Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10908
Revised: May 9, 2014
Accepted: July 11, 2014
Published online: August 21, 2014
Processing time: 181 Days and 22.7 Hours
AIM: To investigate if loss of epithelial cell adhesion molecule (EpCAM) is associated with microinvasion in hepatocellular carcinomas (HCCs) in the presence of chronic hepatitis B.
METHODS: The expression of EpCAM, cytokeratin 7 (CK7) and CK19 in 112 hepatic nodules was studied, including 20 HCCs with nodules ≤ 3 cm, 26 HCCs with nodules > 3 cm, 20 high-grade dysplastic nodules, 26 cirrhotic, large regenerative nodules and 20 cases of cirrhosis.
RESULTS: Membranes of ductular reaction (DR) hepatobiliary cells, interlobular bile duct and some hepatic cells were positive for EpCAM expression. Active expression of DR/EpCAM was observed in the majority of noninvasive nodules (50/66, 75.76%); however, expression was absent in the major area of invasion in HCCs (42/46, 91.30%). DR/EpCAM loss in HCCs ≤ 3 cm was higher than in high-grade dysplastic nodules (HGDNs) (P < 0.05), cirrhotic, large regenerative nodules and cirrhosis (P < 0.01). Furthermore, patients (20 HCCs ≤ 3 cm, 26 HCCs > 3 cm, 20 HGDNs) with DR/EpCAM expression had a higher overall survival rate (P < 0.01) and lower early recurrence rate (P < 0.01). DR/EpCAM expression showed a close relationship with DR/CK7 and DR/CK19 expression (P < 0.01). The area under the receiver operating characteristic (ROC) curve of DR/EpCAM was similar to that of DR/CK7 and DR/CK19 (P > 0.05). The diagnostic specificity and diagnostic accuracy were both increased when DR/EpCAM, DR/CK7 and DR/CK19 were combined (P < 0.01).
CONCLUSION: DR/EpCAM loss may be a useful marker for determining microinvasion in HCCs ≤ 3 cm, but also for predicting prognosis.
Core tip: Epithelial cell adhesion molecule (EpCAM) may be a new marker of ductular reaction (DR) in routine pathology. We observed the morphological features of DR/EpCAM in 112 small hepatic nodules and compared this with DR/cytokeratin 7 (CK7) and DR/cytokeratin 19 (CK19). The diagnostic value of DR/EpCAM was similar to DR/CK7 and DR/CK19; however, the diagnostic accuracy and specificity increased when these parameters were combined. Therefore, DR/EpCAM loss was confirmed to be a useful marker not only for determining microinvasion in HCCs ≤ 3 cm, but also for predicting prognosis.
- Citation: Zhang Q, Zhang CS, Xin Q, Ma Z, Liu GQ, Liu BB, Wang FM, Gao YT, Du Z. Perinodular ductular reaction/epithelial cell adhesion molecule loss in small hepatic nodules. World J Gastroenterol 2014; 20(31): 10908-10915
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10908
Distinguishing well-differentiated hepatocellular carcinomas (HCCs) from high-grade dysplastic nodules (HGDNs) or cirrhotic, large regenerative nodules (CLRNs) can be difficult in patients with small liver nodules. It has been suggested that invasion is a vital diagnostic feature of HCCs[1]. The appearance of microinvasion, however, particularly for the inexperienced liver pathologist without extensive exposure to resected hepatocellular nodules, may be similar to that of regenerative intraseptal hepatocyte buds. Consequently, new methods to identify invasion, particularly small foci of invasion, are required. Intraseptal hepatocyte buds are contiguous with ductular reactions (DRs) which are indicative of regeneration from intrabiliary progenitors[2,3]. DR is lost in the area of invasion in HCCs, but abundant in the majority of noninvasive nodules. It is hypothesized that DR immunostaining in small liver nodules may be a useful method for differential diagnosis. Recent studies of DR/cytokeratin 7 (CK7)[4] and DR/CK19[5,6], have supported this theory. DR can be expressed as CK7 or CK19; however, the diagnosis of small hepatic nodules remains a dilemma.
Epithelial cell adhesion molecule (EpCAM) is a cell surface protein expressed in normal epithelia, with the exception of squamous epithelia, epidermal keratinocytes, gastric parietal cells, myoepithelial cells, thymic cortical epithelium and hepatocytes[7,8]. Adult hepatocytes are EpCAM negative, with only bile duct epithelium being positive in liver tissue. During the regeneration and repair of liver tissues associated with focal nodular hyperplasia and cirrhosis, activation of EpCAM expression was observed, with high expression levels in so-called “ductular proliferations”[9]. The recent discovery of the expression of EpCAM in hepatocytes in the presence of chronic hepatitis B, indicates that these cells are a novel progeny of the hepatobiliary stem/progenitor cell compartment. Furthermore, transit amplifying DR hepatobiliary cells act as intermediates[4,10]. In other words, EpCAM-positive cells are associated with the differentiation of hepatocyte precursors, which are present in the cirrhotic liver, dysplastic nodules or HCCs as tubular structures[11,12]. Consequently, it was hypothesized that EpCAM staining surrounding neoplastic nodules would not only be a marker for DR, but also a diagnostic method for invasion.
To investigate whether loss of DR/EpCAM is associated with invasion in HCCs in the presence of chronic hepatitis B and whether the expression of DR/EpCAM is superior to that of DR/CK7 and DR/CK19, a series of studies were performed to confirm the diagnostic value of DR/EpCAM.
The study was approved by the ethics committee of The Third Central Hospital of Tianjin Medical University. Written informed consent was obtained from each participant. As mentioned in the 2010 Barcelona Clinic Liver Cancer (BCLC) approach, it is crucial to make a diagnosis as early as possible for liver nodules ≤ 3 cm to achieve higher 5-year survival rates[13]. Small hepatic nodules (≤ 3 cm) diagnosed as HCCs, HGDNs or CLRNs following resection were selected from archival files. The size of the liver nodules was determined during surgical resection and small hepatic nodules were defined as a single tumor or 2 tumors (all < 3 cm). All patients with small hepatic nodules were diagnosed as having chronic hepatitis B, Child-Pugh A liver function and serum α-fetoprotein (AFP) < 400 ng/mL, and were followed up for a minimum of 24 mo. Patients with chronic hepatitis C, alcoholic hepatitis or autoimmune hepatitis and pathologically confirmed cholangiocarcinoma were excluded from the study. The specimens were selected from the Department of Pathology in our hospital. The HCCs with nodules > 3 cm and biopsy-proven cirrhosis (CIR) tissue on splenectomy for hypersplenism due to cirrhosis, were selected as the control groups. In total, 112 cases were assessed during HCC surveillance in hepatitis B virus-associated liver cirrhosis patients from Jan 1, 2005 to Feb 11, 2010, including 20 HCCs with nodules ≤ 3 cm, 26 HCCs with nodules > 3 cm, 20 HGDNs, 26 CLRNs and 20 cases of cirrhosis. Tumor recurrence was followed until patient death, or to the end of the study (Feb 1, 2013) using a serum AFP assay, chest radiography and ultrasound scanning or computed tomography every 3 mo after surgery. When recurrence was strongly suspected, selective hepatic angiography and ultrasound-guided biopsy were conducted for definitive diagnosis.
The pathological features of all small liver nodules were evaluated by two senior pathologists blinded to patient clinical information. The criteria for HCC and HGDN diagnosis were according to the World Health Organisation and International Consensus Group for Hepatocellular Neoplasia guidelines[1,14]. According to the American Association for the Study of Liver Diseases guidelines[15] for the management of HCC, serum AFP, abdominal ultrasound examination and enhanced computed tomography (CT) or magnetic resonance imaging were used to diagnose HCC. Liver cirrhosis was diagnosed based on histological, serological and radiological tests. Small HCCs and HGDNs were accepted. Patients were followed up for a minimum of 24 mo (Table 1).
| Group | Follow-up time (mo) | Tumor-free survival time (mo) | Tumor-free survival rate | Early recurrence | Recurrence rate | Metastasis rate | Mortality rate | |
| 1-yr | 3-yr | |||||||
| HCC > 3 cm | 21.7 | 9.0 | 4/26 | 0/26 | 18/26 | 20/26 | 3/26 | 20/26 |
| HCC ≤ 3 cm | 60.1 | 35.5 | 18/20 | 7/20 | 7/20 | 14/20 | 3/20 | 1/20 |
| HGDN | 41.9 | 35.4 | 20/20 | 10/20 | 3/20 | 3/20 | 0/20 | 0/20 |
All samples were fixed with neutral 4% formaldehyde solution and 4 μm thick continuous sections were obtained for Hematoxylin and eosin (HE) staining, reticular fiber staining and CD34 staining for pathological diagnosis. The categorical diagnostic assignments for each of the hepatic nodules in this study were determined by consensus between 2-3 participating pathologists.
Immunohistochemical staining for EpCAM (VU1D9, Cell Signaling, United States; 1:500), CK7 and CK19 (OV-TL 12/30 and RCK108, Shanghai Biosun Sci & Tech Co., Ltd, Shanghai, China, Ready-to-Use) was performed according to the manufacturers’ instructions. Briefly, 4 μm sections from formalin-fixed, paraffin-embedded tissue blocks were deparaffinized, rehydrated, and treated with 3% hydrogen peroxide for 15 min to inhibit endogenous peroxidase. Following heat-induced epitope retrieval in 0.1 mol/L of citrate buffer at pH 6.0 in a pressure cooker for 20 min, the slides were incubated with a mouse monoclonal antibody specific for each protein for 1 h at room temperature. Only CK7 received trypsinase-induced epitope retrieval. After incubation with a mouse anti-human secondary antibody, a reaction was performed using the EnVision plus detection system that contained biotin-free horseradish peroxidase-labeled polymers (Biosun Sci & Tech Co., Ltd). Staining was visualized using 3,39-diaminobenzidine substrate-chromogen (DAB) solution and counterstained with hematoxylin.
The DR of EpCAM, CK7, and CK19 was semiquantified as follows: the ”-” label represents less than 25% DR positive cells (diffuse loss of DR); the label of “+/-” represents 26%-75% DR positive cells (focal loss of DR) and the label of “+” represents more than 76% DR positive cells (active DR). Cases were evaluated by independent reviewers along with 2 experienced observers.
The percentage of DR/EpCAM focal loss (+/-) and diffuse loss (-) in all HCCs represented the sensitivity, and the percentage of active DR in non-HCCs represented the specificity of immunostaining. The paired χ2 test and Fisher’s exact test were used for group comparisons. Pearson’s correlation coefficient was used to determine the relationship between antibodies and clinical data. The receiver operating characteristic (ROC) curve was plotted for each biomarker. The area under the ROC curve (AUC) was calculated to compare the values of DR/EpCAM, CK7 and CK19 as diagnostic biomarkers. Traditionally, a poorly designed experiment has an AUC of 0.5, whereas a well-designed experiment (one that has zero false positives and zero false negatives) has an AUC of 1.0. A Z test was used to compare between the two groups. A follow-up comparison between the two groups was performed and analyzed with the independent-samples Student’s t test. The tumor-free survival time was measured from the date of resection to the detection of recurrent tumor or the end point of this study. Recurrent tumor within two years after surgery was considered early recurrence. The survival curves were generated by the Kaplan-Meier method and compared by the log rank test. A P value of < 0.05 was considered statistically significant. A u test was used to compare two rates. Statistical analyses were performed using the SPSS software (Version 16.0; SPSS, Inc., Chicago, IL, United States).
Patients with small HCC nodules were classified as early stage BCLC, while patients with nodules > 3 cm were classified as intermediate or advanced BCLC. A total of 112 cases participated in the current study (20 HCCs ≤ 3 cm, 26 HCCs > 3 cm, 20 HGNs, 26 CLRNs and 20 CIRs).
The mean age of the 73 male and 39 female patients was 52.68 years. Patients were followed up for 3 to 90 mo. The follow-up time was shorter than 1 year in patients with HCCs due to mortality. A total of 18 patients died, including 1 who died of causes unrelated to HCC or cirrhosis. The death rate, 1-year and 3-year tumor-free survival rate, were significantly different between patients with HCCs ≤ 3 cm and HCCs > 3 cm (P < 0.01), and the early recurrence rate was also significantly different between the two groups (P < 0.05). Only the recurrence rate was significantly different between patients with HCCs ≤ 3 cm and HGDNs (P < 0.01). Twenty-six cases of CLRN and 20 cases of cirrhosis were followed up for at least 24 mo (24-77 mo, mean 45.59 mo), and no malignancy was observed.
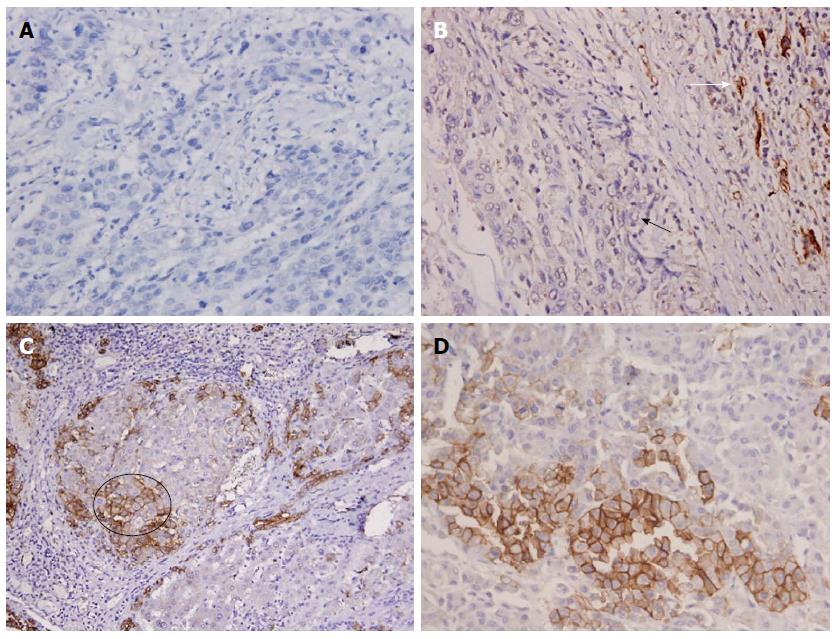
EpCAM-immunoreactive DRs were analyzed at the epithelial-stromal boundaries between the neoplastic tissue and/or paraneoplastic tissue of each nodule. In this study, EpCAM staining was positive on the membrane of hepatic cells and biliary cells. There was diffuse loss of DR/EpCAM in HCCs > 3 cm (Figure 1A). In the HCC ≤ 3 cm group, there were twelve well differentiated HCCs (Figure 2A) and eight moderately differentiated HCCs (Figure 1B). The expression of DR was negative around the HCCs ≤ 3 cm (Figures 1B, 2A, 3), but positive between the dysplastic nodules (Figure 2B, C) and cirrhotic nodules (Figure 1C). A significant number of HCCs were positive for EpCAM (Figure 1D) and some liver cells were EpCAM positive (Figure 1C, circle). These cells showed spotted or focal staining; however, the positive cells were located inside neoplastic cells and not in the boundaries between neoplastic cells.
Diffuse loss or focal loss of DR/EpCAM was evident in most HCCs ≤ 3 cm and in HCCs with nodules > 3 cm (42/46, 91.30%). However, only 8, 5 and 3 cases presented with diffuse loss or focal loss of DR/EpCAM in HGDNs, CLRNs and CIRs, respectively. Therefore, the positive rate of active DR/EpCAM was 75.76% (50/56) in non-invasive hepatic nodules. DR/EpCAM staining was significantly different between HCCs ≤ 3 cm and HGDNs (P < 0.05), HCCs ≤ 3 cm and CLRNs or CIRs (P < 0.01). Specimens from HCCs ≤ 3 cm showed greater DR/CK19 loss than specimens from HGDNs, CLRNs and CIRs (all P < 0.01). DR/CK7 loss in HCCs ≤ 3 cm was less than that in HCCs with nodules > 3 cm ( P < 0.05), and more than CLRNs and CIRs (both P < 0.01). The distribution of DR among the different groups is listed in Table 2.
| Group | DR/EpCAM | DR/CK7 | DR/CK19 | ||||||
| - | +/- | + | - | +/- | + | - | +/- | + | |
| HCC1 | 17 | 8 | 1 | 13 | 11 | 2 | 16 | 9 | 1 |
| HCC2 | 11 | 6 | 3 | 7 | 5 | 8 | 9 | 7 | 4 |
| HGDN | 1 | 7 | 12 | 2 | 5 | 13 | 1 | 5 | 14 |
| CLRN | 0 | 5 | 21 | 0 | 4 | 22 | 0 | 5 | 21 |
| CIR | 0 | 3 | 17 | 0 | 2 | 18 | 0 | 4 | 16 |
Semiquantitative analysis of DR/EpCAM expression showed a significant correlation between DR/CK7 and DR/CK19 (P < 0.01). The sensitivity of DR/EpCAM loss, DR/CK7 loss and DR/CK19 loss in all HCCs (HCCs ≤ 3 cm and HCCs > 3 cm) was 91.30%, 78.26%, and 89.13%, respectively, and the specificity was 75.76%, 80.30%, and 77.27%, respectively. The ROC curve showed that the area under the ROC curves of DR/EpCAM loss (0.864) was similar to DR/CK7 loss (0.727), DR/CK19 loss (0.831) and active GPC3 (0.914) (Z = 1.51, 0.41, and 0.69, respectively; P > 0.05). The diagnostic accuracy of the loss of DR/EpCAM, DR/CK7 and DR/CK19 was 82.14%, 79.46% and 82.14%, respectively, and the negative predictive value (NPV) was 94.20%, 84.13% and 91.07%, respectively. The results were then combined into a new group. If 2 or more results were positive for DR/EpCAM, DR/CK7 and DR/CK19, then the tumor was considered to be DR positive. If two or more results showed focal/diffuse loss of DR/EpCAM, DR/CK7 or DR/CK19, the tumor was considered to be DR negative. Using this method, the sensitivity was 91.30%, but the specificity increased to 98.48%. The specificity of the new group was higher than that for DR/EpCAM, DR/CK7 or DR/CK19 (u = 3.90, 3.73, and 3.39 respectively; P < 0.01). The area under the ROC curves of the combined group was 0.924 and was similar to DR/EpCAM and DR/CK19 (Z = 1.23 and 1.88, respectively; P > 0.05), but significantly higher than DR/CK7 (Z = 3.36, P < 0.01). The diagnostic accuracy of this method was 95.54% and the NPV was 94.20%. The diagnostic accuracy was increased by this method (u = 3.18, 3.64, and 3.18, respectively, P < 0.01), but the NPV was similar to DR/EpCAM and DR/CK19 (u = 0.06, and 0.90, P > 0.05) and higher than DR/CK7 (u = 2.42, P > 0.05).
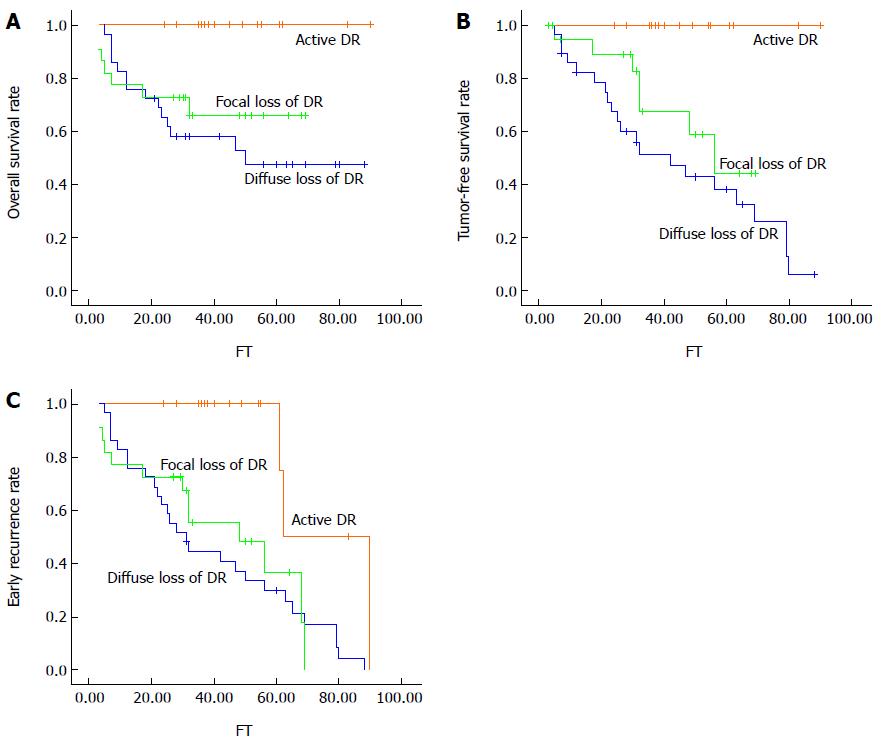
Of 66 patients (20 HCCs ≤ 3 cm, 26 HCCs > 3 cm, 20 HGDNs), 37 (16 DR/EpCAM positive and 21 DR/EpCAM negative) exhibited tumor recurrence during the follow-up period. Thirty-two patients had intrahepatic tumor recurrence only, while 6 had extrahepatic metastasis. Of 16 patients with DR/EpCAM positive nodules, none showed early recurrence. Of 50 patients with DR/EpCAM negative nodules, the overall survival rate and tumor-free survival rate were significantly lower than those with DR/EpCAM positive nodules (overall survival rate: χ2 = 8.285, P = 0.004; tumor-free survival rate: χ2 = 14.400, P = 0.000) (P < 0.01) (Figure 3A, B). The incidence of early recurrence in patients with DR/EpCAM negative nodules was significantly higher than in those with DR/EpCAM positive nodules (χ2 = 10.773, P = 0.001) (P < 0.01) (Figure 3C).
The most proximal branches of the biliary tree (i.e., the canals of Hering and ductules) comprise, or at least harbor, facultative hepatic stem cells[2,3]. These intraseptal hepatocytes most likely represent buds of newly formed hepatocytes arising from branches of the biliary tree[3]. It was previously demonstrated that DR is a sign of newly regenerating hepatocytes in chronic hepatitis B, and therefore did not develop from either biliary metaplasia of malignant hepatocytes, or from the outgrowth of biliary cells[4]. The absence of DR is a useful marker for characterizing the areas of microinvasion in HCCs ≤ 3 cm[4,5], especially for HCCs in the presence of chronic hepatitis B cirrhosis. Based on this understanding, the loss of DR/EpCAM is also helpful in defining microinvasion and distinguishing HCC ≤ 3 cm from other hepatic nodules.
The results from the current study confirm that DR/EpCAM underwent focal or diffuse loss in the majority of invasive hepatocellular nodules (HCCs ≤ 3 cm and HCCs > 3 cm), showing an overtly invasive phenotype. The loss of DR/EpCAM was associated with the areas of morphologically identified microinvasion, whereas noninvasive areas showed abundant DR/EpCAM expression at hepatocellular-stromal boundaries in the same tissue section. In contrast, DR/EpCAM expression was evident in the majority of noninvasive nodules, such as HGDNs, CLRNs and cirrhotic nodules. The degree of DR/EpCAM loss differed between HCCs ≤ 3 cm and HGDNs, indicating that DR/EpCAM may be absent in the small foci of invasive areas around cancerous nodules. The absence of DR/EpCAM in the foci of invasion suggests that immunostaining for these structures may be a useful diagnostic tool and may assist pathologists in identifying the appearance of the histologic lesion. These results are in accord with the study of CK19 expression. Perinodular CK19 loss was consistently observed in HCCs and the altered expression of CK19 in cirrhotic nodules, dysplastic nodules and HCCs was an underlying mechanism for the reproducible extralesional CK19 pattern that paralleled progressive stages of intranodular hepatocarcinogenesis[5]. Results from the current study showed that EpCAM was expressed in both proliferating bile ducts and interlobular bile ducts. A considerable overlap between DR/EpCAM and both DR/CK7 and DR/CK19 was observed. However, when DR/EpCAM was combined with DR/CK7 and DR/CK19, the diagnostic accuracy and diagnostic specificity were significantly increased.
EpCAM was originally identified as a marker of carcinoma, attributable to its high expression in rapidly proliferating tumors of epithelial origin[16]. EpCAM positive HCCs were a subset of cells with cancer stem cell features[12], which was similar to CK19[17]. However, the positive expression of EpCAM and CK19 in tumor cells was low in HCCs[5,12]. Therefore, only the morphological features of EpCAM, CK7 and CK19 positive HCCs were observed, and these cells showed spotted or focal staining in neoplastic cells, but not in the boundaries between neoplastic cells. The morphological features and the distribution of these positive cells were different from the cells of DR. This characteristic of HCCs did not result in an adverse effect on the role of DR as a marker of EpCAM, CK7 and CK19.
Moreover, patients with active DR/EpCAM nodules (in HGDNs, HCCs ≤ 3 cm and HCCs > 3 cm) had a better prognosis, including a higher overall survival rate, 1-year and 3-year tumor-free survival rate, and lower early recurrence rate. Consequently, the loss of DR/EpCAM had a close relationship with invasive HCCs and predicted an increased incidence of recurrence, regardless of HCCs ≤ 3 cm or HCCs > 3 cm.
In conclusion, DR loss is an important feature of the epithelial-stromal compartment in the malignant progression of HCCs with cirrhosis. DR/EpCAM expression may be used as a diagnostic marker. The histological pattern of stromal invasion and altered expression of DR/EpCAM at epithelial-stromal boundaries was determined by DR immunostaining. In particular, the differential diagnosis of HCCs ≤ 3 cm and HGDNs may improve diagnostic confidence in pathologists faced with the spectrum of lesions which occur in small hepatocellular nodules. In addition, the loss of DR/EpCAM is associated with increased invasiveness of HCC and poor prognosis.
The first author of the paper would like to express her sincere thanks to her supervisors, Drs. Gao, Zhang and Du, for their useful advice on preparation of the manuscript, and her colleagues for offering help with pertinent references and information in this paper.
Distinguishing a well-differentiated hepatocellular carcinoma (HCC) from high-grade dysplastic nodules (HGDNs) or cirrhotic, large regenerative nodules can be difficult in patients with small liver nodules. It has been suggested that invasion is a vital diagnostic feature of HCC. However, the appearance of microinvasion, particularly for the inexperienced liver pathologist without extensive exposure to resected hepatocellular nodules, may be similar to that of regenerative intraseptal hepatocyte buds. Thus, new methods to identify invasion, particularly small foci of invasion, are required. These intraseptal hepatocyte buds are contiguous with ductular reactions (DRs) which are indicative of regeneration from intrabiliary progenitors. DR is lost in the area of invasion in HCCs, whilst abundant in the majority of noninvasive nodules. It is hypothesized that DR immunostaining in small liver nodules may be a useful method for differential diagnosis. Recent studies of DR/cytokeratin 7 (CK7) and DR/CK19, have supported this theory. DR can be expressed as CK7 or CK19, however, the diagnosis of small hepatic nodules remains a dilemma.
Epithelial cell adhesion molecule (EpCAM) positive cells are associated with the differentiation of hepatocyte precursors, which are present in the cirrhotic liver, dysplastic nodules or HCCs as tubular structures. Consequently, it was hypothesized that EpCAM staining surrounding the neoplastic nodules would not only be a marker for DR, but also a diagnostic method for invasion recognition.
The authors observed the morphological features of DR/EpCAM in 112 small hepatic nodules and compared these with DR/CK7 and DR/CK19. It has been proved that the diagnostic value of DR/EpCAM was similar to DR/CK7 and DR/CK19, but the diagnostic specificity was increased by the combination of DR/CK7 and DR/CK19. Furthermore, DR/EpCAM loss may predict poor prognosis.
This study provides new knowledge on the differential diagnosis of small liver nodules and may be useful for daily routine work in pathology. The study results suggest that DR/EpCAM loss may be a new useful marker not only for recognizing microinvasion in small HCCs, but also for differentiating HCCs with nodules ≤ 3 cm from HGDNs.
This is a very interesting report. A lot of hard work was done and new information was added to our knowledge. The paper was written very well and provided a set practical procedure for daily routine work. This study can be a guiding method for the differential diagnosis among different liver nodular lesions with their immunohistochemical features.
P- Reviewer: Chiu KW, Fouad YM, Rajeshwari K, Sazci A, Soares RLS, Sonzogni A S- Editor: Nan J L- Editor: O’Neill M E- Editor: Liu XM
| 1. | International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 2. | Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte ‘buds’ in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 522] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 4. | Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, Theise ND, Roncalli M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Bioulac-Sage P, Balabaud C. Perinodular CK19 loss in hepatocarcinogenesis. Clin Res Hepatol Gastroenterol. 2011;35:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lennerz JK, Chapman WC, Brunt EM. Keratin 19 epithelial patterns in cirrhotic stroma parallel hepatocarcinogenesis. Am J Pathol. 2011;179:1015-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Schmelzer E, Reid LM. EpCAM expression in normal, non-pathological tissues. Front Biosci. 2008;13:3096-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Spizzo G, Fong D, Wurm M, Ensinger C, Obrist P, Hofer C, Mazzoleni G, Gastl G, Went P. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol. 2011;64:415-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201-206. [PubMed] |
| 10. | Tanaka M, Okabe M, Suzuki K, Kamiya Y, Tsukahara Y, Saito S, Miyajima A. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: drastic change of EpCAM expression during liver development. Mech Dev. 2009;126:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN, Theise ND. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;53:964-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 958] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 13. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 14. | Theise ND, Curado MP, Franceschi S, Hytiroglou P, Kudo M, Park YN, Sakamoto M, Torbenson M, Wee A. Hepatocellular carcinoma. WHO Classification of Tumours of the Digestive System, 4th ed. Lyon: IARC 2010; 205-216. |
| 15. | Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 16. | Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386-395. [PubMed] |
| 17. | Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |