Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11297
Revised: May 30, 2014
Accepted: July 16, 2014
Published online: August 28, 2014
Processing time: 162 Days and 11.5 Hours
AIM: To investigate the efficacy of moxibustion in ulcerative colitis (UC) rats from morphological, immunological and molecular biological perspectives.
METHODS: Thirty-two Sprague-Dawley rats were randomly assigned to a blank control group (normal rats, n = 6) and a model replication (MR) group (UC rats, n = 26). A UC model was established by 2,4,6-trinitrobenzenesulfonic acid/dextran sulfate sodium enema. Rats in the MR group were further randomly assigned to a 9-min moxibustion (9M) group (9 moxa-cone, n = 6), 6-min moxibustion (6M) group (6 moxa-cone, n = 6), 3-min moxibustion (3M) group (3 moxa-cone, n = 6), and a waiting list control (WLC) group (no moxibustion treatment, n = 6). Rats in the moxibustion treatment group were treated in 14 sessions over 28 d. Disease activity, local tissue morphology, serum level of interleukin (IL)-8 and IL-10, and expression of Toll-like receptor (TLR)9 as well as nuclear factor (NF)-κB p65 in colonic tissue were determined by disease activity index (DAI), hematoxylin and eosin staining, electron microscopy, enzyme-linked immunosorbent assay and Western blotting, respectively.
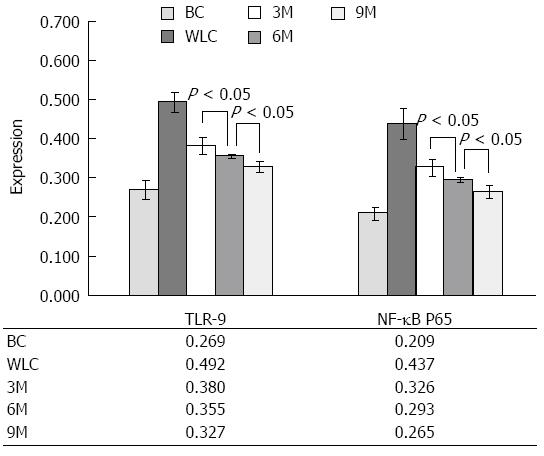
RESULTS: DAI was lowest in the 9M group and highest in the WLC group. The differences in DAI between the moxibustion treatment (3M, 6M, 9M) and no treatment groups were significant for all one-to-one comparisons (0.60 ± 0.54 vs 1.20 ± 0.44, 0.60 ± 0.54 vs 1.80 ± 0.45, 0.60 ± 0.54 vs 3.0 ± 0.45, respectively, P < 0.05). Light and electron microscopy showed that the neatness of the glandular arrangement in colonic mucosal epithelia gradually increased in the WLC, 3M, 6M to 9M groups. IL-8 level successively decreased while IL-10 level increased from the WLC to 3M, 6M and 9M groups. The differences among these groups were significant for all comparisons (105.46 ± 8.75 vs 76.61 ± 3.58, 105.46 ± 8.75 vs 69.78 ± 1.87, 105.46 ± 8.75 vs 67.41 ± 1.84, respectively, P < 0.01 for IL-8; and 30.83 ± 1.29 vs 75.64 ± 1.90, 30.83 ± 1.29 vs 80.90 ± 3.16, 30.83 ± 1.29 vs 83.46 ± 2.37, respectively, P < 0.01 for IL-10), except comparison of 6M vs 9M. Expression of TLR9 and NF-κB p65 decreased in order: highest in the WLC group and lowest in the 9M group. In addition, the differences among the WLC, 3M, 6M and 9M groups were significant for all comparisons (0.492 ± 0.026 vs 0.380 ± 0.022, 0.492 ± 0.026 vs 0.355 ± 0.005, 0.492 ± 0.026 vs 0.327 ± 0.015, respectively, P < 0.05 for TLR9; and 0.436 ± 0.041 vs 0.326 ± 0.022, 0.436 ± 0.041 vs 0.293 ± 0.006, 0.436 ± 0.041 vs 0.265 ± 0.017, respectively, P < 0.05 for NF-κB p65).
CONCLUSION: Moxibustion repairs damaged colonic mucosa, suppresses serum IL-8, activates serum IL-10 level, and decreases expression of TLR-9 and NF-κB p65 in UC rats.
Core tip: We investigated the effectiveness of moxibustion treatment in ulcerative colitis rats from a modern medicine perspective. In addition, we correlated the effects of moxibustion therapy with immune or inflammatory responses by observing levels of interleukin (IL)-8, IL-10, Toll-like receptor 9, and nuclear factor-κB p65, and the underlying mechanisms were suggested.
- Citation: Han Y, Ma TM, Lu ML, Ren L, Ma XD, Bai ZH. Role of moxibustion in inflammatory responses during treatment of rat ulcerative colitis. World J Gastroenterol 2014; 20(32): 11297-11304
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11297
Moxibustion (form of acupuncture point stimulation using heat) is a traditional healing technique that is a useful and important therapy in Asian medicine, including in China, South Korea, and Japan[1-4]. In the western world, moxibustion and acupuncture are gaining more attention as alternative and complementary therapeutic interventions, due to relatively low clinical side effects and a higher compliance in patients compared to drug therapy or surgical procedures[5-8]. Recently, acupuncture and moxibustion have been used worldwide as alternative treatments for chronic back pain[9-11], asthma[12,13], stroke rehabilitation[8,14,15], and gastrointestinal disease[7,16].
Ulcerative colitis (UC) is a nonspecific inflammatory bowel disease that afflicts millions of people worldwide. The pathogenesis of UC mainly involves erosions and ulcers with common clinical manifestations of diarrhea, weight loss, abdominal pain, bloody stools, fever, and fatigue[17]. Although the exact cause of UC remains uncertain, most scientists and scholars agree on a combination of genetic and environmental factors. On the basis of various genetic abnormalities, an immune reaction is triggered by environmental factors that transiently break the mucosal barrier with abnormal responses to pathogenic enteric bacteria, and further give rise to onset and reactivation of disease[17-19]. The pathogenesis of UC is associated with immunological abnormalities, and various factors involved in the immune system may directly/indirectly relate to UC. For instance, two inflammatory mediators, interleukin (IL)-8 (neutrophil chemotactic factor) and IL-10 (human cytokine synthesis inhibitory factor) are genetically linked to the inflammation of UC[20,21]. Toll-like receptor (TLR) 9 and nuclear factor (NF)-κB p65 are also intrinsically associated with the inflammatory reaction of UC[22,23].
In addition to the above research on UC from a modern medicine perspective, there is also some evidence that moxibustion and acupuncture are effective in UC. For example, Zhang[24] found that UC symptoms are improved after moxibustion or acupuncture. Ma[25] showed that moxibustion or acupuncture was effective for treating UC by observing the symptoms in 76 patients. Joos et al[6] have suggested that acupuncture offers an additional therapeutic benefit in patients with mild to moderately active UC. Most research has focused on whether moxibustion and acupuncture are effective, and only rarely has it investigated the mechanistic connection between moxibustion or acupuncture therapy and drug therapy. Wu et al[26] have suggested that moxibustion and acupuncture inhibit expression of inflammatory cytokines by observing IL-1β and IL-6 mRNA expression in the spleen and colonic mucosa of UC rats. Although their work investigated the mechanisms of moxibustion or acupuncture treatment for UC, their results were limited to qualitative observation of electrophoresis patterns. Further research, such as quantitative research, is necessary to shed some light on the role of moxibustion and acupuncture in the treatment of UC, and to elucidate why moxibustion and acupuncture achieve their therapeutic effects.
In this study, we quantitatively investigated the efficacy of moxibustion in the treatment of UC rats by observing changes in physical and biological parameters [disease activity index (DAI), colonic epithelial glandular arrangement, levels of IL-8, IL-10, TLR9, and NF-κB P65]. The purpose of this study was to obtain quantitative information about the efficacy of moxibustion to provide useful information for future clinical practice. In addition, we investigated a comprehensive theoretical system of moxibustion treatment for UC by providing direct scientific evidence of its therapeutic effects from a modern medicine perspective.
Thirty-two Sprague-Dawley rats (150 ± 20 g, 16 female and 16 male) were provided by the Laboratory Animal Center of China Medical University. Rat IL-8 and IL-10 ELISA kits were purchased from Shanghai Yueyan (China). Rat anti-mouse TLR9 antibody and rat anti-mouse NF-κB p65 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Goat anti-goat anti-rabbit IgG/HRP antibody was purchased from Beijing Biosynthesis Biotechnology Co. Ltd. (China). Protein Miniprep kit was purchased from Tiandz (Beijing, China). Protein assay kit was purchased from Beyotine Institute of Biotechnology (Shanghai, China).
The rat model of UC was established using an immunological method. Acute colitis was induced by rectal administration of 100 mg/kg 2,4,6-trinitrobenzenesulfonic acid in 50% ethanol. After the fecal occult blood test showed “1+”, rats with acute colitis were treated with 5% dextran sulfate sodium solution or normal saline to induce inflammation for the second time. UC was induced after 21 d. Colonic tissue stained by HE was examined by light microscopy (Olympus BX41, Tokyo, Japan). Morphology of colonic mucosa was studied by electron microscopy (Olympus CHA). The colonic tissue and mucosal epithelium indicated successful establishment of UC.
Home-made moxa cones (refined mugwort floss: 6 mm in diameter, 7 mm in base diameter, 8 mm in height, 50 mg in weight) were placed on Daheng (SP15, bilateral) and Tianshu (ST25, bilateral) and ignited. UC rats received moxibustion treatment for 3 min (3M group), 6 min (6M group), and 9 min (9M group). Three, six and nine moxa cones were used for each treatment session in the 3M, 6M and 9M groups. The whole treatment course comprised 14 sessions.
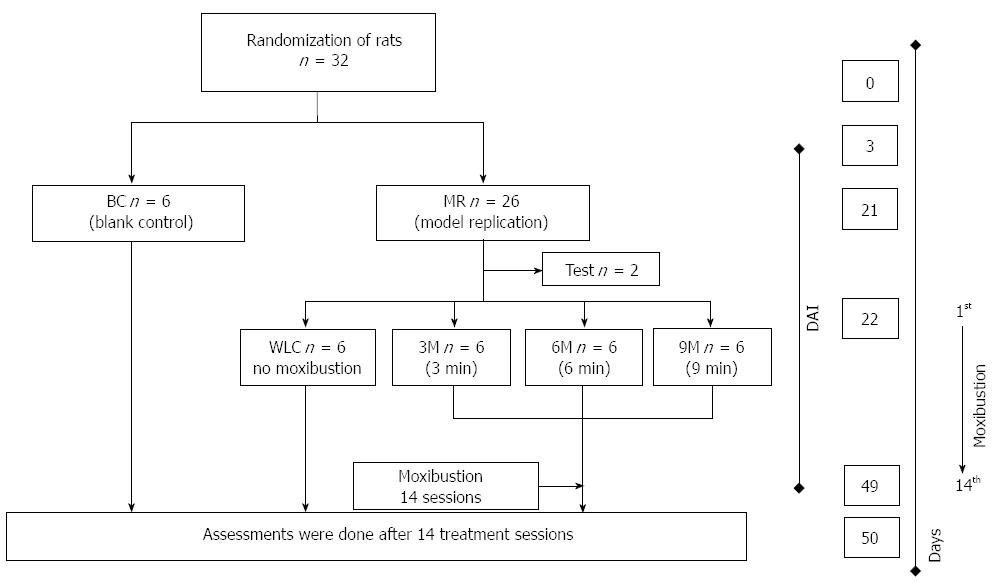
Figure 1 shows the study design for moxibustion treatment of UC rats. Thirty-two normal rats (16 male and 16 female) were randomly assigned to two groups. One was named the blank control (BC) group and consisted of six normal rats without moxibustion treatment. The other was the model replication (MR) group that consisted of 26 rats with UC. Establishment of the UC model lasted 21 d. After that, two rats were randomly selected to determine whether UC had been induced. The observation of disease activity, reflected in DAI, started from day 3 and ended after the moxibustion course. After UC was induced, the MR group was further randomly assigned into three subgroups (3M, 6M and 9M) with six rats each. 3M, 6M and 9M were named according to the timespan of moxibustion treatment in each session, which was 3, 6 and 9 min, respectively. Each session lasted 2 d, in which UC rats only received moxibustion treatment. The whole moxibustion course included 14 sessions (28 d in total). After the entire treatment course, all rats were killed simultaneously. The assessments including IL-8, IL-10, TLR9, and NF-κB p65 measurement were conducted on the next day.
The disease activity of UC rats was observed from day 3 to the last day of 14 moxibustion sessions (Figure 1) and was evaluated based on the index shown in Table 1. The index assessed four variables, which included rate of body mass loss, fecal viscosity, rectal bleeding, and the corresponding disease activity in terms of each variable. Normal fecal viscosity means solid shaped feces, medium fecal viscosity indicates semi-formed feces, and low fecal viscosity represents watery loose feces that can attach to the anus. The physician’s overall assessment of disease activity was determined by the average rating of body mass loss, fecal viscosity and rectal bleeding, which is expressed as the equation: DAI = (body mass loss + fecal viscosity + rectal bleeding)/3.
| Rate of body mass loss | Fecal viscosity | Rectal bleeding | Rating |
| None | Normal | None | 0 |
| 1%-5% | Low to medium | Concealed hemorrhage | 1 |
| 5%-10% | Medium | Concealed hemorrhage | 2 |
| 10%-15% | Slightly low | Revealed hemorrhage | 3 |
| > 15% | Low | Revealed hemorrhage | 4 |
The levels of IL-8 and IL-10 in the serum were detected by typical enzyme-linked immunosorbent assay, according to the manufacturer’s instructions (Shanghai Yueyan, China). Expression of TLR9 and NF-κB p65 in the colonic tissue was detected by western blotting, according to the protocol available in the Protein Miniprep kit and protein assay kit.
Data analysis was performed using SPSS version 12 software. Least significant difference was used to evaluate the differential efficacy of moxibustion treatment in UC rats. P values < 0.05 (two-sided) were regarded as statistically significant.
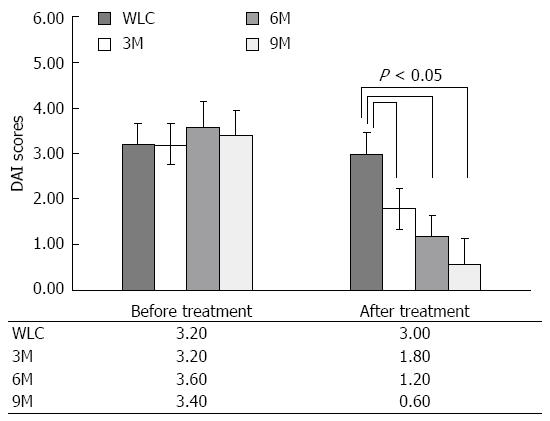
DAI has been widely used for the evaluation of disease activity, allowing the integration of various aspects of the disease into a single value[27,28]. To investigate whether moxibustion is effective in improving the disease activity of UC rats, we evaluated the DAI by continuously observing the rate of body mass loss, fecal viscosity, and rectal bleeding for 14 sessions with 2 d/session. Figure 2 shows the DAI of UC rats in the different groups before and after moxibustion treatment, and comparison of disease activity between the moxibustion-treated rats (3M, 6M and 9M groups) and moxibustion-free rats [waiting list control (WLC) group]. The values given were the average of DAI scores obtained in 14 sessions. From the pre-treatment data, DAI was relatively static among the different groups (3.20 for WLC, 3.20 for 3M, 3.60 for 6M, and 3.40 for 9M), which indicated the legitimacy of sampling randomization. In contrast, DAI represented a significant change between each moxibustion group and the moxibustion-free group (3M vs WLC, 6M vs WLC, and 9M vs WLC, all P < 0.05). The significant decrease in DAI between the moxibustion-free and moxibustion-treated groups indicated the efficacy of moxibustion in UC rats. In the moxibustion-treated groups, the duration of treatment gradually increased from 3 to 6 and 9 min. We also noticed that DAI decreased from 1.80 to 0.60 as the duration of treatment increased from 3 to 9 min. This implies that 9 min moxibustion probably yields better improvement of UC disease activity, while taking physical tolerance into account.
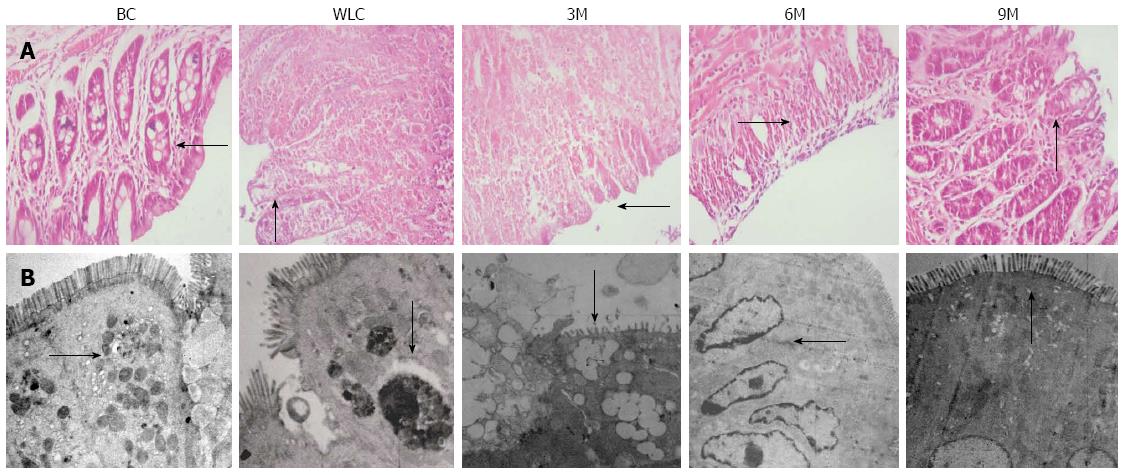
In order to visualize the efficacy of moxibustion treatment in UC rats, we compared the condition of colonic tissue stained by HE and observed by light microscopy (Figure 3A). For normal rats in the BC group, colonic tissue showed intact mucosal epithelia, ordered glands, clear structure, and no ulceration, as well as no inflammatory cell infiltration. In contrast, for rats in the WLC group, colonic tissues showed ulcers, damaged mucosa, disordered glandular structure, apparent edema in and underneath the mucosa, and infiltration of inflammatory cells. The above symptoms were gradually improved after moxibustion treatment, as shown in the three pictures on the right in Figure 3A. The 9M group showed the best improvement: epithelial mucosa and glands had an ordered structure, ulcer surface had new epithelial cells, and most of the infiltrating inflammatory cells disappeared. The condition of the colonic tissue in the 9M group was close to that in the BC group.
We further scrutinized the morphology of colonic mucosal epithelia by electron microscopy (Figure 3B). Our observations under electron microscopy were consistent with those of colonic tissue under light microscopy. For normal rats in the BC group, colonic mucosal epithelia showed intact microvilli in good order, tight junctions between cells, and clear mucous granules in the cytoplasm. On the contrary, colonic mucosal epithelia in the WLC group (without moxibustion treatment) contained damaged microvilli with uneven length, loose cell junctions, and unclear dissolved mucous granules in the cytoplasm. Symptoms of UC were greatly improved after moxibustion treatment. In particular, in the 9M group, colonic mucosal epithelia showed a similar condition to that in the rats without UC: relatively intact and ordered microvilli, tight cell junctions, and even cellular matrix.
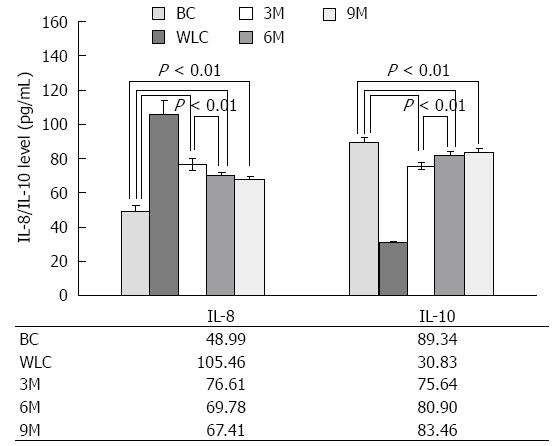
In order to determine whether the effectiveness of moxibustion treatment on inflammation of UC was linked to immunological abnormalities, we measured the levels of two inflammatory mediators, IL-8 and IL-10, which are genetically related to the immune system[20,21]. Serum levels of IL-8 and IL-10 in normal and UC rats are summarized and shown in Figure 4. IL-8 level in the WLC, 3M, 6M and 9M groups was significantly increased compared with that in the BC group (P < 0.01). In order to quantify the efficacy, we compared the IL-8 level among the different groups. The results in the 3M vs 6M and 3M vs 9M groups indicated significant differences in IL-8 (P < 0.01), and a tendency to decrease gradually to the normal level when UC rats received 9 min moxibustion treatment. The difference between the 6M and 9M groups was no longer significant (P > 0.05).
The results for IL-10 levels were similar to those for IL-8, except for the increasing/decreasing tendency. IL-10 level showed a tendency to increase to the normal level as the duration of moxibustion treatment increased, whereas IL-8 level had a tendency to decrease to the normal level. For the parallel comparison of 6M vs 9M, although the difference was no longer significant (P > 0.05), the tendency for the level of IL-10 to approach the normal level was still noticeable when the duration of treatment increased from 6 to 9 min. Due to the physical tolerance of UC rats, we reached 9 min of moxibustion treatment and did not increase the duration.
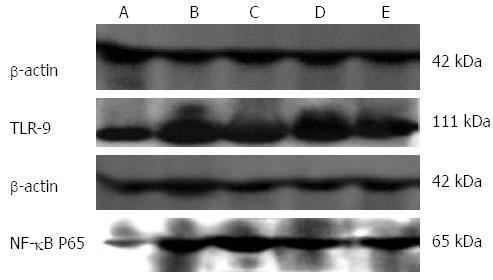
The other two factors that play significant roles in immune responses, TLR9 and NF-κB p65, are also intrinsically associated with the inflammatory reaction of UC[22,23,29]. In order to investigate the intrinsic connection between effectiveness of moxibustion treatment in UC rats and expression of those two factors, we compared their expression among the different groups of normal and UC rats. Figures 5 show direct visualization of the differences of both TLR9 and NF-κB p65 expression in colonic tissue of normal rats (BC), moxibustion-free UC rats (WLC), and moxibustion-treated UC rats (3M, 6M and 9M).
We further summarized and compared such differences quantitatively, as shown in Figure 6. Expression of both TLR9 and NF-κB p65 in UC rats (WLC, 3M, 6M and 9M) showed a significant decrease (P < 0.01), compared to that in normal rats (BC). For UC rats, the differences of 3M vs WLC, 6M vs WLC, and 3M vs WLC were also significant (P < 0.05). The above results implied that moxibustion had an effect on expression of TLR9 and NF-κB p65 in UC rats. We further compared their expression in UC rats receiving different durations of moxibustion treatment. As the duration of moxibustion treatment in UC rats increased, the amount of TLR9 and NF-κB p65 expression decreased simultaneously. Significant differences (P < 0.05) still existed among all different comparisons (3M vs 6M, 3M vs 9M, and 6M vs 9M). The level of expression in UC rats that received 9 min moxibustion was the lowest, which was closest to the levels in normal rats without UC. On the contrary, the level of expression in UC rats without moxibustion treatment was the highest, which differed most from the level in normal rats. The above comparisons of TLR9 and NF-κB p65 among different groups regardless of moxibustion imply that moxibustion is an effective alternative treatment for UC in rats.
The significant decrease in DAI indicates that moxibustion is effective in improving the disease activity of UC rats. To be specific, moxibustion treatment can increase body mass, improve fecal viscosity, and reduce rectal bleeding. Our comparison of DAI among moxibustion-treated groups suggests that the efficacy of moxibustion depends on the duration of treatment, and 9 min treatment gave the best improvement of disease activity.
Our histological observations of colonic tissue and mucosal epithelia agree with the disease activity results: (1) without moxibustion treatment, ulcers, damaged mucosa, disordered glandular structure, edema, and infiltration of inflammatory cells appeared in the colonic tissue of UC rats; (2) damaged microvilli with uneven length, loose cell junctions, and unclear dissolved mucous granules in the cytoplasm were seen in colonic mucosal epithelia; (3) with moxibustion treatment, the above abnormalities showed significant improvements (P < 0.05); and (4) after 9 min treatment in each session, the features of colonic tissue and mucosal epithelia of UC rats showed the closest similarity to those of normal rats without UC.
UC is one type of nonspecific inflammatory bowel disease. A combination of genetic and environmental factors is widely accepted as the main cause of UC. The pathogenesis of UC is closely associated with immune and inflammatory responses. Inflammatory mediators IL-8, IL-10, TLR9 and NF-κB p65 are closely connected with immune and inflammatory responses[29-38].
IL-8 is a proinflammatory cytokine and a major chemoattractant and activator of neutrophils. IL-8 is closely connected to neutrophil recruitment, activation of the immune response, and promotion of inflammation. It activates immune responses through recruiting neutrophils to a site of inflammation as well as inducing neutrophils to bind to the extracellular matrix of cells, leading to inflammation[30,31]. IL-10 is an anti-inflammatory molecule that can suppress in vitro production of cytokines, and is involved in regulating immune and inflammatory responses[32]. Our comparison of the levels of IL-8/IL-10 among different groups suggest: (1) IL-8 level is correlated positively with severity of UC, whereas IL-10 level has a negative correlation; (2) moxibustion is effective in treating UC by significantly reducing IL-8 level while increasing IL-10 level; (3) within the physical tolerance limit of rats, a longer duration of moxibustion treatment achieves better efficacy; and (4) moxibustion treatment suppresses secretion of proinflammatory cytokine IL-8 while activating secretion of anti-inflammatory cytokine IL-10, thereby inhibiting the release of inflammatory cells and further interrupting the inflammatory response of UC.
In mammals, TLR9 plays a role in recognizing conserved pathogen-associated molecular patterns and further in inducing the inflammatory innate immune responses[33,34]. More specifically, TLR9 mediates CpG-DNA signaling involved in signal transduction pathways[37,38]. NF-κB p65, as a subunit of the NF-κB transcription complex, plays a crucial role in inflammatory and immune responses[29,35,36]. Activation of NF-κB p65 is the last step of the intracellular signaling pathway from the cell surface to the nucleus. Increased expression of NF-κB p65 has been reported in patients with UC[39]. By comparing the expression of TLR9 and NF-κB p65 in rat colonic tissue among different groups, several observations were made: (1) expression of NF-κB p65 in colonic tissue of UC rats was higher than in normal rats, indicating activation of NF-κB p65 in UC; (2) moxibustion treatment reduced expression of TLR9 and NF-κB p65 in UC; and (3) the degree of expression decreased significantly with duration of treatment, and 9 min treatment yielded the best results. In addition to its effectiveness, our results suggest that moxibustion treatment inhibits activation of NF-κB p65, thereby blocking the signal transduction pathways of TLR9.
In summary, in this study, we investigated the effectiveness of moxibustion treatment in UC rats, and quantitatively assessed the efficacy from a modern medicine perspective. In addition, we established the relation between moxibustion therapy and immune or inflammatory responses by observing IL-8, IL-10, TLR9 and NF-κB p65 expression. Our results indicate that moxibustion treatment improves disease activity, repairs damaged colonic mucosa, suppresses secretion of serum IL-8 while activating that of IL-10, inhibits activation of NF-κB p65, and decreases expression of TLR-9 in UC rats. We propose that the effect of moxibustion therapy in UC rats is probably related to inhibition of inflammatory cells by suppressing secretion of proinflammatory cytokine IL-8 and activating secretion of anti-inflammatory cytokine IL-10, and blocking of the inflammatory signaling transduction pathway by inhibiting activation of transcription factor NF-κB p65 and repressing the pattern-recognition receptor TLR9. Our results provide direct evidence of why moxibustion is effective in the treatment of UC from a modern medicine perspective.
Moxibustion (application of heat to acupuncture points), as an alternative and complementary medicine, has been widely used for treating inflammatory bowel disease, due to its relatively low level of clinical side effects and higher compliance in patients compared to drug therapy or surgical procedures. Ulcerative colitis (UC) is a nonspecific inflammatory bowel disease that afflicts millions of people worldwide. Many studies have reported that moxibustion is effective in UC. Nevertheless, there is little or insufficient information for evaluation of the effectiveness and therapeutic mechanism of moxibustion.
The combination of genetic and environmental factors is widely accepted as the cause of UC. In the area of moxibustion treatment of UC, the research hotspot is to establish whether moxibustion is effective, and only rarely do studies focus on the underlying mechanisms of its therapeutic effects.
The authors quantitatively investigated the efficacy of moxibustion in the treatment of UC rats by observing the changes in physical and biological parameters related to the immune system: disease activity index, colonic epithelial glandular arrangement, interleukin (IL)-8, IL-10, Toll-like receptor (TLR)9, and nuclear factor (NF)-κB p65. The results indicate that moxibustion improves disease activity of UC rats, repairs damaged colonic mucosa, suppresses secretion of serum IL-8 while activating that of IL-10, inhibits activation of NF-κB p65, and decreases expression of TLR-9 in UC rats. They also propose a connection between the therapeutic efficacy of moxibustion therapy effect and inflammatory responses. The results provide direct scientific evidence of why moxibustion is effective in the treatment of UC.
This study provides quantitative information about the efficacy of moxibustion for future clinical practice. The efforts to probe into the comprehensive theoretical system of moxibustion treatment for UC offer direct scientific evidence of its therapeutic effects from a modern medicine perspective.
Moxibustion is a traditional Chinese medicine technique that involves burning of mugwort (a small spongy herb) to facilitate healing, strengthen the blood, stimulate the flow of qi, and maintain general health. UC is one of the most common types of inflammatory bowel disease. UC affects the colon and rectum. The pathogenesis of UC mainly involves erosion and ulceration, with common clinical manifestations of diarrhea, weight loss, abdominal pain, bloody stools, fever, and fatigue.
This was an interesting study about the roles of moxibustion in regulating IL-8, IL-10, TLR9 and NF-κB p65 in UC. The paper is well written.
P- Reviewer: Beissner F S- Editor: Gou SX L- Editor: Logan S E- Editor: Zhang DN
| 1. | Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374-383. [PubMed] |
| 2. | Okada K, Kawakita K. Analgesic action of acupuncture and moxibustion: a review of unique approaches in Japan. Evid Based Complement Alternat Med. 2009;6:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Kawakita K, Shinbara H, Imai K, Fukuda F, Yano T, Kuriyama K. How do acupuncture and moxibustion act? - Focusing on the progress in Japanese acupuncture research. J Pharmacol Sci. 2006;100:443-459. [PubMed] |
| 4. | Zhou EH, Liu HR, Wu HG, Shi Y, Wang XM, Tan LY, Yao LQ, Zhong YS, Jiang Y, Zhang LL. Suspended moxibustion relieves chronic visceral hyperalgesia via serotonin pathway in the colon. Neurosci Lett. 2009;451:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Paterson C, Britten N. Acupuncture as a complex intervention: a holistic model. J Altern Complement Med. 2004;10:791-801. [PubMed] |
| 6. | Joos S, Wildau N, Kohnen R, Szecsenyi J, Schuppan D, Willich SN, Hahn EG, Brinkhaus B. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Shiflett SC. Does acupuncture work for stroke rehabilitation: what do recent clinical trials really show? Top Stroke Rehabil. 2007;14:40-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Inoue M, Kitakoji H, Ishizaki N, Tawa M, Yano T, Katsumi Y, Kawakita K. Relief of low back pain immediately after acupuncture treatment--a randomised, placebo controlled trial. Acupunct Med. 2006;24:103-108. [PubMed] |
| 10. | Furlan AD, van Tulder MW, Cherkin DC, Tsukayama H, Lao L, Koes BW, Berman BM. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005;CD001351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety, and cost of acupuncture, massage therapy, and spinal manipulation for back pain. Ann Intern Med. 2003;138:898-906. [PubMed] |
| 12. | Chen R, Chen M, Xiong J, Yi F, Chi Z, Zhang B. Comparison of heat-sensitive moxibustion versus fluticasone/salmeterol (seretide) combination in the treatment of chronic persistent asthma: design of a multicenter randomized controlled trial. Trials. 2010;11:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Fung KP, Chow OK, So SY. Attenuation of exercise-induced asthma by acupuncture. Lancet. 1986;2:1419-1422. [PubMed] |
| 14. | Lee MS, Shin BC, Kim JI, Han CH, Ernst E. Moxibustion for stroke rehabilitation: systematic review. Stroke. 2010;41:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Tabosa A, Yamamura Y, Forno ER, Mello LE. A comparative study of the effects of electroacupuncture and moxibustion in the gastrointestinal motility of the rat. Dig Dis Sci. 2004;49:602-610. [PubMed] |
| 17. | Head KA, Jurenka JS. Inflammatory bowel disease Part 1: ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247-283. [PubMed] |
| 18. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1276] [Article Influence: 67.2] [Reference Citation Analysis (2)] |
| 19. | Rutgeerts P, Geboes K. Understanding inflammatory bowel disease--the clinician’s perspective. Eur J Surg Suppl. 2001;66-72. [PubMed] |
| 20. | Tedde A, Laura Putignano A, Bagnoli S, Congregati C, Milla M, Sorbi S, Genuardi M, Papi L. Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand J Gastroenterol. 2008;43:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. [PubMed] |
| 23. | Li Z, Zhang de K, Yi WQ, Ouyang Q, Chen YQ, Gan HT. NF-kappaB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch Med Res. 2008;39:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Zhang X. 23 cases of chronic nonspecific ulcerative colitis treated by acupuncture and moxibustion. J Tradit Chin Med. 1998;18:188-191. [PubMed] |
| 25. | Ma X. Acupuncture treatment for 76 cases of ulcerative colitis. J Tradit Chin Med. 2005;25:264-265. [PubMed] |
| 26. | Wu HG, Zhou LB, Pan YY, Huang C, Chen HP, Shi Z, Hua XG. Study of the mechanisms of acupuncture and moxibustion treatment for ulcerative colitis rats in view of the gene expression of cytokines. World J Gastroenterol. 1999;5:515-517. [PubMed] |
| 27. | Maunder RG, Greenberg GR. Comparison of a disease activity index and patients’ self-reported symptom severity in ulcerative colitis. Inflamm Bowel Dis. 2004;10:632-636. [PubMed] |
| 28. | Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, Vogel I, Böcker U, Böhm S, Büning C. Significant differences between Crohn’s disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: results from a European multicenter study in 1,176 patients. Dig Dis Sci. 2010;55:1066-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3173] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 30. | Reddy KP, Markowitz JE, Ruchelli ED, Baldassano RN, Brown KA. Lamina propria and circulating interleukin-8 in newly and previously diagnosed pediatric inflammatory bowel disease patients. Dig Dis Sci. 2007;52:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Ina K, Kusugami K, Yamaguchi T, Imada A, Hosokawa T, Ohsuga M, Shinoda M, Ando T, Ito K, Yokoyama Y. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1342-1346. [PubMed] |
| 32. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [PubMed] |
| 33. | Ashkar AA, Rosenthal KL. Toll-like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002;2:545-556. [PubMed] |
| 34. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6738] [Article Influence: 449.2] [Reference Citation Analysis (0)] |
| 35. | Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649-683. [PubMed] |
| 36. | Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2972] [Cited by in RCA: 3213] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 37. | Chuang TH, Lee J, Kline L, Mathison JC, Ulevitch RJ. Toll-like receptor 9 mediates CpG-DNA signaling. J Leukoc Biol. 2002;71:538-544. [PubMed] |
| 38. | Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16:17-22. [PubMed] |
| 39. | Neurath MF, Fuss I, Schürmann G, Pettersson S, Arnold K, Müller-Lobeck H, Strober W, Herfarth C, Büschenfelde KH. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann N Y Acad Sci. 1998;859:149-159. [PubMed] |