Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.11012
Revised: February 16, 2014
Accepted: April 30, 2014
Published online: August 21, 2014
Processing time: 249 Days and 18.9 Hours
AIM: To evaluate the effect of muscovite in preventing small bowel injury induced by nonsteroidal anti-inflammatory drugs (NSAIDs).
METHODS: We recruited and screened thirty-two healthy volunteers who were randomly allocated equally into two groups: an NSAID control group, who received 75 mg slow-release diclofenac, twice daily for 14 d; and an NSAID-muscovite group, who received 3 g of muscovite in addition to the 75 mg of slow-release diclofenac, twice daily for 14 d. For gastroprotection, both groups were administered 20 mg/d of the proton pump inhibitor omeprazole. All eligible subjects underwent video capsule endoscopy (CE) prior to and 14 d after treatment.
RESULTS: Thirty subjects (NSAID-muscovite group, n =16; NSAID control group, n =14) finally completed the whole trail. At the baseline CE examination, no statistically significant differences between the two groups have been observed. However, after 14 d of drug treatment, a significant difference was observed in the percentage of subjects with mucosal breaks when comparing the NSAID-muscovite group with the NSAID control group. While 71.4% (10/14) of subjects in the NSAID control group had at least one mucosal break, co-administration of muscovite in the NSAID-muscovite group reduced the rate to 31.3% (5/16) (P = 0.028). Moreover, higher number of mucosal breaks was found in the NSAID control group vs that in the NSAID-muscovite group (P < 0.05).
CONCLUSION: Muscovite co-therapy reduced the incidence of small intestinal injury after 14 d of diclofenac administration.
Core tip: This is a randomized, open-label, controlled clinical trial to evaluate the incidence of small bowel damage by capsule endoscopy in healthy participants who received treatment with the nonsteroidal anti-inflammatory drug (NSAID) diclofenac and the effect of muscovite in preventing NSAID-induced small bowel injury.
- Citation: Huang C, Lu B, Fan YH, Zhang L, Jiang N, Zhang S, Meng LN. Muscovite is protective against non-steroidal anti-inflammatory drug-induced small bowel injury. World J Gastroenterol 2014; 20(31): 11012-11018
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/11012.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.11012
Nonsteroidal anti-inflammatory drugs (NSAIDs), one of the most commonly used classes of drugs worldwide, are widely accepted for their anti-inflammatory and analgesic properties. Although NSAIDs are beneficial in reducing pain and inflammation, they are also known to have adverse gastrointestinal (GI) effects. As cyclooxygenase-2 inhibitors were associated with an increased risk of cardiovascular events, conventional NSAIDs are more frequently prescribed by clinicians[1]. After the introduction of capsule endoscopy (CE) and due to the increased use of aspirin and NSAIDs, NSAID-induced gastric and duodenal mucosa damage has gained more attention. CE now allows for a full investigation and visualization of the entire small intestine. CE in patients has revealed that NSAID-induced lower GI injury is more common than NSAID-associated gastropathy[2-9]. The same CE studies showed that in up to 55% of healthy volunteers, co-administration of proton pump inhibitors (PPIs) with NSAIDs failed to prevent NSAID-induced small intestinal damage[10]. Co-administration of NSAIDs and misoprostol, a mucosal protective agent for the management of gastric ulcers, could attenuate mucosal damage, though this study lacked a large clinical sample[11]. Medications that prevent or treat NSAID-induced intestinal injuries are not currently available. It is critical to further understand the small intestinal damage induced by NSAIDs, because all clinicians, particularly gastroenterologists, should have a comprehensive understanding of the gastrointestinal adverse effects associated with NSAIDs.
Muscovite, a kind of natural clay or traditional Chinese medicine, is composed of insoluble double silicate of aluminum and magnesium. It has served in the management of gastric diseases in China for many years. Previous research from our lab has demonstrated that muscovite can reduce intestinal permeability in rats with NSAID-induced enteropathy. Moreover, muscovite also provides a protective effect against acute and sub-acute injuries of the intestinal mucosa[12,13]. The aim of the current study was to evaluate the effect of intragastric muscovite administration on intestinal injury induced by diclofenac treatment in healthy volunteers. This two-week, single-center study was a prospective, single-blinded, randomized, controlled study that utilized CE to evaluate the incidence of small bowel damage induced by NSAIDs in healthy subjects undergoing concomitant therapy with muscovite or not.
From December 2012 through June 2013, we recruited and screened 32 healthy volunteers by CE and laboratory tests. Subjects that met the following criteria were eligible for inclusion in our study: no history of surgery, between the ages of 18 and 70 years, not taking any medication during the month prior to enrolment, and no abnormal findings from physical examinations or laboratory tests. All the subjects received a CE examination before enrolment. Subjects were excluded for the following reasons: (1) failure to traverse the full length of the small intestine; (2) the presence of stenosis, tumors, or ulcers; and (3) the number of mucosal breaks in the small intestine more than 5. Subjects with active gastrointestinal disease, ulcer and bleeding history, fecal occult blood test (+) or hemoglobin levels < 12 g/dL were further excluded from this study. This study was approved by the ethics committee of the First Affiliated Hospital of Zhejiang Chinese Medical University. Informed consent was obtained from each subject enrolled in this study before undergoing baseline CE examination.
All the eligible subjects were randomly allocated equally into two groups using a sorted random number generator. The subjects in the control group received 75 mg of diclofenac twice per day for 14 d, while the experimental group was co-administered the same dosage of diclofenac along with 3 g of muscovite twice daily for 14 d. Both groups were also given 20 mg of omeprazole daily for gastroprotection. All eligible subjects underwent CE prior to and 14 d after treatment. Post-treatment CE was conducted within 24 h after treatment was completed. Participants who discontinued treatment due to adverse effects or had incomplete post-treatment CE examination were also excluded.
We used the OMOM video capsule system (Jinshan Science and Technology, Chongqing, China) in the current study. The CE procedures and methodology for image review were performed according to the study by Li et al[14]. After a 12-h fast, the subjects were requested to drink 50% magnesium sulfate 50 mL and 40 mg/mL simethicone 30 mL, respectively, 10 h and 15 min before the CE examination. All the participants were provided with recorder-battery belt pack and a sensor array. The capsule was swallowed with a cup of warm water, and two images were taken per second within 8 h. All the frames are transmitted continuous video images, and processed after unloading onto a computer. Following the preliminary CE examination, we briefly analyzed the results to determine whether participants were eligible for the further study. Two skilled technical reviewers independently screened per video for GI pathology, and the detected pathologies were further evaluated by two endoscopists who were blinded to the exact treatment protocol as well as participant characteristics. We saved all the images for a thorough analysis when all post-treatment CE examinations were accomplished.
The primary end point was the mean number of small intestinal mucosal breaks per subject. Table 1 describes the definition of any mucosal breaks as categories 1-5. The secondary end points included (1) the percentage of participants with at least one mucosal break of the small bowel; (2) the severity of injury (categories 0-4 in Table 1); and (3) the type of injury (categories 1-5 in Table 1) in the small intestine. A post-hoc analysis was used to analyze the distribution of small intestinal mucosal breaks across intestinal tertiles. To do this, we grouped three equal areas between the cecum and duodenum according to the small bowel transit time of each participant. The participants were excluded from this particular specific analysis when the cecum was not clearly identified. Safety was assessed according to physical and laboratory findings, or observed and self-reported side-effects.
| 0 | Normal |
| 1 | Petechiae |
| 2 | Erosion |
| 3 | Ulcer (< 3) |
| 4 | Ulcer (≥ 3) |
| 5 | Other: denuded mucosa and lymphangiectasis |
Age, sex, height, body weight and the number of mucosal breaks at baseline CE between the experiment and the control groups were analyzed by the Student’s t-test. The percentage of subjects with at least one mucosal break between the two groups was analyzed by the Pearson χ2 test. The injury severity and mean number of mucosal breaks per subject between the experiment and the control groups were evaluated by the Wilcoxon signed rank test. Data are presented as mean ± standard deviation (SD) if the values were normally distributed. P < 0.05 was set as the threshold for statistical significance.
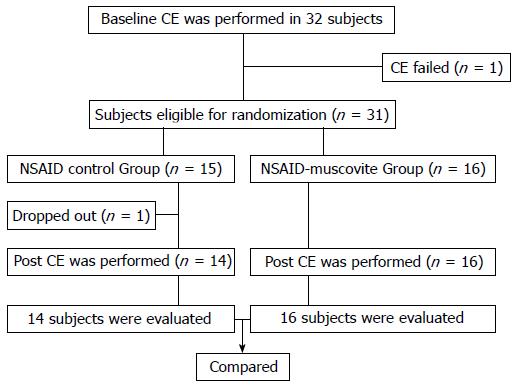
A flow chart depicting the study organization is presented in Figure 1. Thirty-two subjects underwent a baseline CE. Of the initial 32 participants, the entire small intestine was unable to observe in one participant, who was excluded from this study. There was no significant GI pathology in the remaining 31 participants, and they thus entered the study. Eligible subjects were then randomly assigned to either the NSAID control group or the NSAID-muscovite group. In the NSAID control group, one participant withdrew for personal reasons, and the remaining 14 participants accomplished the final study. All the 16 participants completed their therapy regimens in the NSAID-muscovite group. Thus, 14 participants in the NSAID control group and 16 subjects in the NSAID-muscovite group were finally evaluated for the presence of any mucosal break of the small bowel.
The basic characteristics of each participant are shown in Table 2. There were no statistically significant differences in the baseline characteristics between the two groups during the initial CE examination. We observed 7 mucosal breaks in 2 of 16 participants (number of mucosal breaks: 0.5 ± 1.4) in the NSAID-muscovite group during the initial CE examination. No mucosal breaks were identified in the NSAID control group.
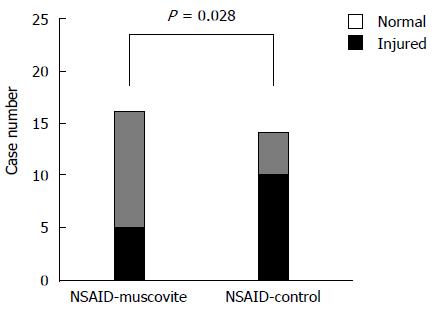
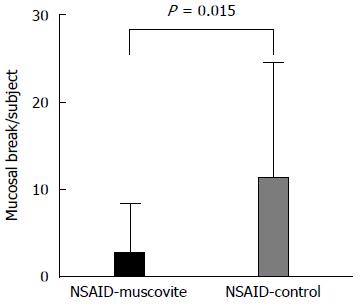
After 14 d of treatment, the percentage of participants with at least one mucosal break of the small bowel was significantly higher in the NSAID control group [71.4% (10/14) of subjects] than in the NSAID-muscovite group [31.3% (5/16) of subjects] at the post-treatment CE (P = 0.028) (Figure 2). No statistically significant difference in the incidence of mucosal breaks was observed (12.5% before treatment and 31.3% after treatment; P = 1.00) in the NSAID-muscovite group (Table 3). We next analyzed the mean number of mucosal breaks in the participants who developed one or more mucosal breaks. The mean number of mucosal breaks in each participant increased in response to NSAID treatment in the NSAID control group; there were zero mucosal breaks at the baseline CE and 11.1 ± 13.5 at the end of treatment (P = 0.005). While there was no significant change in the number of mucosal breaks found (0.5 ± 1.4 before treatment and 2.5 ± 5.7 after treatment; P = 0.270) in the NSAID-muscovite group. Therefore, the mean number of mucosal breaks in each participant was increased in the NSAID control group vs the NSAID-muscovite group at the post-treatment CE examination (P = 0.015) (Figure 3, Table 4).
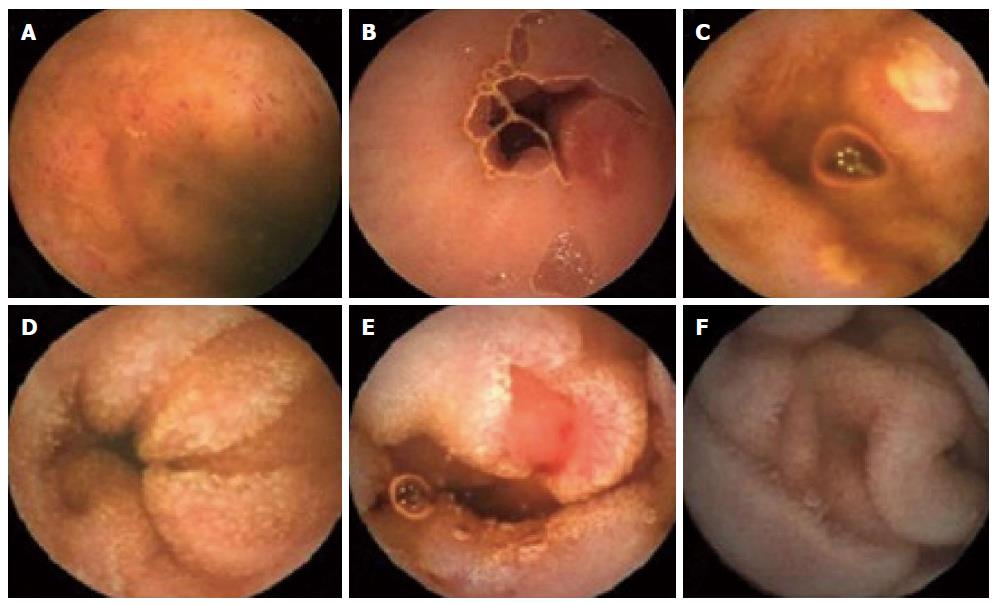
In the NSAID control group, we observed 28 (2.0 ± 3.0 per subject) episodes of petechiae in 7/14 subjects, 47 (3.4 ± 4.1 per subject) erosions in 10/14 subjects and 80 (5.7 ± 9.8 per subject) ulcers in 8/14 subjects after two weeks of NSAID administration. Treatment with muscovite reduced the incidence of mucosal breakdown to 14 (0.9 ± 2.5 per subject), episodes of petechiae in 3/16 subjects, 14 (0.9 ± 2.1 per subject) erosions in 4/16 subjects, and 12 (0.8 ± 2.0 per subject) ulcers in 4/16 subjects in the NSAID-muscovite group (Table 5). Representative examples of mucosal breaks observed in this study are shown in Figure 4.
| Type of injury | NSAID-muscovite group | NSAID control group | P value1 |
| Petechiae | 3 (19) | 7 (50) | 0.070 |
| Erosion | 4 (25) | 10 (71) | 0.011 |
| Ulcer | 4 (25) | 8 (57) | 0.073 |
| Denuded areas | 1 (6) | 3 (21) | 0.315 |
| Lymphangiectasis | 1 (6) | 8 (57) | 0.004 |
We divided mucosal break severity into five levels (levels 0-4; Table 1): level 0: normal; level 1: petechiae; level 2: erosion; level 3: less than three ulcers; level 4: three or more ulcers observed. If the subjects had more than one type of mucosal break, we scored them at the highest level. In the NSAID control group, 57% (8/14) of the subjects had ulcers, with 43% (6/14) having at least three or more ulcers. In contrast, only 6% (1/16) of subjects in the NSAID-muscovite group had three or more ulcers. Thus, the severity of mucosal breaks observed in the NSAID control group was significantly greater compared with the NSAID-muscovite group (P = 0.017) at the post-treatment CE (Table 6).
We performed a post-hoc analysis and the distribution of participants with at least mucosal breaks is listed in Table 7. We observed no statistically significant difference in the distribution of small intestinal mucosal breaks across intestinal tertiles in the NSAID-muscovite group (P = 0.939). On the contrary, we observed a significant difference in the distribution of mucosal break across the tertiles of the small bowel in the NSAID control group (P = 0.027). Moreover, within each tertile, the difference between the NSAID-muscovite group and NSAID control group was statistically significant in the first and third tertiles, with the exception of the second tertile, probably due to fewer mucosal breaks observed in this tertile.
Mild diarrhea during the first few treatment days was reported in 4 participants in the NSAID control group. We chose to keep these participants in the study, however, for the period of this study due to the mild nature of their symptoms. The remaining subjects experienced no complications for the duration of the study.
Subjects in the NSAID-muscovite group, who received muscovite in addition to diclofenac and omeprazole, had five-fold fewer number of small intestinal mucosal breaks after two weeks of treatment in comparison to the NSAID control group (2.5 vs 11.1, P = 0.015). In addition, participants in the NSAID-muscovite group were associated with a significantly lower percentage of subjects with one or more mucosal breaks (31.3% vs 71.4%, P = 0.028). Moreover, subjects in the NSAID-muscovite group had significantly lower injury severity of the small bowel in comparison to the NSAID control group. While 43% (6/14) of the NSAID control subjects had three or more ulcers, only 6% (1/16) of subjects in the NSAID-muscovite group had three or more ulcers (P = 0.017). In our study, we observed various NSAID-induced small bowel damages, such as petechia, erosions, ulcers, denuded areas or lymphangiectasis. Co-administration of muscovite resulted in a lower mean number of erosions and ulcerations induced by the short-term administration of NSAIDs. Although we did not observe a protective effect of muscovite against all observed intestinal damages, to the best of our knowledge, this study for the first time demonstrated by CE that treatment with muscovite could prevent or attenuate the severity of small bowel injury induced by some forms of NSAID. To determine if the treatment had a localized effect on any portion of the small bowel, we also conducted a post-hoc analysis of the distribution of mucosal breaks across the intestinal tertiles. There was a significant difference in the mucosal break distribution across the intestinal tertiles in the NSAID control group (P = 0.027).
It has been well established regarding the use of NSAIDs and the risk of small bowel damage and complications. Our results (71% of subjects in the NSAID control group developed NSAID-induced small-intestinal injuries; 57% developed ulcers) are consistent with findings recently reported for the small bowel from a video CE study in arthritis patients. Using CE, one study[3] showed that new intestinal damages developed in 68% of healthy subjects who received NSAIDs for 2 wk[2]. Another study[10] indicated that 55% of participants developed small bowel damages after the NSAID naproxen was administered for two weeks, with a mean of 2.99 mucosal breaks in each participant. In the majority of the end points measured in this study, the NSAID-muscovite group was statistically significantly different from the NSAID control group.
Although the cause of intestinal injury is not well understood, it is hypothesized that an aberrant increase in intestinal permeability promotes susceptibility to NSAID-induced inflammation and damage in the small intestine. As a type of traditional Chinese medicine, muscovite has served in the management of gastric diseases in China for many years. Pharmacological studies have confirmed that the layered structure of muscovite, with natural and special physical properties, may uniformly coat the surface of the gastric mucosa through the stimulation of mucus secretion to enhance intestinal mucosal barrier function. Alternatively, muscovite may effectively protect the mucosa by reducing the amount of direct contact with harmful luminal factors (e.g., drugs, bile and various enzymes), thereby reducing membrane permeability. In addition, previous research has also shown that muscovite can effectively stimulate secretion of endogenous epidermal growth factor, which is known to promote mucosal repair and healing[15-17].
Many studies found that administration of omeprazole is ineffective in preventing injury in the small intestine[3,18]. In contrast, celecoxib, a cyclooxygenase-2 inhibitor, could effectively reduce the number of mucosal breaks each participant and the percentage of participants with one or more mucosal break[10]. Because cyclooxygenase-2 inhibitors may be associated with an increased risk of adverse cardiovascular events, many clinicians prefer to prescribe the traditional NSAIDs in combination with PPIs instead of cyclooxygenase-2 inhibitors in the management of NSAID-induced GI damages. Previously, no therapeutic agents existed to protect against NSAID-induced small bowel injury. Our paper broadens the understanding of the impacts of NSAIDs in the small bowel injury and explores the mechanisms of administration of traditional Chinese medicine (muscovite) on small bowel health. We found that participants who received muscovite treatment had a significantly lower number of small bowel mucosal break compared with those who received NSAIDs alone.
Although our study found that administration of muscovite could effectively prevent small intestinal damages induced by NSAIDs, some potential limitations should be mentioned. First, sample size of our study was relatively small and only healthy volunteers were included. Second, the short-term administration of NSAIDs and muscovite is not a typical course of treatment. In the clinical setting, patients often require long-term administration of NSAIDs. Third, our study had an inherent bias against neutrality because of its open-label trial design character. So, future trials with larger sample sizes are required to further evaluate the beneficial effect of muscovite identified in the present study.
Non-steroidal anti-inflammatory drugs (NSAIDs), one of the most commonly used classes of drugs worldwide, are widely accepted for their anti-inflammatory and analgesic properties. Although NSAIDs are beneficial in reducing pain and inflammation, they may induce fatal complication, such as ulcerations, perforation, bleeding or diaphragm-like strictures.
Due to the increased use of aspirin and NSAIDs and the introduction of capsule endoscopy (CE), a new diagnostic modality, NSAID-induced enteropathy has gained much attention. NSAID-induced lower gastrointestinal (GI) injury is more common than NSAID-associated gastropathy, and has been underestimated or ignored in clinical practice prior to the wide use of CE.
This is the first try to use the traditional Chinese medicine (muscovite) to prevent NSAID-induced enteropathy in clinical trial. This study broadens the understanding of the impacts of NSAIDs in the small bowel injury and explores the mechanisms of administration of traditional Chinese medicine (muscovite) on small bowel health.
In this study, the authors found that participants who received muscovite treatment had a significantly lower number of small bowel mucosal break than participants who received NSAIDs alone, which means that traditional Chinese medicine (muscovite) co-therapy may have a brilliant future in preventing NSAID-induced lower GI injury.
It is a very well designed and conducted study examining the potential protective effects of muscovite on the small bowel in those healthy volunteers taking NSAID for 2 wk.
P- Reviewer: Butterworth J, Maehata Y, Mizukami K S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119:521-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 4. | Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, Bjarnason I. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Fujimori S, Gudis K, Takahashi Y, Seo T, Yamada Y, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010;40:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Matsumoto T, Kudo T, Esaki M, Yano T, Yamamoto H, Sakamoto C, Goto H, Nakase H, Tanaka S, Matsui T. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Smale S, Tibble J, Sigthorsson G, Bjarnason I. Epidemiology and differential diagnosis of NSAID-induced injury to the mucosa of the small intestine. Best Pract Res Clin Gastroenterol. 2001;15:723-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Zuccaro G. Epidemiology of lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 10. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG; Investigators. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 451] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Wu WF, Lu B, Fang L, Zhang S. Effect of mica on intestinal in experimental NSAIDs enteropathy in rats. Zhongguo Weichangbingxue Zazhi. 2009;14:478-480. |
| 13. | Wu WF, Lu B, Zhang S, Yu LM. Prevention of mica on intestinal mucosal damage induced by diclofenac in rats. Yiyao Daobao. 2009;128:1127-1130. |
| 14. | Li CY, Zhang BL, Chen CX, Li YM. OMOM capsule endoscopy in diagnosis of small bowel disease. J Zhejiang Univ Sci B. 2008;9:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wang LJ, Zhou QY, Chen Y, Chen SJ, Xu M, Du Q, Zhu FS, Si JM. Muscovite reverses gastric gland atrophy and intestinal metaplasia by promoting cell proliferation in rats with atrophic gastritis. Digestion. 2009;79:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wang LJ, Chen SJ, Si JM, Xu M. Effects of Muscovite on cell proliferation of gastric mucosa in rats with chronic atrophic gastritis. Zhongguo Yaoxue Zazhi. 2005;40:1226-1229. |
| 17. | Chao G, Zhang S. Therapeutic effects of muscovite to non-steroidal anti-inflammatory drugs-induced small intestinal disease. Int J Pharm. 2012;436:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Zhang S, Chao GQ, Lu B. Proton pump inhibitors are not the key for therapying non-steroidal anti-inflammatory drugs-induced small intestinal injury. Rheumatol Int. 2013;33:2513-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |