzed using Kendall’s W test, with W > 90% considered as having homogeneity.
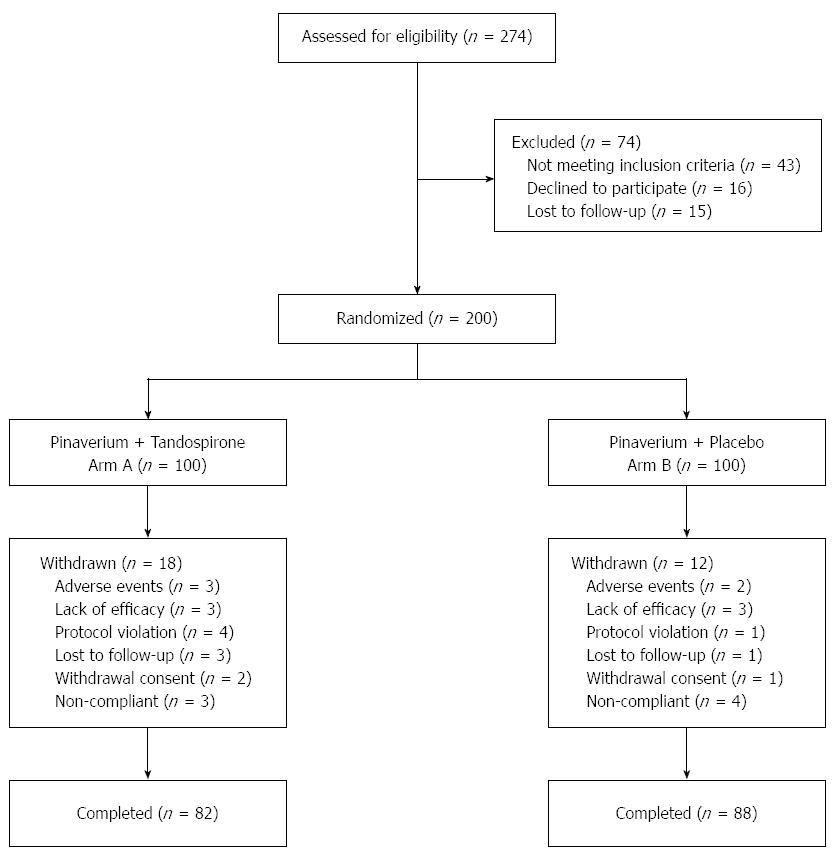
The study flowchart is shown in Figure 1. Among 274 subjects screened, 200 were eligible for the study. One hundred patients were assigned to receive tandospirone and pinaverium, and 100 to receive tandospirone. Thirty patients (18 in arm A and 12 in arm B) withdrew due to adverse events, lack of efficacy, protocol violation, lost to follow-up or withdrawal consent. In total, 82 patients in arm A and 88 patients in arm B completed the study. Demographic and baseline characteristics of the 170 participants are shown in Table 1. The median age of the study subjects was 45.6 (range: 19-65) years and there were slightly more male patients (54.1%, 92/170) than female patients (45.9%, 78/170). Patients in the two arms were well balanced in demographic characteristics. The mean baseline HAM-A score was 21.8 ± 5.2 for arm A and 20.9 ± 4.7 for arm B with no apparent difference between the two arms (P = 0.21). The mean baseline abdominal pain and diarrhea score was comparable between arm A (8.3 ± 2.0) and arm B (8.0 ± 2.1) (P = 0.18).
| Characteristic | Arm A (n = 100) | Arm B (n = 100) |
| Age (yr) | ||
| mean ± SD | 46.6 ± 12.9 | 44.7 ± 12.8 |
| Range | 20-65 | 19-64 |
| Female gender | 35 (42.7) | 43 (48.9) |
| Anxiety | ||
| mean ± SD | 21.8 ± 5.2 | 20.9 ± 4.7 |
| Range | 14-24 | 14-24 |
| Abdominal pain and diarrhea score | ||
| mean ± SD | 8.3 ± 2.0 | 8.0 ± 2.1 |
| Range | 4-12 | 4-14 |
Forty-three patients in arm A and twenty-nine patients in arm B had a 50% or greater reduction in the abdominal pain score at week 8. The response rate was 43.0% for arm A and 29.0% for arm B in the intention-to-treat population (P < 0.05), and was 52.4% for arm A and 33.0% for arm B in the per-protocol sets (P < 0.05) (Table 2).
| Arm | n | CR | PR | SR | NR | Response rate | |
| Abdominal pain | |||||||
| Per protocol | A | 82 | 22 (26.8) | 21 (25.6) | 27 (32.9) | 12 (14.6) | 43 (52.4)a |
| B | 88 | 14 (15.9) | 15 (17.0) | 28 (31.8) | 31 (35.2) | 29 (33.0) | |
| Intention-to-treat | A | 100 | 22 (22) | 21 (21) | 27 (27) | 30 (30) | 43 (43)a |
| B | 100 | 14 (14) | 15 (15) | 28 (28) | 43 (43) | 29 (29) | |
| Diarrhea | |||||||
| Per protocol | A | 82 | 25 (30.5) | 25 (30.5) | 23 (28) | 9 (11) | 50 (61.0)b |
| B | 88 | 13 (14.8) | 21 (23.9) | 30 (34.1) | 24 (27.3) | 34 (38.6) | |
| Intention-to-treat | A | 100 | 25 (25) | 25 (25) | 23 (23) | 27 (27) | 50 (50)a |
| B | 100 | 13 (13) | 21 (21) | 30 (30) | 36 (36) | 34 (34) | |
| Abdominal pain plus diarrhea | |||||||
| Per protocol | A | 82 | 24 (29.3) | 28 (34.1) | 21 (25.6) | 9 (11) | 52 (63.4)b |
| B | 88 | 11 (12.5) | 26 (29.5) | 15 (17.0) | 36 (40.9) | 37 (42.0) | |
| Intention-to-treat | A | 100 | 24 (24) | 28 (28) | 21 (21) | 27 (27) | 52 (52)a |
| B | 100 | 11 (11) | 26 (26) | 15 (15) | 48 (48) | 37 (37) | |
Fifty patients in arm A and 34 patients in arm B had a 50% or greater reduction in the diarrhea score at week 8. The response rate was 50.0% for arm A and 34.0% for arm B in the intention-to-treat population (P < 0.05), and was 61.0% for arm A and 38.6% for arm B per-protocol sets (P < 0.01) (Table 2).
The overall response rate in patients with abdominal pain and diarrhea was 52.0% for arm A and 37.0% for arm B in the intention-to-treat population (P < 0.05), and was 63.4% for arm A and 42.0% for arm B in the per-protocol sets (P < 0.01) (Table 2).
The Kendall coefficient was > 90%, indicating the homogeneity of the HAM-A score among investigators. Sixty-one patients in arm A and 21 patients in arm B had a 50% or greater reduction in the HAM-A score at week 8. The response rate was 61.0% for arm A and 21.0% for arm B in the intention-to-treat population (P < 0.01), and was 74.4% for arm A and 23.9% for arm B in the per-protocol sets (P < 0.01) (Table 3).
The treatments were well tolerated and no significant adverse events were reported. Treatment-emergent adverse events included somnolence (four in arm A), vertigo (two in arm A), vomiting (one in arm A and one in arm B) and aggravated abdominal pain (one subject in arm B), which resulted in discontinuation (vertigo, vomiting and aggravated abdominal pain) or was resolved two weeks later (somnolence).
IBS is a functional gastrointestinal disorder (FGID) characterized by symptoms including abdominal pain, distention and abnormal defecation habit and feces appearance[8]. Despite absence of organic disease, IBS may have a notable adverse effect on the quality of life of patients and lead to the exhaustion of medical resources[15]. The etiology of IBS is unknown. However, it has been demonstrated that IBS is the GID which was most strongly associated with mental health conditions[16]. Mental stress and psychological distress are correlated with development of IBS[17]. Psychological and social factors can interfere with the communication between the central and enteric nervous systems, and there is proof that they are involved in the onset of IBS and influence the response to treatment and outcome[18]. Anxiety or depression may influence autonomic nervous system balance in women with IBS[19]. Gastrointestinal (GI)-specific anxiety seems to be an important factor in GI symptom severity and quality of life in patients with IBS[20]. No effective therapy is currently available for IBS-D, and commonly used medications including pinaverium and trimebutine are unfavorable in refractory IBS and are associated with frequent recurrences. Patients with refractory IBS usually experience negative mood and sleep disturbance[21], indicating the importance of psychological intervention and anti-anxiety/-depressive therapy in the treatment of IBS[22-25].
As a third-generation anti-anxiety agent, tandospirone is a novel partial agonist of 5-HTlA receptor and modulates 5-HT projected from the raphe nuclei to the hippocampus by selectively activating 5-HTlA receptor in a postsynaptic manner, thus exerting its anti-anxiety activity[26]. In addition, tandospirone has an anti-depressive effect by down-regulating presynaptic 5-HTlA receptor density[27]. Therefore, tandospirone has dual anti-anxiety and anti-depressive effects, particularly in anxiety and takes effect at 1 to 2 wk after administration. 5-HTlA receptor is located at the cholinergic nerve terminal and the presynaptic component of the neuromuscular junction, and may lead to relaxation of smooth muscle when it is activated[28,29]. Therefore, tandospirone can not only act on psychological symptoms including anxiety and depression, but also improve autonomic nerve disorder-related physical symptoms, such as abnormalities of appetite, sexual behavior, body temperature and blood pressure[30]. Therefore it can be used to treat eating and GI disorders[31,32]. As reported previously[28,32,33], tandospirone can be used in patients with combined functional dyspepsia and emotional disorders, particularly those with treatment-refractory FD.
This study demonstrated that the combination of tandospirone and pinaverium was associated with a significantly increased overall response rate in patients with abdominal pain and diarrhea. Furthermore, we found that the combination of tandospirone and pinaverium was associated with a markedly higher response rate. These findings demonstrate that tandospirone is effective in patients with IBS-D with anxiety. Fukudo et al[34] showed that IBS is related to brain-gut interactions, emotional dysregulation, and illness behaviors. Corticotropin-releasing hormone and 5-HT are candidate substances which regulate exaggerated brain-gut response. Therefore, it is possible that tandospirone as a partial 5-HTlA agonist, can regulate brain-gut response and have an anti-spasmodic effect on the colon by binding 5-HTlA receptor, thereby producing an improvement in abdominal pain and diarrhea.
We also found that the drug was overall well tolerated by IBS-D patients and showed a benign safety profile with no major treatment-emergent adverse events. This compares favorably to selective serotonin reuptake inhibitors (SSRIs), anti-anxiety/-depressive agents used in the clinic, which also exert their effects via the 5-HT system. However, as an SSRI blocks the reuptake of 5-HT, thus increasing 5-HT levels in the synaptic cleft, this may lead to agitation and increase the frequency of adverse events[35]. In addition, tandospirone is associated with less somnolence and dependency than other anti-anxiety agents due to the absence of non-anti-anxiety effects associated with benzodiazepines, such as muscular relaxation, anti-convulsion and sedation[28], and can be withdrawn when the symptoms are resolved[22].
Clinically, IBS is primarily treated with drugs acting on GI motility, spasmolysis and analgesia, and psychological disorders are suspected only if the above-mentioned treatments are ineffective. However, in IBS patients, as an interaction between psychological disorders and physical symptoms may exacerbate the condition, delaying anti-anxiety/-depressive intervention until poor efficacy of conventional treatments is confirmed may extend the distress of these patients[22]. In our opinion, anti-anxiety/-depressive treatment should be administered at an early period for IBS patients who present with anxiety and depression in order to avoid chronic stress[36].
Our prospective randomized controlled multicenter trial has demonstrated that tandospirone is effective and safe in IBS-D patients with anxiety. Prompt anti-anxiety therapy in IBS-D patients with anxiety could lead meaningful improvements in anxiety as well as a significant reduction in abdominal pain and diarrhea. Further larger-scale, long-term clinical trials are warranted to confirm our findings.
We acknowledge all site investigators for their participation in the study: Jing Yu, Chang-He Jia, Bo-Wei Liu, Yuan Yuan. We also thank the Endoscope Center of the People’s Hospital of Zhengzhou University, Zhengzhou, China.
The etiology of irritable bowel syndrome (IBS) is unknown. IBS-diarrhea (IBS-D) is an isotype of IBS. No effective therapy is available for IBS-D, and commonly used medications including pinaverium and trimebutine are unfavorable for refractory IBS and are associated with frequent recurrences. Along with the “biomedical model” changing to the “biopsychosocial medical model”, the influence of psychological stress on IBS has been followed with interest.
Recently, many studies found that IBS is associated with psychological stress, anxiety and depression. IBS-D is often accompanied by anxiety. More researchers are now attempting to determine whether IBS-D may respond to anti-anxiety/-depression therapies.
Few randomized, controlled and multicenter studies have focused on the efficacy of anti-anxiety agents in IBS-D patients. As a third-generation anti-anxiety agent, tandospirone has not been used for the treatment of IBS in studies. The authors conducted a prospective, randomized, controlled study to evaluate the efficacy of tandospirone in patients with IBS-D and anxiety.
Tandospirone treatment in IBS-D patients with anxiety could lead meaningful improvements in anxiety as well as a significant reduction in abdominal pain and diarrhea. This may provide a novel therapeutic option for IBS-D patients with anxiety.
This is a well-written paper. The topic is timely and of general interest. This randomized controlled trial could have contribution to the management of IBS-D.
P- Reviewer: Henderson P, Kuo SM, Poli-Neto OB S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Chang FY. Irritable bowel syndrome: the evolution of multi-dimensional looking and multidisciplinary treatments. World J Gastroenterol. 2014;20:2499-2514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Thijssen AY, Jonkers DM, Leue C, van der Veek PP, Vidakovic-Vukic M, van Rood YR, Clemens CH, Masclee AA. Dysfunctional cognitions, anxiety and depression in irritable bowel syndrome. J Clin Gastroenterol. 2010;44:e236-e241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Kabra N, Nadkarni A. Prevalence of depression and anxiety in irritable bowel syndrome: A clinic based study from India. Indian J Psychiatry. 2013;55:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Dragoş D, Ionescu O, Ojog DG, Tănăsescu MD. Psychoemotional features in irritable bowel syndrome. J Med Life. 2012;5:398-409. [PubMed] |
| 5. | Dekel R, Drossman DA, Sperber AD. The use of psychotropic drugs in irritable bowel syndrome. Expert Opin Investig Drugs. 2013;22:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Grover M, Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J Gastroenterol. 2013;48:177-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Pae CU, Lee SJ, Han C, Patkar AA, Masand PS. Atypical antipsychotics as a possible treatment option for irritable bowel syndrome. Expert Opin Investig Drugs. 2013;22:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, Guo X, Sun G, Shang J, Wang WF. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2012;27:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Lee S, Wu J, Ma YL, Tsang A, Guo WJ, Sung J. Irritable bowel syndrome is strongly associated with generalized anxiety disorder: a community study. Aliment Pharmacol Ther. 2009;30:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Tao M, Han YJ, Gao JF, Tang WX, Zhu XZ, Tao DH, Zhang P, Zou Y, Chen H, Tao SF. [Efficacy and safety of tandospirone in the treatment of patients with anxiety disorder]. Zhonghua Yi Xue Zazhi. 2012;92:920-923. [PubMed] |
| 11. | Nishitsuji K, To H, Murakami Y, Kodama K, Kobayashi D, Yamada T, Kubo C, Mine K. Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression: results of a comparatively high dosage trial. Clin Drug Investig. 2004;24:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Rome Foundation. Guidelines--Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointestin Liver Dis. 2006;15:307-312. [PubMed] |
| 13. | The American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn, Text revision. Washington, DC: American Psychiatric Association; 2000; . |
| 14. | Matza LS, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Naliboff BD, Kim SE, Bolus R, Bernstein CN, Mayer EA, Chang L. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: a structural equation modeling analysis. Am J Gastroenterol. 2012;107:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Maguen S, Madden E, Cohen B, Bertenthal D, Seal K. Association of mental health problems with gastrointestinal disorders in Iraq and Afghanistan veterans. Depress Anxiety. 2014;31:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Surdea-Blaga T, Băban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World J Gastroenterol. 2012;18:616-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Jarrett ME, Burr RL, Cain KC, Hertig V, Weisman P, Heitkemper MM. Anxiety and depression are related to autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2003;48:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Jerndal P, Ringström G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, Simrén M. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22:646-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 22. | Labus J, Gupta A, Gill HK, Posserud I, Mayer M, Raeen H, Bolus R, Simren M, Naliboff BD, Mayer EA. Randomised clinical trial: symptoms of the irritable bowel syndrome are improved by a psycho-education group intervention. Aliment Pharmacol Ther. 2013;37:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Kearney DJ, McDermott K, Martinez M, Simpson TL. Association of participation in a mindfulness programme with bowel symptoms, gastrointestinal symptom-specific anxiety and quality of life. Aliment Pharmacol Ther. 2011;34:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Gros DF, Antony MM, McCabe RE, Lydiard RB. A preliminary investigation of the effects of cognitive behavioral therapy for panic disorder on gastrointestinal distress in patients with comorbid panic disorder and irritable bowel syndrome. Depress Anxiety. 2011;28:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Enck P, Junne F, Klosterhalfen S, Zipfel S, Martens U. Therapy options in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:1402-1411. [PubMed] |
| 26. | Kawana S, Kato Y, Omi T. Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin Exp Dermatol. 2010;35:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Shimizu H, Karai N, Hirose A, Tatsuno T, Tanaka H, Kumasaka Y, Nakamura M. Interaction of SM-3997 with serotonin receptors in rat brain. Jpn J Pharmacol. 1988;46:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Miwa H, Nagahara A, Tominaga K, Yokoyama T, Sawada Y, Inoue K, Ashida K, Fukuchi T, Hojo M, Yamashita H. Efficacy of the 5-HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. Am J Gastroenterol. 2009;104:2779-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Yamada K. Clinical application of 5-HT1A agonist tandospirone in mind and physique domain. Med Adv. 2007;223:653-657. |
| 31. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1131] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 32. | Kinoshita Y, Hashimoto T, Kawamura A, Yuki M, Amano K, Sato H, Adachi K, Sato S, Oshima N, Takashima T. Effects of famotidine, mosapride and tandospirone for treatment of functional dyspepsia. Aliment Pharmacol Ther. 2005;21 Suppl 2:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Seno H, Nakase H, Chiba T. Usefulness of famotidine in functional dyspepsia patient treatment: comparison among prokinetic, acid suppression and antianxiety therapies. Aliment Pharmacol Ther. 2005;21 Suppl 2:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Boyer WF. Potential indications for the selective serotonin reuptake inhibitors. Int Clin Psychopharmacol. 1992;6 Suppl 5:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |