Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11273
Revised: March 11, 2014
Accepted: May 12, 2014
Published online: August 28, 2014
Processing time: 229 Days and 2.6 Hours
The intestinal epithelium constitutes a physical and functional barrier between the external environment and the host organism. It is formed by a continuous monolayer of intestinal epithelial cells maintained together by intercellular junctional complex, limiting access of pathogens, toxins and xenobiotics to host tissues. Once this barrier integrity is disrupted, inflammatory disorders and tissue injury are initiated and perpetuated. Beneath the intestinal epithelial cells lies a population of astrocyte-like cells that are known as enteric glia. The morphological characteristics and expression markers of these enteric glia cells were identical to the astrocytes of the central nervous system. In the past few years, enteric glia have been demonstrated to have a trophic and supporting relationship with intestinal epithelial cells. Enteric glia lesions and/or functional defects can be involved in the barrier dysfunction. Besides, factors secreted by enteric glia are important for the regulation of gut barrier function. Moreover, enteric glia have an important impact on epithelial cell transcriptome and induce a shift in epithelial cell phenotype towards increased cell adhesion and cell differentiation. Enteric glia can also preserve epithelial barrier against intestinal bacteria insult. In this review, we will describe the current body of evidence supporting functional roles of enteric glia on intestinal barrier.
Core tip: This review offers a state-of-the-art discussion on the role of enteric glial cells (EGCs), an intriguing population of astrocyte-like cells within the gastrointestinal tract, on the regulation of intestinal epithelial barrier. The discussion will shed light on the novel mechanisms of EGC-intestinal epithelial cells interactions, which is invaluable in ultimately developing new therapeutic tools for the restoration of the intestinal barrier functions.
- Citation: Yu YB, Li YQ. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol 2014; 20(32): 11273-11280
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11273
The intestinal epithelial barrier (IEB) serves as the first boundary of defense between the organism and the luminal environment. It consists of a continuous monolayer of proliferating and differentiating intestinal epithelial cells (IECs) maintained together by intercellular junctional complex which establishes the cellular polarity and reduces the space between adjacent cells. Therefore, the IEB provides a highly selective permeability that prevents the passage of pathogens[1,2]. Loss of this barrier integrity would allow the translocation of the normally excluded luminal contents (microbes, food antigens, etc.) into the mucosa, where inflammatory disorders and tissue injury are initiated and perpetuated[3-5]. Considerable evidence indicates that intestinal barrier dysfunction plays a pathogenic role in diseases such as inflammatory bowel disease, celiac disease and irritable bowel syndrome[6-8]. Therefore, mediators associated with reinforcing or re-establishing IEB functions could be of great interest in the intervention of these barrier dysfunction diseases.
Beneath the intestinal epithelial cells lies a population of astrocyte-like cells that are known as enteric glia cells (EGCs). The morphological characteristics and expression markers [glial fibrillary acidic protein (GFAP), S-100β] of these enteric glia cells were identical to the astrocytes of the central nervous system[9,10]. Within the central nervous system, the blood-brain barrier, which shelters the nervous system from circulating blood, is maintained via interactions between astrocytes and cerebral endothelia[11]. Whether similar interactions between enteric glia and epithelia regulate intestinal barrier function has moved into the spotlight in recent years. In this review, we will summarize the current evidence supporting functional roles of enteric glia in the control of IEB functions and gut homeostasis.
The important component of the intestinal barrier is the intercellular junctional complex, which consists of the tight junctions (TJs), gap junctions, adherens junctions and desmosomes[12-14]. TJs seal the space between adjacent epithelial cells near the apical surface. Structurally, the TJs are composed of membrane-spanning proteins, including claudins, occludin, and zona occludens[15,16]. Claudins, in particular, play a critical role in barrier function. Claudin-1, claudin-4 and claudin-5 reduce paracellular diffusion by sealing neighbor epithelial cells. Conversely, claudin-2 forms channels or pores contributing to epithelial leakiness[17,18]. Occludin, and the zona occludens could link the cytoplasmic component of the TJs to the actin-myosin cytoskeleton[19,20]. Adherens junctions are located beneath the TJs and are involved in cell-cell adhesion and intracellular communication[21]. As for gap junctions and desmosomes, they are reported to be involved in cell-cell adhesion and intracellular communication, respectively[22,23]. The intercellular junctional complex forms a selective barrier which allows nutrient absorption and defends against entry of infectious agents and foreign antigens into the body. Candidate mediators are reported to affect the intercellular junctional complex in two ways, first by expression regulation[24] and second, perhaps more importantly, by affecting the redistribution processes[25]. Further, the balance between apoptosis and regeneration of epithelial cells is also crucial for the maintenance of the intact mucosal barrier function[26]. Both disruption of the intercellular junctional complex and abnormal epithelial cell apoptosis have been reported to be involved in the development of a “leaky” gut, which may promote the translocation of luminal antigens into the colonic mucosa and subsequently destroy gut mucosal homeostasis.
The first description of EGCs within the gut was made in 1899[27]. However, for decades, the role of EGCs in gut was largely ignored, and was considered merely as foster cells accompanying and supporting enteric neurons. Interestingly, the current body of evidence expands the functional role of these cells within the gut.
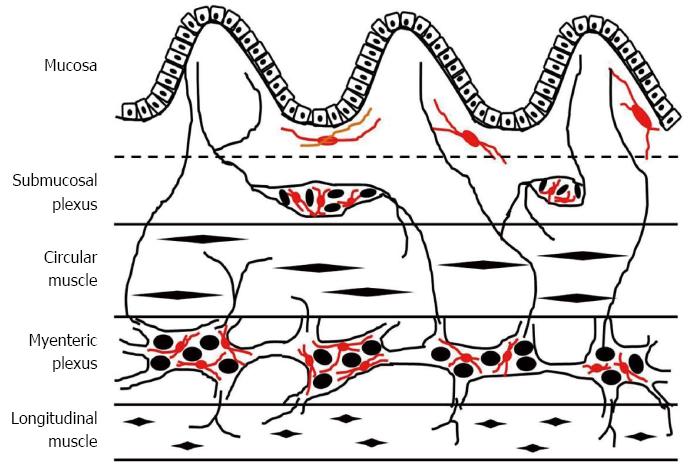
In the intestine, the EGCs are the major constituent of the enteric nervous system and outnumber enteric neurons by a factor of 4 to 10[28]. They possess a densely integrated array of intermediate filaments rich in GFAP[29] and express the calcium-binding protein S-100β[30]. The mucosal EGC population are in close proximity (< 1 μm) to the epithelial cells of the colonic crypts and their terminal end-feet processes often extend to the epithelial basement membrane and blood capillaries in the intestinal mucosa[10,28]. Meanwhile, major populations of enteric glia are found in enteric ganglia in the submucosal and myenteric plexuses of the enteric nervous system. These EGCs can ensheath the neuronal cell bodies within the enteric ganglia, as well as the connecting enteric neuronal interganglionic processes and the processes extending from the enteric plexi to the muscularis mucosae and externae, blood vessels, and mucosal glands[10,31]. The EGCs are typically described as highly irregular, stellate-shaped, small cells which provide regulatory signals for the development and function of neurons and ganglions in the gastrointestinal tract[32,33]. As is known, enteric glia share many structural and functional similarities to astrocytes in central nervous system. Amounts of evidence indicate the critical role of astrocytes in the maintenance of the blood-brain barrier. As for EGCs, it has been demonstrated that EGCs actively receive and propagate signals, both to and from nearby enteric neurons and the intestinal epithelium[27,34]. Thus, EGCs may be an ideal candidate cell type to maintain proper intestinal epithelial barrier integrity (Figure 1).
Several lines of evidence implicate an essential role of mucosal EGCs for the integrity of the gut epithelium. Examination of noninvolved intestinal tissue from patients with Crohn’s disease demonstrated that the EGC network was significantly disrupted, and the diminished EGC network appeared to respond poorly to inflammatory signals in these patients[7,35]. Animal studies demonstrated that the conditional genetic ablation of EGC in mice could induce the disruption of the intestinal integrity and vascular disturbances, and ultimately lead to fatal hemorrhagic jejuno-ileitis[6,36,37]. Further observations showed that the destruction of the EGC network by chemical or autoimmune T-cell-targeted methods resulted in a collapse of the epithelial lining, and the mucosa healing was obviously delayed[7,38,39]. In a mice model of intestinal injury caused by severe burns, stimulation of the vagus nerve could activate the EGCs, and the activated EGCs subsequently prevented burn-induced intestinal permeability and attenuated histological gut injury[40]. Hence, it is imaginable that EGCs are a major constituent of the IEB microenvironment favoring barrier protection.
Mucosal EGCs lie in the mucosa directly beneath the epithelial cells, suggesting that regulation of IEB functions by EGC might be via paracrine pathways[41]. As is known, mucosal EGCs are producers of several mediators implicated in mucosal barrier function[42], such as glial-derived neurotrophic factor (GDNF), transforming growth factor-β1 (TGF-β1), 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2), glial-derived s-nitrosoglutathione (GSNO) and neurotrophins.
GDNF potently promotes the survival and differentiation of many types of neurons[43,44], and is able to prevent apoptosis of neurons induced by axotomy[45]. In the intestine, the main source of GDNF could be identified as the EGCs of the mucosal plexus. It is reported that GDNF immunoreactivity was strongly up-regulated in the colonic epithelium during rat experimental colitis and GDNF had strong anti-apoptotic effects on the colonic epithelial cells[46,47]. Mechanism studies revealed that the GDNF-mediated antiapoptotic properties required the activation of both the MAPK and the PI3K/AKt signaling pathways[46,47]. Recent evidence further supports the anti-apoptotic role of GDNF on mucosal EGCs. It showed that GDNF could feed back in an autocrine manner to protect EGCs from apoptosis in Crohn’s disease patients[48]. Disruption of this protective network could contribute to a higher susceptibility towards EGC apoptosis, and subsequently induce alteration of the mucosal integrity. Abnormal mucosal immune responses are considered as a contributing event in the mucosal barrier dysfunction. At this point, role of GDNF in the mucosal inflammatory responses was also investigated. Interestingly, GDNF could inhibit the expression of pro-inflammatory cytokines [interleukin (IL)-1β, tumor necrosis factor (TNF)-α] and myeloperoxidase activity in the rat colon[47,49]. In addition, administration of the recombinant adenoviral vectors encoding GDNF (Ad-GDNF) via the rectum could significantly ameliorate the severity of dextran sodium sulfate-induced rat colitis[47].
TGF-β1 has been reported to be secreted by astrocytes and plays a key role in neuronal homeostasis[50]. Interestingly, phenotypic studies of EGCs revealed that TGF-β1 could also be synthesized and released by EGCs[28,51]. Moreover, TGF-β1 was reported to account for approximately 12%-30% of the effects of EGCs on intestinal epithelial cell proliferation[52,53]. Growing evidence demonstrates that TGF-β1 inhibits epithelial cell proliferation while stimulates epithelial cell migration in a dose-dependent manner[54,55]. TGF-β1 mediates the anti-proliferative effects through either a down-regulation of cyclin-dependent kinases or an up-regulation of cyclin-dependent kinase inhibitors, and consequently induces a cell cycle blockade in the G1/S phase[56,57]. Interestingly, the effect assessment of cultured EGCs on cultured epithelial cell lines supports the concept that EGCs could significantly inhibit intestinal epithelial cell proliferation and concomitantly increase the cell surface of epithelial cells partly through a TGF-β1-dependent pathway[28].
15dPGJ2, a cellular source of the natural peroxisome proliferator-activated receptor gamma (PPARγ) ligand, could also be provided by EGCs[58]. Through activation of PPARγ, 15dPGJ2 mediated the inhibitory effects of EGC on epithelial cells proliferation and the positive effects of EGCs on epithelial differentiation, which promises the continuous renewal process of the intestinal epithelium[59]. As is known, the renewal process of IECs involves the epithelial cell emergence from the mucosal crypts and subsequent cell migration along the crypt-villus axis, during which the IECs cease to proliferate and acquire differentiated function. However, it should be noteworthy that EGC-derived 15dPGJ2 had no effect on the colonic paracellular permeability[60]. Further evidence showed that the anti-proliferative effects of EGCs might be attributed to its induction of a cell cycle blockade at G0/G1 phase in epithelial cells[61,62]. Besides, Krüppel-like factor 4, which is expressed in IECs and plays major roles in IEC differentiation and maturation, was supposed to be the candidate cellular target of PPARγ following activation by EGCs[63].
GSNO is another potent barrier-inducing factor present in enteric glial cell-conditioned media. Interestingly, GSNO is the nitrosylated form of reduced glutathione (GSH), and nitrosylation of GSH is responsible for an antioxidant cytoprotective action[64]. It has been demonstrated that intraperitoneal administration of GSNO obviously inhibited the increased intestinal permeability induced by enteric glial cell ablation in transgenic mice. This barrier-inducing effect of GSNO might be associated with the up-regulated expression of peri-junctional F-actin and TJ-associated proteins such as zonula occludens-1 (ZO-1) and occludin[65]. GSNO may also maintain the epithelial barrier function by improving the localization of the intestinal tight junction proteins, such as ZO-1, occudin and phosphorylated MLC[66]. In addition, GSNO may inhibit the gut inflammatory response through redox-sensitive s-nitrosylation of nuclear factor κB (NF-κB) inflammatory signaling, suppressing the transcription of pro-inflammatory mediators such as TNF-α[67]. Altering NF-κB inflammatory signaling also has important effects on the down-regulation of endothelial cell adhesion molecules that promote leukocyte infiltration[68]. However, it should be noteworthy that GSNO did not regulate the epithelial barrier in a dose-dependent manner. It was reported that disruptive effect of GSNO on the epithelial integrity was obtained at relatively higher concentrations[69]. The molecular mechanism remains unclear, but may be attributed to altered NO production. As is known, GSNO is a potent nitric oxide donor, which can function to S-nitrosylate proteins and play an important role in proper epithelial ion transport[70,71].
In concert with the barrier-inducing effects of EGCs, microarray analysis was carried out to further identify the EGC influence on the intestinal epithelial cells transcriptome. The study was performed to identify statistically significant differences in gene expression profiling in Caco-2 cells cultured alone or in presence of EGCs. The results showed that EGCs could regulate the expression of various genes involved in the control of IEC adhesion, differentiation, motility, cell cycle and proliferation. These collective gene-related data reinforces the concept that EGCs play a major protective role upon IEB homeostasis[72]. Besides the protective role, a repair process-inducing role of EGCs has recently been put forward. The study showed that EGCs could promote mucosa healing by increasing epithelial restitution and cell spreading after mechanical injury to IEC monolayers. Epidermal growth factor precursor (proEGF), as a novel glial mediator, was confirmed to be involved in the EGC-mediated epithelial restitution[73]. Indeed, proEGF exhibits a lower wound healing ability compared with EGF. However, subsequent studies showed that EGC-derived proEGF could be activated by concomitant release of MMPs or proteases during inflammatory or infectious insults of IEB, which would process proEGF into mature EGF and therefore enhance mucosa repairing[73,74].
Recently, a protective role of EGCs on the mucosal barrier during enteric bacterial insult has drawn increasing interest, which may also provide new therapeutic tools in the protection and regeneration of intestinal barrier[75]. As is known, Shigella flexneri (S. flexneri) is one of the major enteroinvasive pathogens which are responsible for the destruction of the intestinal epithelium[76]. Flamant demonstrated for the first time that the protective effects of EGCs on the IEB could be due in part to its ability to inhibit S. flexneri invasion. Further, cdc42, a key molecular factor for S. flexneri invasion, was significantly downregulated by performing co-culture experiments between IECs and EGCs. In addition, EGCs prevents tight-junction disruption during S. flexneri infection, and diminishes mucosal secretion of the pro-inflammatory cytokine IL-8[77]. Indeed, these EGC-mediated effects could also be reproduced by GSNO[78]. Under the stimulation of lipopolysaccharide (LPS), EGCs could play the protective effect on IEB functions by inhibiting the increase of inducible nitric oxide synthase activity induced by LPS[79]. A recent study has shown that Toll-like receptor 2 (TLR2), which plays key roles in sensing microbial structures, is expressed on glial cells. In the intestine, TLR2 exerts cytoprotective effects in intestinal epithelial cells and regulates epithelial barrier function. Besides, TLR2 could stimulate the intestinal expression of GDNF through NF-κB and p38 mitogen-activated protein kinase signaling pathway. In this context, the TLR2-GDNF axis might represent an attractive regulator for gut homeostasis[80].
Because gut inflammation accompanies changes in intestinal permeability, roles of EGCs in mucosal inflammation have also been investigated. Similar to CNS astrocytes, EGCs are recognized as immunocompetent cells that have the ability to express major histocompatibility complex class I and class II molecules, and to produce and respond to a variety of chemokines and cytokines[10]. Co-cultured with interferon-gamma and TNF-α, EGCs acquire the ability to process and present antigens efficiently to specific T-cells, indicating that EGCs can act as antigen-presenting cells[7]. EGCs express substance P, which can induce the activation of mast cells and macrophages and promote lymphocyte proliferation[81]. S100B protein, specifically expressed by EGCs, can orchestrate a wide range of signal activation pathways which are directly correlated with the severity of gut inflammatory processes[82,83]. Palmitoylethanolamide can exert anti-inflammatory effects through the selective targeting of the S100B/TLR4 axis on EGCs, causing a downstream inhibition of nuclear factor kappa B-dependent colonic inflammation[84]. Further, EGCs have the ability to respond to inflammatory stimuli through the production of pro-inflammatory cytokines, such as IL-6[85], TGF-β1[28] etc. EGCs could also inhibit inflammation in animal models of colitis as they produce mediators such as nerve growth factor and neurotrophin-3 which have anti-inflammatory properties[86,87]. In Cytomix-stimulated intestinal epithelial cells while EGCs were removed from the culture, the anti-inflammatory effects of nicotine were lost and consequently resulted in increased in vitro epithelial permeability[88]. These data support the hypothesis that EGCs are likely immune mediators in the gastrointestinal tract. However, so far, limited information is available to indicate the exact mechanisms of EGCs in the regulation of mucosal inflammation-induced permeability alterations.
Collectively, EGCs, intriguing cellular populations within the gastrointestinal tract, might be of interest as a source of novel molecules aiming at preventing relapse or increasing IEB repair. However, the precise mechanism of EGCs on the regulation of intestinal barrier is still partly unclear. Future research identifying precisely how EGCs participate in intestinal epithelium physiology and pathophysiology will be beneficial for our understanding of EGC-IEC interactions, which is also invaluable in ultimately developing new therapeutic tools for the restoration of the barrier functions.
P- Reviewer: Bashashati M S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 463] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Jankowski JA, Bedford FK, Boulton RA, Cruickshank N, Hall C, Elder J, Allan R, Forbes A, Kim YS, Wright NA. Alterations in classical cadherins associated with progression in ulcerative and Crohn’s colitis. Lab Invest. 1998;78:1155-1167. [PubMed] |
| 4. | Suenaert P, Bulteel V, Vermeire S, Noman M, Van Assche G, Rutgeerts P. Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis. 2005;11:667-673. [PubMed] |
| 5. | Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [PubMed] |
| 7. | Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci USA. 2001;98:13306-13311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437-1439. [PubMed] |
| 9. | Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3452] [Cited by in RCA: 3878] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 12. | Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 771] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 13. | Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3-20; quiz 21-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1247] [Article Influence: 77.9] [Reference Citation Analysis (2)] |
| 14. | Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930-6938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (1)] |
| 16. | Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Overgaard CE, Daugherty BL, Mitchell LA, Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal. 2011;15:1179-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Kotler BM, Kerstetter JE, Insogna KL. Claudins, dietary milk proteins, and intestinal barrier regulation. Nutr Rev. 2013;71:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031-1049. [PubMed] |
| 20. | Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851-G859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 472] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 21. | Balda MS, Matter K. Transmembrane proteins of tight junctions. Semin Cell Dev Biol. 2000;11:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025-G1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 23. | Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 26. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 952] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 27. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 28. | Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231-G241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736-737. [PubMed] |
| 30. | Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409-410. [PubMed] |
| 31. | Nanda S. Neurogastroenterology: A role for enteric glial cells in mucosal healing. Nat Rev Gastroenterol Hepatol. 2011;8:242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | De Giorgio R, Giancola F, Boschetti E, Abdo H, Lardeux B, Neunlist M. Enteric glia and neuroprotection: basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol. 2012;303:G887-G893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035-4041. [PubMed] |
| 34. | Neunlist M, Van Landeghem L, Mahé MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 35. | von Boyen GB, Schulte N, Pflüger C, Spaniol U, Hartmann C, Steinkamp M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Bush TG. Enteric glial cells. An upstream target for induction of necrotizing enterocolitis and Crohn’s disease? Bioessays. 2002;24:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Aubé AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 38. | Savidge TC. S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci. 2011;5:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3031] [Cited by in RCA: 3709] [Article Influence: 247.3] [Reference Citation Analysis (0)] |
| 40. | Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest. 2007;87:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Cirillo C, Sarnelli G, Esposito G, Turco F, Steardo L, Cuomo R. S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J Gastroenterol. 2011;17:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (3)] |
| 43. | Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 928] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 44. | Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 45. | Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920-6928. [PubMed] |
| 46. | Steinkamp M, Geerling I, Seufferlein T, von Boyen G, Egger B, Grossmann J, Ludwig L, Adler G, Reinshagen M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology. 2003;124:1748-1757. [PubMed] |
| 47. | Zhang DK, He FQ, Li TK, Pang XH, Cui de J, Xie Q, Huang XL, Gan HT. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol. 2010;222:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Steinkamp M, Gundel H, Schulte N, Spaniol U, Pflueger C, Zizer E, von Boyen GB. GDNF protects enteric glia from apoptosis: evidence for an autocrine loop. BMC Gastroenterol. 2012;12:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 547] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 50. | Xin H, Chopp M, Shen LH, Zhang RL, Zhang L, Zhang ZG, Li Y. Multipotent mesenchymal stromal cells decrease transforming growth factor β1 expression in microglia/macrophages and down-regulate plasminogen activator inhibitor 1 expression in astrocytes after stroke. Neurosci Lett. 2013;542:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Zajzon K, Pröls F, Heermann S. Concerted interaction of TGF-β and GDNF mediates neuronal differentiation. Neuroreport. 2013;24:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 53. | Muñoz NM, Baek JY, Grady WM. TGF-beta has paradoxical and context dependent effects on proliferation and anoikis in human colorectal cancer cell lines. Growth Factors. 2008;26:254-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Gatza CE, Holtzhausen A, Kirkbride KC, Morton A, Gatza ML, Datto MB, Blobe GC. Type III TGF-β receptor enhances colon cancer cell migration and anchorage-independent growth. Neoplasia. 2011;13:758-770. [PubMed] |
| 55. | Bulut K, Meier JJ, Ansorge N, Felderbauer P, Schmitz F, Hoffmann P, Schmidt WE, Gallwitz B. Glucagon-like peptide 2 improves intestinal wound healing through induction of epithelial cell migration in vitro-evidence for a TGF--beta-mediated effect. Regul Pept. 2004;121:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Ali NA, Molloy MP. Quantitative phosphoproteomics of transforming growth factor-β signaling in colon cancer cells. Proteomics. 2011;11:3390-3401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Wang J, Sergina N, Ko TC, Gong J, Brattain MG. Autocrine and exogenous transforming growth factor beta control cell cycle inhibition through pathways with different sensitivity. J Biol Chem. 2004;279:40237-40244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Bull AW, Steffensen KR, Leers J, Rafter JJ. Activation of PPAR gamma in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR gamma. Carcinogenesis. 2003;24:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Bach-Ngohou K, Mahé MM, Aubert P, Abdo H, Boni S, Bourreille A, Denis MG, Lardeux B, Neunlist M, Masson D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J Physiol. 2010;588:2533-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 60. | Ponferrada A, Caso JR, Alou L, Colón A, Sevillano D, Moro MA, Lizasoain I, Menchén P, Gómez-Lus ML, Lorenzo P. The role of PPARgamma on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology. 2007;132:1791-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Neunlist M, Van Landeghem L, Bourreille A, Savidge T. Neuro-glial crosstalk in inflammatory bowel disease. J Intern Med. 2008;263:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem. 2001;276:29681-29687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Rageul J, Mottier S, Jarry A, Shah Y, Théoleyre S, Masson D, Gonzalez FJ, Laboisse CL, Denis MG. KLF4-dependent, PPARgamma-induced expression of GPA33 in colon cancer cell lines. Int J Cancer. 2009;125:2802-2809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Yap LP, Sancheti H, Ybanez MD, Garcia J, Cadenas E, Han D. Determination of GSH, GSSG, and GSNO using HPLC with electrochemical detection. Methods Enzymol. 2010;473:137-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 66. | Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1308-G1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA. 2004;101:8945-8950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 297] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 68. | Awad EM, Khan SY, Sokolikova B, Brunner PM, Olcaydu D, Wojta J, Breuss JM, Uhrin P. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 2013;11:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912-4916. [PubMed] |
| 70. | Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1129] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 71. | Marshall HE, Hess DT, Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci USA. 2004;101:8841-8842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | Van Landeghem L, Mahé MM, Teusan R, Léger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics. 2009;10:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Le Gall SM, Meneton P, Mauduit P, Dreux C. The sequential cleavage of membrane anchored pro-EGF requires a membrane serine protease other than kallikrein in rat kidney. Regul Pept. 2004;122:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Matteoli G. Enteric glial cells: new players in mucosal defence against bacteria? Gut. 2011;60:429-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 741] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 77. | Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, Meurette G, Marteyn B, Savidge T, Galmiche JP. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 581] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 79. | Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci. 2011;46:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 81. | Rühl A, Franzke S, Stremmel W. IL-1beta and IL-10 have dual effects on enteric glial cell proliferation. Neurogastroenterol Motil. 2001;13:89-94. [PubMed] |
| 82. | Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Calì G, D’Armiento FP, Rocco A, Nardone G. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil. 2009;21:1209-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil. 2011;23:e372-e382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 85. | Rühl A, Franzke S, Collins SM, Stremmel W. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1163-G1171. [PubMed] |
| 86. | Reinshagen M, Rohm H, Steinkamp M, Lieb K, Geerling I, Von Herbay A, Flämig G, Eysselein VE, Adler G. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology. 2000;119:368-376. [PubMed] |
| 87. | von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol. 2006;18:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, Coimbra R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One. 2013;8:e69042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |