Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10960
Revised: April 7, 2014
Accepted: May 19, 2014
Published online: August 21, 2014
Processing time: 181 Days and 4.1 Hours
AIM: To compare the efficacy of different chemotherapeutic agents during conventional transarterial chemoembolization (cTACE) in the treatment of unresectable hepatocellular carcinoma (HCC).
METHODS: A retrospective review was undertaken of patients with unresectable HCC undergoing cTACE from May 2003 to November 2011. A total of 107 patients were treated with at least one cTACE session. Irinotecan (CPT-11) was used as a chemotherapeutic agent in 24 patients, gemcitabine (GEM) in 24 and doxorubicin in 59.
RESULTS: The time to progression and overall survival rates were significantly superior in patients treated with CPT-11 compared with the GEM or doxorubicin treated groups (11.4, 8.2, 9.5 mo, P = 0.02 and 21.7, 12.7, 14.5 mo, P = 0.004, respectively). Subgroup analysis showed that for intermediate-stage HCC, CPT-11 resulted in a significantly longer time to progression and overall survival compared with the GEM or doxorubicin treated groups (P = 0.022; P = 0.003, respectively). There were no significant differences in adverse events among the three groups (P > 0.05).
CONCLUSION: For patients treated with cTACE, the chemotherapeutic agent CPT-11 was significantly associated with improved overall survival and delayed tumor progression compared with GEM or doxorubicin. There were no significant differences in clinical adverse events between the three agents. CPT-11 thus appears to be a promising agent when combined with cTACE for the treatment of HCC.
Core tip: In the present study, we aimed to compare the efficacy of different chemotherapeutic agents during conventional transarterial chemoembolization (cTACE) in the treatment of unresectable hepatocellular carcinoma. Our study indicated that for patients treated with cTACE, the chemotherapeutic agent irinotecan (CPT-11) was significantly associated with improved overall survival and longer time to progression compared with gemcitabine or doxorubicin. There were no significant differences in clinical adverse events between the three agents. CPT-11 thus appears to be a promising agent when combined with cTACE for the treatment of hepatocellular carcinoma.
- Citation: Wu J, Song L, Zhao DY, Guo B, Liu J. Chemotherapy for transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. World J Gastroenterol 2014; 20(31): 10960-10968
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10960
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. The annual incidence ranges from < 10 cases per 100000 persons in North America and Western Europe to 50-150 cases per 100000 persons in parts of Africa and Asia, where HCC is responsible for a large proportion of cancer-related deaths[1,2]. The Barcelona Clinic Liver Cancer (BCLC) staging system directs therapy according to tumor stage, liver function status, physical status and cancer-related symptoms[3]. However, over 60% to 70% of patients with HCC are diagnosed at a late stage and therefore curative therapies such as resection, liver transplantation or local ablation therapy are not appropriate[4]. Transarterial chemoembolization (TACE) is the primary treatment used most frequently for unresectable HCC. TACE has been shown to improve survival when compared with best supportive care for unresectable HCC[5,6]. The rationale for using TACE is that intra-arterial chemotherapy using lipiodol and chemotherapeutic agents followed by selective vascular embolization will result in a strong cytotoxic effect combined with ischemia (conventional TACE or cTACE)[7,8].
However, there is a lack of data to support the use of one chemotherapeutic agent or combination of agents over another. Doxorubicin as a single agent is the most common chemotherapeutic agent used worldwide. In the United States, combination therapy is more often used, typically consisting of doxorubicin, mitomycin C and cisplatin. An adenosine triphosphate tumor chemosensitive assay system is a new promising regime as a single chemotherapeutic treatment for HCC. Cells of HCC are highly sensitive to various chemotherapy drugs: taxol 46%, CPT-11 (irinotecan) 44%, gemcitabine (GEM) 36%, mitomycin 14%, adriamycin 12%, cisplatin 8%, 5-fluorouracil oxalate (5-FU) 4%[9]; the higher the percentage, the higher the sensitivity. Thus, it is indicated that CPT-11 might be a potential drug for the treatment of HCC and prolong survival time of HCC patients.
CPT-11, a drug used for the treatment of cancer, prevents DNA unwinding by inhibition of topoisomerase 1. It is a semi-synthetic analogue of the natural alkaloid camptothecin and is activated by hydrolysis to SN-38, an inhibitor of topoisomerase 1. Inactivation follows by uridine diphosphate glucoronosyltransferase 1A1 glucuronidation. The inhibition of topoisomerase 1 by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription. In 2007, Takeba et al[10] suggested that the antitumor effects of SN-38 might include the mechanism of the mitochondria-apoptotic pathway inducing p53 activation. This newly discovered mechanism of action of CPT-11 might be useful as a treatment for patients with HCC. Currently, there are limited data available regarding the use of chemotherapeutic agents administered via cTACE in patients with HCC. This study evaluated the efficacy, tumor response, clinical adverse events, time to progression and overall survival benefit of three chemotherapy agents: CPT-11, GEM and doxorubicin.
This study was approved by the ethics committees of the Dalian Medical University (No. 2013.012). As a retrospective medical records study, consents were not obtained. The records and personal information of all patients were anonymized prior to analysis.
This retrospective analysis was conducted on 107 patients with HCC who were treated with TACE-based therapy from May 2003 to November 2011 at the Second Hospital of Dalian Medical University of China. There were 95 men and 12 women with a mean age of 57 years (± 11 years). Hepatitis B virus was present in 81 of the 107 patients. The primary tumor was verified in all patients either by biopsy and histopathology or according to EASL criteria[11]. Briefly, non-invasive diagnosis of HCC was verified if a nodule of more than 2 cm within existing liver cirrhosis appeared arterially hypervascularized and with an enhanced venous “wash-out” on one contrast-enhanced imaging modality, with an AFP level exceeding 400 ng/mL. In patients with AFP levels below 400 ng/mL, a tumor greater than 2 cm had to show the above-mentioned dynamics of the contrast agent in two different imaging modalities.
Data evaluation was performed retrospectively and data were reported according to the standards defined by the Society of Interventional Radiology[12]. The study was performed in accordance with guidelines of the local institutional review board. A computed tomography (CT) scan was performed before the first chemoembolization to assess tumor size, multifocality, vascular invasion, morphological signs of liver cirrhosis and the presence of ascites. Etiology of liver cirrhosis, laboratory results including bilirubin, albumin, liver enzymes, prothrombin time (as Quick value or INR), thrombocytes, AFP and Eastern Cooperative Oncology Group status were retrieved from patient records. Based on these data, all patients were rated according to Child-Pugh[13,14], The Model for End-stage Liver Disease (MELD)[15], Cancer of the Liver Italian Program (CLIP)[16] and the BCLC[17]. Survival data were based on patients’ records from our institution and follow-up information from their families.
CPT-11 and 5-Fu were used as chemotherapy agents in the CPT-11 group, GEM and 5-Fu in the GEM group, and doxorubicin and 5-Fu in the doxorubicin group. The doses were CPT-11 130-180 mg/m2, GEM 1000 mg/m2, doxorubicin 30-40 mg/m2, 5-Fu 500-600 mg/m2. Physical condition of patients was also considered in the determination of the final doses.
Digital subtraction angiography (DSA, Multistar, Siemens, Erlangen, Germany) was performed before TACE to show vascular anatomy of the liver and to identify arterial feeders of the tumor. TACE was performed by selective catheterization of the hepatic segmental arteries nourishing the lesions. A 3-F coaxial microcatheter (TurboTracker 18; Boston Scientific, Cork, Ireland) was utilized. A co-mixture of iodised oil (Lipiodol UltraFluid; Laboratories Guerbet, Aulnay-sous-Bois, France) and chemotherapeutic agent (CPT-11, GEM or doxorubicin) with gelatine sponge particles (Spongostan Standard; Johnson and Johnson Medical Limited, Gargrave, Skipton, United Kingdom) was injected until a complete blockage of the tumor feeding branch was demonstrated. The doses of anticancer agent and lipiodol and the pieces of gelatine sponge particles used for TACE were determined based on the tumor size and extension of the lesions.
TACE was considered to be technically successful when target lesions were fully embolized and a complete blockage of the tumor feeding branch was demonstrated in the absence of immediate technical complications requiring treatment interruption. Complications were defined according to the Society of Interventional Radiology guidelines[18].
After the TACE procedure, patients recovered with approximately 12 h of bed rest in hospital. During the first 6 h, a clinical examination (abdominal evaluation and measurements of pulse rate, arterial blood pressure and body temperature) was performed every two hours. All patients underwent routine laboratory tests (liver enzyme biochemistry, AFP, routine blood) to assess peri-procedural complications and impact on liver function 7 d later after TACE.
One month after each cTACE procedure, a CT scan was performed in order to evaluate the tumor radiological response and then in all cases with complete response, scans were performed every three months in order to monitor the appearance of recurrence. Tumor response was assessed at CT by two expert abdominal radiologists according to the amended RECIST criteria[19,20]. Complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target lesions. All the other radiological responses were considered non-complete (non-CR) and categorized as partial response (PR), progressive disease (PD) and stable disease (SD) according to mRECIST criteria.
Viable tumor was defined as contrast uptake in the arterial phase and wash-out in portal venous and/or late venous phases. Contrast enhancement was visually assessed in the majority of cases. However, in doubtful cases at CT, quantitative measurements were obtained by placing a region-of-interest in specific areas in all phase images, according to Kim et al[21]. Repeated cTACE cycles were performed ‘‘on demand’’ upon the demonstration of viable tumour (non-CR) or intrahepatic recurrences in patients of Child-Pugh A and B.
The primary endpoint of our study was overall survival. Secondary endpoints were: (1) safety and liver toxicity; (2) tumor response at one month; and (3) time to local tumor recurrence (within target lesion) and intrahepatic tumor recurrence (new lesions).
Continuous variables were reported as median and range. Comparisons among groups were calculated using non-parametric tests (Mann-Whitney and Wilcoxon). Categorical variables were compared with the χ2 test. Survival analysis was performed with Kaplan-Meier statistics for all the patients as well as for the different Child-Pugh, MELD, CLIP, and BCLC stages. Median survival and CI were calculated. Differences in survival between the groups were assessed for statistical significance with the log-rank test. SPSS-software (version 15.0, SPSS Inc., Chicago, United States) was used for data evaluation and statistical analysis. A two-sided P value of less than 0.05 was considered statistically significant.
Baseline patient characteristics are shown in Table 1. The primary tumor was verified histopathologically in 17/107 of patients. In 90 patients, HCC was diagnosed based on radiological imaging procedures and AFP levels according to EASL criteria. A total of 53 patients were AFP-positive with levels greater than 400 ng/mL. Cirrhosis of the liver was present in 62 patients (58%) and thrombosis of a portal vein branch was present in 33 patients (31%). The mean tumor maximal diameter was 7.8 ± 4.1 cm. A mean of 2.0 ± 2.0 selective chemoembolization sessions were performed in each patient and the total number for all patients was 264.
| Total | CPT-11 | GEM | DDP+5-FU | P value | ||
| (n = 107) | (n = 24) | (n = 24) | (n = 59) | OS | PFS | |
| Mean age ± SD (yr) | 57.0 ± 11.0 | 61.0 ± 8.7 | 57.5 ± 12.4 | 56.0 ± 11.4 | ||
| Sex (M:F) | 95:12 | 22:2 | 24:0 | 49:10 | ||
| HBV | ||||||
| Absent/Present | 26/81 | 7/17 | 6/18 | 13/46 | 0.583 | 0.734 |
| Cirrhosis of the liver | ||||||
| Absent/Present | 45/62 | 13/11 | 6/18 | 26/33 | 0.003 | 0.011 |
| Tumor maximal diameter (cm) | 7.8 ± 4.1 | 8.0 ± 3.9 | 7.7 ± 4.1 | 7.4 ± 4.2 | ||
| ≤ 5/> 5 | 30/77 | 4/20 | 5/19 | 21/38 | 0.361 | 0.165 |
| Pathological T | ||||||
| T1/T2/T3/T4 | 8/32/50/17 | 3/8/10/3 | 0/9/10/5 | 5/15/30/9 | 0.070 | 0.052 |
| Pathological Stage | ||||||
| I/II/III/IV | 7/26/57/17 | 3/6/10/5 | 0/6/13/5 | 4/14/34/7 | 0.013 | 0.022 |
| TACE Sessions | ||||||
| ≤ 2/> 2 | 73/34 | 8/16 | 18/6 | 47/12 | 0.001 | 0.009 |
| Initial AFP (ng/dL) | ||||||
| ≤ 400/> 400 | 59/48 | 18/6 | 11/13 | 16/43 | 0.095 | 0.157 |
| Number of Tumor Single/Multiple | 57/50 | 12/12 | 13/11 | 32/27 | 0.017 | 0.039 |
| Vascular invasion | ||||||
| Absent/Present | 74/33 | 18/6 | 15/9 | 41/18 | 0.014 | 0.090 |
| Child-Pugh | ||||||
| A/B | 98/9 | 20/4 | 23/1 | 55/4 | 0.746 | 0.930 |
| BCLC Stage | ||||||
| A/B/C | 15/59/33 | 2/16/6 | 1/14/9 | 12/29/18 | 0.005 | 0.006 |
| CLIP Score | ||||||
| ≤ 2/> 2 | 77/20 | 20/4 | 18/6 | 39/20 | 0.013 | 0.013 |
| MELD Score | ||||||
| ≤ 6/> 6 | 74/33 | 17/7 | 16/8 | 41/18 | 0.914 | 0.610 |
| ALB (g/L) | ||||||
| ≤ 40/> 40 | 49/58 | 9/15 | 7/17 | 33/26 | 0.033 | 0.073 |
| TB (μmol/L) | ||||||
| ≤ 17/> 17 | 55/52 | 14/10 | 12/12 | 29/30 | 0.440 | 0.808 |
| AST (U/L) | ||||||
| ≤ 40/> 40 | 23/84 | 8/16 | 8/16 | 7/52 | 0.947 | 0.958 |
| ALT (U/L) | ||||||
| ≤ 40/> 40 | 32/75 | 11/13 | 8/16 | 13/46 | 0.456 | 0.522 |
| Lipiodol (mL) | ||||||
| ≤ 10/> 10 | 62/45 | 11/13 | 15/9 | 36/32 | 0.997 | 0.369 |
Treatment response was evaluated one month after the first TACE session. In the CPT-11 group, 4 (16.7%) and 16 (66.7%) patients showed a CR and PR respectively, two patients (8.3%) progressed and two (8.3%) had SD. In the GEM group, 3 (12.5%) and 16 (66.7%) patients showed a CR and PR respectively, 2 (8.3%) progressed and 3 (12.5%) had SD. In the doxorubicin group, 3 (12.5%) and 16 (66.7%) patients showed a CR and PR respectively, 2 (8.3%) progressed and 3 (12.5%) had SD. There was no significant difference in treatment responses among the three groups.
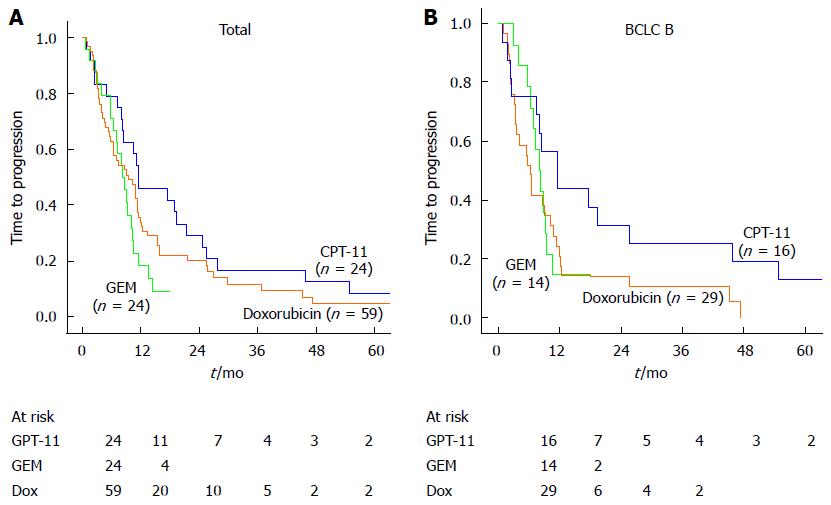
During follow-up, the median time to progression in the CPT-11, GEM and doxorubicin groups was 11.41, 8.25 and 9.46 mo respectively. The time to progression was significantly longer in the CPT-11 group than the other two groups (P = 0.02, Figure 1A). Furthermore, subgroup analysis according to BCLC stage showed that for intermediate-stage HCC, time to progression was significantly longer in the CPT-11 group compared with the GEM or the doxorubicin groups (P = 0.022, Figure 1B). Univariate analysis revealed eight prognostic factors affecting tumor progression were recognized: cirrhosis of the liver, BCLC stage, CLIP stage, pathological stage, number of tumors (single/multiple), TACE sessions (≤ 2/> 2), PS score and chemotherapy agent used. In multivariate analysis, pathological stage (P = 0.021) and PS score (P = 0.032) were significant independent factors for tumor progression (Table 2).
| Factors | Univariate (P value) | Multivariate (P value) | Exp(B) | 95%CI |
| Cirrhosis of the liver | ||||
| Absent/Present | 0.025 | 0.928 | 1.070 | 0.242-4.729 |
| Pathological stage | ||||
| I/II/III/IV | 0.022 | 0.021 | 0.643 | 0.442-0.936 |
| TACE Sessions | ||||
| ≤ 2/> 2 | 0.009 | 0.083 | 1.272 | 0.969-1.670 |
| BCLC Stage | ||||
| A/B/C | 0.006 | 0.666 | 1.241 | 0.465-3.316 |
| Number of Tumor | ||||
| Single/Multiple | 0.039 | 0.734 | 0.839 | 0.305-2.309 |
| CLIP Score | ||||
| ≤ 2/> 2 | 0.013 | 0.466 | 0.822 | 0.485-1.392 |
| Chemotherapy agent | ||||
| CPT-11/GEM/Doxorubicin | 0.020 | 0.648 | 0.869 | 0.474-1.591 |
| PS score | ||||
| 1/2/2000 | 0.029 | 0.032 | 0.095 | 0.011-0.818 |
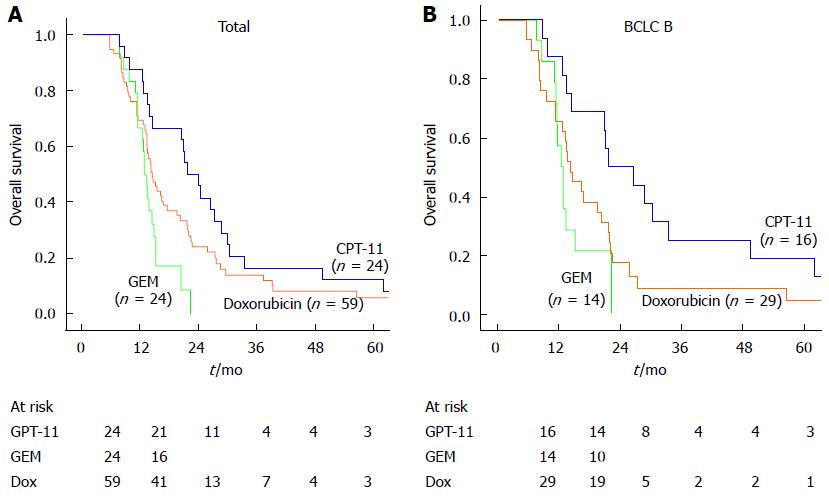
Overall survival was evaluated from the time of first TACE session to the endpoint of death or the last follow-up time (31st December, 2012). In patients who died, the cause of death was progression of liver disease (74.8%), rupture of esophageal varices (18.7%) and others (6.5%). There were no treatment related deaths. There was a lower rate of death in the CPT-11 group compared with the GEM or doxorubicin group (P = 0.02) due to less tumor progression. The median overall survival times in the CPT-11, GEM and doxorubicin groups were 21.68, 12.72 and 14.46 mo respectively. The cumulative survival rates at 12 and 24 months were 87.5% and 45.8% in the CPT-11 group, 66.7% and 0% in the GEM group and 69.5% and 22.0% in the doxorubicin group (Figure 2A). The overall survival was significantly higher in the CPT-11 group compared with the GEM or doxorubicin groups (P = 0.004). Subgroup analysis showed that the difference between the three groups was also significant in patients with intermediate-stage HCC (P = 0.003, BCLC B stage, Figure 2B). Univariate analysis revealed eight prognostic factors affecting overall survival: cirrhosis of the liver, BCLC stage, CLIP stage, pathological stage, number of tumors (Single/Multiple), TACE sessions (≤ 2/> 2), ALB and chemotherapy agent used. In multivariate analysis, the chemotherapy agent used was a significant independent factor for overall survival (P = 0.016, Table 3). In addition, ALB (P = 0.030), pathological stage (P = 0.012) and number of TACE sessions (P = 0.001) were related to survival. These results suggest that the use of CPT-11 may be associated with a better prognosis in patients with HCC.
| Factors | Univariate (P value) | Multivariate (P value) | Exp(B) | 95%CI |
| Cirrhosis of the liver | ||||
| Absent/Present | 0.003 | 0.083 | 5.114 | 0.806-32.436 |
| Pathological Stage | ||||
| I/II/III/IV | 0.013 | 0.012 | 0.485 | 0.276-0.851 |
| TACE Sessions | ||||
| ≤ 2/> 2 | 0.001 | 0.001 | 1.964 | 1.311-2.942 |
| BCLC Stage | ||||
| A/B/C | 0.005 | 0.061 | 0.183 | 0.031-1.078 |
| Number of Tumor | ||||
| Single/Multiple | 0.017 | 0.460 | 1.651 | 0.437-6.235 |
| CLIP Score | ||||
| ≤ 2/> 2 | 0.013 | 0.982 | 0.992 | 0.496-1.985 |
| Chemotherapy agent | ||||
| CPT-11/GEM/Doxorubicin | 0.004 | 0.019 | 0.407 | 0.192-0.863 |
| ALB (g/L) | ||||
| ≤ 40/> 40 | 0.033 | 0.030 | 0.834 | 0.709-0.982 |
Overall, adverse events were transient and tolerable and successfully managed with conservative treatment. Post-embolization symptoms, such as fever or pain, occurred in 23 patients and were reported as mild. There were no major complications or grade 4 liver toxicity[22] in either group within one week after cTACE. The most common adverse event was bone marrow suppression (37 patients) in the CPT-11, GEM and doxorubicin groups. Grade IV of bone marrow suppression was experienced in 1, 2 and 0 patients; 2, 3 and 4 patients had grade III; and mild elevation was seen in 3, 9 and 13 patients (grade I and II), respectively. Elevation of bilirubin was documented in three patients. Four patients experienced mild gastrointestinal symptoms (nausea or vomiting). Diarrhea occurred in only two patients treated with CPT-11 (Table 4).
| Grade I/II/III/IV | |||
| GEM | CPT-11 | Doxorubicin | |
| Aminotransferase elevation | 2/4/2/0 | 4/2/0/0 | 7/2/1/0 |
| Hyperbilirubinemia | 1/0/0/0 | 1/0/0/0 | 2/0/0/0 |
| Gastrointestinal toxicity | 1/0/0/0 | 2/0/0/0 | 0/1/0/0 |
| Post-embolization symptom | 3/2/0/0 | 3/3/0/0 | 7/5/0/0 |
| Bone marrow inhibition | 4/5/3/2 | 2/1/2/1 | 8/5/4/0 |
| Diarrhea | 0/0/0/0 | 1/1/0/0 | 0/0/0/0 |
Conventional transarterial chemoembolization is widely accepted as a predominantly palliative approach for patients with HCC when surgical intervention is not appropriate. The rationale for TACE is that a powerful cytotoxic effect combined with ischemia followed by chemoembolization of the hepatic artery will result in therapeutic efficacy and survival benefit compared with supportive care[23]. If performed in a selective and sequential way, high concentrations of embolic and chemotherapeutic agents may offer effective local tumor control, whilst maintaining tolerable systemic concentrations reducing the risk of significant adverse events, such as liver failure and other clinical adverse events. This study demonstrated that local tumor control translates into long survival times for patients treated with more sessions of cTACE[23,24]. However, there is insufficient evidence of chemotherapeutic agents used with cTACE to allow informed comparisons. Doxorubicin has been widely used as the chemotherapeutic agent of choice in cTACE, but with the development of new chemotherapeutic agents, such as CPT-11, GEM and oxaliplatin, comparative studies are needed to find the optimum agent for use in cTACE for the treatment of HCC.
This study is based on previous research on the application of the adenosine triphosphate tumor chemosensitive assay system as sole chemotherapy for HCC[9]. A comparison of CPT-11, GEM and doxorubicin agents used in cTACE for the treatment of HCC was performed. The time to progression and overall survival were significantly longer in patients treated with CPT-11. Additionally, liver toxicity or other clinical adverse events were not significantly different among the groups.
For tumor response, there was no significant difference among the groups. This may be explained by the hypothesis that embolization is more important than the chemotherapeutic agent used, but these agents may direct a powerful cytotoxic effect on hepatic cancer cells that determines time to progression and overall survival. Further research in this area is therefore warranted. Moreover, subgroup analysis according to the BCLC stage showed that for intermediate-stage HCC, the time to progression and overall survival were significantly better in the CPT-11 group compared with the GEM or doxorubicin groups (P = 0.022 and P = 0.003). As another new chemotherapy agent which may have potential, GEM in this study showed no advantages in cTACE with regard to the time to progression and overall survival compared with CPT-11 and even doxorubicin. From baseline characteristics in each groups, we found that the stage of patients in the CPT-11 and doxorubicin groups was relatively earlier than that in the GEM group, and more patients received extra gelatin sponge and microcatheter sessions in the CPT-11 and doxorubicin group than in the GEM group. These may be the reasons for the result produced in this study. However, we feel that our result is accurate, and more research should be performed to confirm it.
In this study, pathological stage was a prognostic factor in both the time to progression and overall survival. Earlier stage would be associated with a better prognosis in HCC patients, which is same as the conclusion in authoritative research and in guidelines of the National Comprehensive Cancer Network. Previous studies have shown that higher albumin (ALB) level is independently and significantly associated with improved survival duration[25-27]. From the multivariate analysis, patients with ALB > 40 g/L showed longer times of overall survival. However, due to the lack of ALB post-cTACE, the prognostic significance of ALB was not evaluated. This may be one potential point we can research. And we can see from this study that the patients who received more sessions (> 2) have a significantly different outcome compared with those who received only one or two sessions of cTACE regarding overall survival. This result is the same as reported in the study by Farinati et al[28]: the number of TACE courses and of embolizations is one of the prognostic factors in HCC patients undergoing TACE. This indicates that cTACE is different from the curative treatments, and more sessions should be accepted by patients to control the time to progression. After progression has happened, more cTACE sessions should also be accepted to control the local tumor recurrence or new lesions in the liver, in order to prolong the time of overall survival. However, patients may omit cTACE sessions due to financial reasons, which affects the tumor response and overall survival.
This study has a number of strengths and limitations. Firstly, doxorubicin is widely used as the chemotherapeutic agent in cTACE, but there are few published studies assessing newer chemotherapy agents used with cTACE such as CPT-11 and GEM. Secondly, cTACE with CPT-11 showed improved time to progression and overall survival compared with GEM or doxorubicin. As for limitations, sample sizes of each group were not balanced, with a smaller number in the CPT-11 and GEM groups. Therefore, we can draw only preliminary conclusions regarding the potential value of CPT-11 in cTACE when compared with GEM and doxorubicin. Secondly, our study was a retrospective analysis with selection bias that may have influenced our findings. Further studies in a larger cohort are undoubtedly necessary to confirm these preliminary findings.
In the future, the combination of CPT-11-cTACE with drug-eluting beads or sorafenib is interesting with a view to performing more research. Sorafenib, a new multi-targeting drug, inhibits components of the Raf signaling pathway, VEGF, PDGF and RTKs, resulting in inhibition of tumor angiogenesis and proliferation. The efficacy and safety of sorafenib in the treatment of advanced HCC has been demonstrated in clinical practice[29] and in a phase III trial. Furthermore, it has been found to prolong survival times in patients with advanced HCC[30,31]. Studies are needed to compare the tumor response, time to progression and overall survival of patients treated with cTACE using sorafenib.
This study demonstrated that cTACE with CPT-11 could prolong the time to progression and overall survival in patients with HCC compared with GEM or doxorubicin. There were no significant differences in hepatic treatment-related toxicities and clinic adverse events. CPT-11 thus appears to be a feasible and promising choice of chemotherapy agent to use with cTACE for the treatment of HCC.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Over 60% to 70% of patients with HCC are diagnosed at a late stage and therefore curative therapies are not appropriate. Transarterial chemoembolization (TACE) is the primary treatment used most frequently for unresectable HCC. However, there is a lack of data to support the use of one chemotherapeutic agent or combination of agents over another. Chemotherapeutic agent irinotecan (CPT-11) (irinotecan), a drug used for the treatment of cancer, prevents DNA unwinding by inhibition of topoisomerase 1. Many studies reported that CPT-11 might be a potential drug for the treatment of HCC and prolong survival time of HCC patients, but the effect has not been evaluated in TACE.
Conventional transarterial chemoembolization (cTACE) is widely accepted as a predominantly palliative approach for patients with HCC when surgical intervention is not appropriate. The rationale for TACE is that a powerful cytotoxic effect combined with ischemia followed by chemoembolization of the hepatic artery will result in therapeutic efficacy and survival benefit compared with supportive care.
Doxorubicin has been widely used as the chemotherapeutic agent of choice in cTACE, but with the development of new chemotherapeutic agents, such as CPT-11, gemcitabine (GEM) and oxaliplatin, comparative studies are needed to find the optimum agent for use in cTACE for the treatment of HCC. Currently, there are limited data available regarding the use of chemotherapeutic agents administered via cTACE in patients with HCC. This study evaluated the efficacy, tumor response, clinical adverse events, time to progression and overall survival benefit of three chemotherapy agents: CPT-11, GEM and doxorubicin.
The study results suggest that the chemotherapeutic agent CPT-11 is significantly associated with improved overall survival and delayed tumor progression compared with GEM or doxorubicin. CPT-11 thus appears to be a promising agent when combined with cTACE for the treatment of HCC.
CPT-11: a semi-synthetic analogue of the natural alkaloid camptothecin and activated by hydrolysis to SN-38, an inhibitor of topoisomerase-1. Inactivation follows by uridine diphosphate glucoronosyltransferase 1A1 glucuronidation. The inhibition of topoisomerase 1 by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.
The authors present the scope and limitations of the study and pointed out that the most important items are the small number of cases and the retrospective character of the study.
P- Reviewer: Frider P, Pan WS S- Editor: Qi Y L- Editor: Logan S E- Editor: Ma S
| 1. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2140] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 723] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 5. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1988] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2273] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 9. | Chen T, Chu ZH, Liu JP, Wang J, Zhao HY, Ou QJ. [Application of adenosine triphosphate tumor chemosensitive assay system to individual chemotherapy for hepatocellular carcinoma]. Aizheng. 2005;24:1018-1022. [PubMed] |
| 10. | Takeba Y, Kumai T, Matsumoto N, Nakaya S, Tsuzuki Y, Yanagida Y, Kobayashi S. Irinotecan activates p53 with its active metabolite, resulting in human hepatocellular carcinoma apoptosis. J Pharmacol Sci. 2007;104:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 12. | Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, Rilling WS, Geschwind JF, Salem R, Vedantham S. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2007;18:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5738] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 14. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 15. | Testa R, Testa E, Giannini E, Botta F, Malfatti F, Chiarbonello B, Fumagalli A, Polegato S, Podesta E, Romagnoli P. Trans-catheter arterial chemoembolisation for hepatocellular carcinoma in patients with viral cirrhosis: role of combined staging systems, Cancer Liver Italian Program (CLIP) and Model for End-stage Liver Disease (MELD), in predicting outcome after treatment. Aliment Pharmacol Ther. 2003;17:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 374] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2877] [Article Influence: 110.7] [Reference Citation Analysis (1)] |
| 18. | Brown DB, Cardella JF, Sacks D, Goldberg SN, Gervais DA, Rajan DK, Vedantham S, Miller DL, Brountzos EN, Grassi CJ. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2009;20:S219-S226, S226.e1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1325] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 20. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3305] [Article Influence: 220.3] [Reference Citation Analysis (36)] |
| 21. | Kim SH, Lee WJ, Lim HK, Lim JH. Prediction of viable tumor in hepatocellular carcinoma treated with transcatheter arterial chemoembolization: usefulness of attenuation value measurement at quadruple-phase helical computed tomography. J Comput Assist Tomogr. 2007;31:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 24. | Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, Kawarada Y, Kusano M, Kubo Y, Kuroda C. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma--a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1992;31 Suppl:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi H, Yamamoto Y, Ichikawa S, Hasebe A, Yano M, Miyamoto Y. Risk factors for death in 224 cases of hepatocellular carcinoma after transcatheter arterial chemoembolization. Hepatogastroenterology. 2009;56:213-217. [PubMed] |
| 26. | Wigmore SJ, Redhead DN, Thomson BN, Parks RW, Garden OJ. Predicting survival in patients with liver cancer considered for transarterial chemoembolization. Eur J Surg Oncol. 2004;30:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Farinati F, De Maria N, Marafin C, Herszènyi L, Del Prato S, Rinaldi M, Perini L, Cardin R, Naccarato R. Unresectable hepatocellular carcinoma in cirrhosis: survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Dig Dis Sci. 1996;41:2332-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Di Costanzo GG, Tortora R, Iodice L, Lanza AG, Lampasi F, Tartaglione MT, Picciotto FP, Mattera S, De Luca M. Safety and effectiveness of sorafenib in patients with hepatocellular carcinoma in clinical practice. Dig Liver Dis. 2012;44:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 31. | Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, Wang D, Gao H. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |