Published online Aug 21, 2014. doi: 10.3748/wjg.v20.i31.10790
Revised: February 7, 2014
Accepted: April 5, 2014
Published online: August 21, 2014
Processing time: 295 Days and 16.8 Hours
Pancreatic cancer is one of the most aggressive and lethal malignancies. Despite remarkable progress in understanding pancreatic carcinogenesis at the molecular level, as well as progress in new therapeutic approaches, pancreatic cancer remains a disease with a dismal prognosis. Among the mechanisms responsible for drug resistance, the most relevant are changes in individual genes or signaling pathways and the presence of highly resistant cancer stem cells (CSCs). In pancreatic cancer, CSCs represent 0.2%-0.8% of pancreatic cancer cells and are considered to be responsible for tumor growth, invasion, metastasis and recurrence. CSCs have been extensively studied as of late to identify specific surface markers to ensure reliable sorting and for signaling pathways identified to play a pivotal role in CSC self-renewal. Involvement of CSCs in pancreatic cancer pathogenesis has also highlighted these cells as the preferential targets for therapy. The present review is an update of the results in two main fields of research in pancreatic cancer, pathogenesis and therapy, focused on the narrow perspective of CSCs.
Core tip: Pancreatic cancer is one of the most aggressive and lethal malignancies, despite remarkable progress in understanding pancreatic carcinogenesis and new therapeutic approaches. The presence of highly resistant cancer stem cells (CSCs) and the changes in their signaling pathways lead to drug resistance in pancreatic cancer. CSCs are considered responsible for tumor growth, invasion, metastasis and recurrence. CSC involvement in pancreatic cancer pathogenesis has also highlighted them as preferential targets for therapy. This review is an update of the results in two main fields of research in pancreatic cancer, pathogenesis and therapy, focused on the narrow perspective of CSCs.
- Citation: Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R. Cancer stem cells: Involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol 2014; 20(31): 10790-10801
- URL: https://www.wjgnet.com/1007-9327/full/v20/i31/10790.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i31.10790
Pancreatic cancer is one of the most aggressive and lethal malignancies. Despite remarkable progress in understanding pancreatic carcinogenesis at the molecular level as the identification of new therapeutic approaches, pancreatic cancer remains a disease with a dismal prognosis; the five-year survival rate is approximately 5%[1]. Although several histological subtypes of pancreatic cancer have been described, the most common form is pancreatic ductal adenocarcinoma. According to data published by the International Agency for Research on Cancer, pancreatic cancer death is the eighth or ninth most frequent cause of cancer death worldwide and is the fourth or fifth most common cause of cancer death in developed countries[2,3].
The main risk factors for pancreatic cancer include increasing age, smoking[4], chronic pancreatitis, diabetes mellitus, metabolic syndrome, low levels of serum vitamin D, family history of pancreatic cancer and rare inherited genetic conditions such as Peutz-Jeghers syndrome, familial melanoma and hereditary pancreatitis[5]. Age is a significant risk factor; the median age at diagnosis is 72 years. Pancreatic tumors are rarely diagnosed before the age of 50, and such cases are very likely to be associated with underlying predisposing genetic disorders. Approximately 5%-10% of pancreatic cancer patients report a family history of pancreatic cancer. The genes responsible for a minority of the familial clustering of pancreatic cancer have been identified, including STK11, CDKN2A, PRSS1, BRCA2 and PALB2[1,6].
The high mortality rate of pancreatic cancer is due to difficulty in early diagnosis[7,8] and its notorious resistance to chemotherapy and radiation[9]. Lack of clinical symptoms in early stages leads to delay in tumor detection; thus, approximately 80% of patients with pancreatic cancer have metastatic disease at the moment of diagnosis[10]. Existing systemic therapies for advanced disease are far from effective, and the median survival for patients with metastatic disease remains 6 mo. Surgery offers a better prognosis of a cure, but even those patients who undergo resection and receive adjuvant therapy have a median survival of 12-22 mo and a 5-year survival of 20%-25%[2,11].
Chemoresistance is a critical issue in pancreatic cancer. Among mechanisms responsible for drug resistance, the most relevant are changes in individual genes or signaling pathways, the influence exerted by tumor microenvironment (desmoplastic reaction) and the presence of highly resistant cancer stem cells (CSCs)[9]. The notion of CSCs has gained prominence, and several identified molecules and signaling pathways are relevant for the diagnosis and therapy of cancer. The paradigm of cancer-initiating stem cells has initially been developed with respect to blood cancers, where chronic conditions such as myeloproliferative neoplasms are due to mutations acquired in hematopoietic stem cells[12].
Pancreatic cancer (especially pancreatic ductal adenocarcinoma) is an aggressive malignancy, with one of the worst prognoses among solid tumors. Pancreatic cancer is typically diagnosed in late stages, when most patients are inoperable and when curable treatment is not available. Current therapies (radio- and chemotherapy) may improve prognosis and reduce tumor size but cannot target all pancreatic cancer cells[13,14].
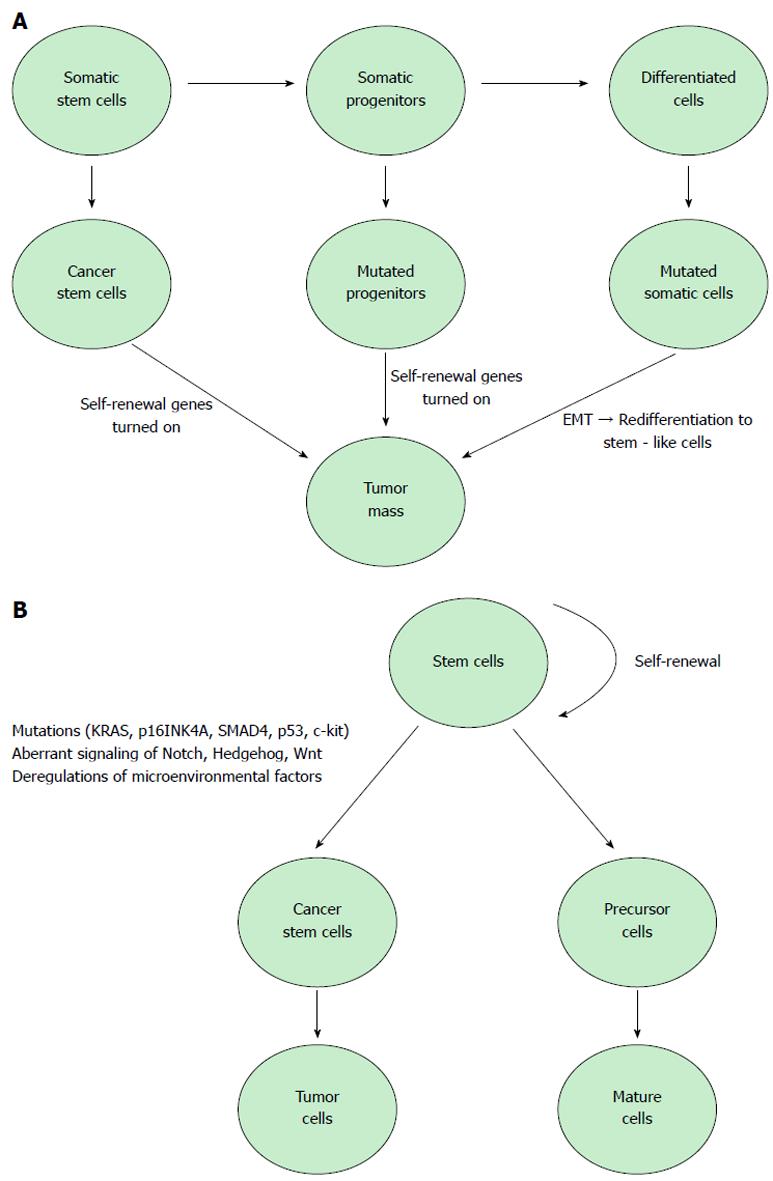
Cancer stem cells, identified in a large number of human malignancies, represent 0.2%-0.8% of pancreatic cancer cells and are considered responsible for tumor growth, invasion, metastasis and recurrence[15]. Currently, there are two models that explain tumor development[16-18]. The stochastic model states that every cancer cell has the ability to initiate and promote tumoral growth. The other model, the “cancer stem cell hypothesis”, proposes that tumor evolution is based on stem cells with a ‘deregulated’ self-renewal pathway. A recent and rapidly growing body of research shows solid evidence in support of the cancer stem cell model against the stochastic model[19,20]. The American Association for Cancer Research defines CSCs as cells within a tumor that have the capacity to generate the heterogeneous cancer cell lineages found in the tumor and that possesses the capacity to self-renew. CSCs also share other several important attributes: active telomerase expression, drug resistance to harming agents, the activation of antiapoptotic pathways, the ability to migrate and to metastasize and increased membrane transporter activity. To date, CSCs have been isolated and characterized only from a relatively small number of tumor types: breast, brain, pancreas, colon, blood and head and neck[21,22]. Several studies argue that cancer stem cells cannot be eradicated by current therapy and thus are responsible for tumor relapse and metastasis[23]. Many studies have demonstrated that multiple critical genes, including K-ras, p53 and p16, and key signaling kinases, such as PI-3K, mTOR, NF-κB, epidermal growth factor receptor (EGFR) and SHH, play important roles in pancreatic tumorigenesis[24].
Several pancreatic cancer stem cell (PCSC) subpopulations have been isolated using flow cytometry and combinations of positive and/or negative membrane surface markers[25-29]. Historically, research on stem cells and cancer stem cells from the hematopoietic system began long before studies in other tissues. As a result, several markers identified in hematopoietic malignancies, such as cluster of differentiation (CDs), were also proposed as potential PCSCs markers. Li et al[30] were the first to identify a population of PCSCs using CD44, CD24 and ESA as separation markers. The cell fraction with the CD44+/CD24+/ESA+ phenotype exhibits several important cancer stem cell characteristics, including a minor population of cells (between 0.2% and 0.8%) that has the potential to form tumors in half of the mice used for transplantation. In vitro studies lend further support to arguments for the use of CD44 and CD24 as cancer stem cell markers. CD44+/CD24- cells isolated from PANC-1, a pancreatic adenocarcinoma cell line, exhibit a much higher tumorigenic potential than cellular subpopulations not expressing the markers[31]. Prominin-1 or CD133 is another important marker used for isolating PCSCs. Hermann et al[32] demonstrated that CD133+ cells form more tumors than CD133- populations. Another important finding of the study is that cells positive for CD133 and for CXCR4 exhibit a higher metastatic potential than other populations from the same tumors, supporting the observation that CXCR4 may be involved in tumor invasion and metastasis. A recent study provided further evidence for the role of CXCR4 in pancreatic cancer, demonstrating that human pancreatic ductal adenocarcinomas contain a side population of cells with CSC properties and high expression levels of CXCR4 and ABCB1[33]. Moreover, these genes correlate with poor patient survival rates. c-Met is a hepatocyte tyrosine kinase growth factor upregulated by CD44[34]. C-Met was also shown to be a PCSC marker[35,36]. Interestingly, cells expressing c-Met have the same tumor-forming potential as CD44+/CD24+/ESA+. Furthermore, CD133+/c-Met-high are less tumorigenic than CD44+/c-Met-high[35]. Aldehyde dehydrogenase 1 is another marker expressed by cancer stem cells. Studies report that ALDH1 can identify PCSCs and protect the tumor pancreatic cells from programmed cell death induced by radiotherapy[35,37]. Other studies demonstrate that pancreatic cancer stem cells are characterized by genetic and epigenetic alterations associated with carcinogenesis and can form xenograft tumors in immunodeficient mice[38,39].
Limitations of the current methods for isolating cancer stem cells from pancreatic cancer include the lack of specific PCSC markers and the needed to understand the molecular mechanisms that regulate the specific biological properties of PCSCs.
Another important line of research focuses on biomarkers that regulate PCSC properties and behavior[40]. Thus, nestin can modulate important characteristics of PCSCs, such as invasion or metastasis, and may represent a viable target for anticancer therapy. A recent study Lu et al[41] reported that Oct 4 and Nanog play important roles in pancreatic cancer by regulating PCSC behavior and suggested that these molecules may represent prognosis markers. Both CD44+/CD24+/ESA+ and pancreatic tumor CD133+ subpopulations are characterized by the overexpression of Nanog, Oct4, Notch1, MDR1 and ABCG2 and are capable of metastasizing to distant sites, such as the liver[33,42]. Moreover, inhibiting their expression impairs PCSC characteristics. Other reports demonstrate that markers such as DCLK1 can discriminate between normal and tumoral stem cells and that knockdown of DCLK1 decreases molecular pathways that control pancreatic tumorigenesis. Another important regulator of stem-like characteristics in PCSCs is SOX2, which controls cellular proliferation and differentiation[43]. C-kit with KRAS were also proven to modulate the progression of pancreatic adenocarcinoma, supporting the assumption that the use of drugs that downregulate the activity of these markers can improve the prognostic of the disease[44].
One of the main causes of high mortality in this pathology is the resistance to chemotherapy, which is also believed to be mediated by cancer stem cells within the tumor mass[45,46]. In 2013, Lu et al[41] demonstrated that in the pancreatic cancer cell line PANC-1, the highly expressed stem cell markers Oct4 and Nanog are associated with chemoresistance, proliferation, migration, invasion, and tumorigenesis in vitro and in vivo. This study also indicated the potential use of these two transcription factors as prognostic markers and targeted therapies in pancreatic cancer. Another study in a murine model reported that the ALDH+ and CD44+CD24+ cell populations are resistant to treatment with gemcitabine, one of the main chemotherapeutic agents[47].
Shah et al[48] has developed a gemcitabine-resistant cell line that exhibits higher expression of the pancreatic CSC markers CD44, CD24, and c-Met, which are also associated with epithelial-mesenchymal transition (EMT).
Aldehyde dehydrogenase (ALDH), considered to be a marker for cancer stem cells, is a detoxification enzyme with increased activity in many cancer types where its presence has been associated with decreased survival[49]. An in vitro study revealed that ALDH expression is correlated with the invasiveness of pancreatic cancer cell lines and that patients with ALDH-positive tumors have poor prognosis[49].
It is unclear what the initial molecular events underlying the conversion of tissue stem cells to cancer stem cells in pancreatic cancer; some studies suggest that appearance of c-kit and KRAS mutations might be the primary events in the initial stages of this disease and have proposed c-kit as a potential therapeutic target[44]. Almost all pancreatic cancers are characterized by activating mutations in KRAS and the loss of p16INK4A, but these cancers are also characterized by mutations in the tumor suppressors SMAD4 and p53. More studies suggest the involvement of these genetic alterations in the development of cancer stem cell properties and surface marker profiles.
Another theory suggests that EMT is responsible for the appearance of cells with stem cell-like properties that are characterized by the activation of many pathways involved in EMT[27]. EMT is a crucial process for tumor progression, involving the transformation of epithelial characteristics into mesenchymal characteristics, which subsequently allow cancer cells to disseminate from the tumor mass[50].
Several signaling pathways are altered in CSCs and EMT-like cells in pancreatic cancer: Hedgehog, Notch, Wnt, AKT and NF-κB (Figure 1). Hedgehog, Notch and Wnt have been shown to be of particular importance in pancreatic cancer stem cells, due to their role in pancreatic embryonic development and differentiation[51]. These signaling pathways play important roles in the self-renewal of CSCs, tumor growth, invasion, metastasis and resistance to therapy[27]. MiRNAs were recently considered to play a role in the regulation of CSCs[15].
Notch signaling is involved in cell proliferation, survival, apoptosis and the differentiation of pancreatic cells and can promote EMT by controlling some transcription factors and growth factors like Snail, Slug, and TGF-β. Some of the Notch target genes are Akt, cyclin D1, c-myc, COX-2 (cyclooxygenase-2), ERK (extracellular signal-regulated kinase), MMP-9 (matrix metalloproteinase-9), mTOR (mammalian target of rapamycin), NF-κB ( nuclear factor-kappa B), VEGF (vascular endothelial growth factor), p21cip1, p27kip1, and p53, all involved in the development and progression of human cancer. Gemcitabine-resistant pancreatic cancer cells exhibit overexpression of Notch-2 and Jagged-1, whereas Notch1, a key downstream mediator of KRAS, is responsible for pancreatic sphere formation[15,28,52]. Many studies found that pancreatic cancer stem cells resistance to chemotherapy is linked to activated Notch signaling, but the exact mechanism remains unclear[9,53]. There is more evidence detailing that the Notch signaling pathway is essential in supporting the ability of KRAS to transform normal cells into tumor stem cells. In this regard, in pancreatic cancer treatment, Notch signaling inhibition can be more attractive, as long as there are no data arguing that Notch signaling has a critical role in normal adult pancreas homeostasis[54].
Hedgehog signaling is another self-renewal pathway allowing normal stem cells to become independent of control signals; as a result of mutations in this signaling, transformed cells can use Hedgehog for tumor initiation, progression and metastasis. In vivo studies revealed that compared with normal pancreatic epithelial cells, CD44+CD24+ESA+ pancreatic cancer stem cells exhibit up-regulation of Shh transcripts, the ligand of Hedgehog signaling[55]. Moreover, 70% of pancreatic cancer tissue exhibits overexpression of Shh, suggesting that Hedgehog signaling may be involved in pancreatic carcinogenesis[15]. Studies in the pancreatic cancer cell line PANC-1 demonstrated that the inhibition of Hedgehog signaling by SMO suppression can reverse EMT, induce apoptosis via PI3K/AKT inhibition and inhibit the invasion of pancreatic cancer cells[56]. Moreover, a combination of focal irradiation with Hedgehog signaling inhibition reduces lymph node metastasis in an orthotopic animal model[57].
Wnt/β-catenin signaling is involved in cell proliferation, migration, apoptosis, differentiation, and stem cell self-renewal in several types of cancers[58]. Wnt/β-catenin signaling pathway dysregulation is also associated with chemoresistance in pancreatic cancer, and recent studies suggest that nuclear β-catenin is essential for EMT[50,59]. In vitro and in vivo studies suggest that activated β-catenin may decrease the differentiation of epidermal stem cells, increase self-renewal capacity and promote epithelial cancers in transgenic mice[60].
In 2013, Sun et al[38] reported that one of the most activated signaling pathways in pancreatic cancer stem cells is NF-κB, whose inhibition leads to loss of stem cell properties. This study also indicated that aberrant epigenetic processes, like CpG promoter methylation, can be involved in carcinogenesis mediated by cancer stem cells.
Cancer stem cells from epithelial tissues were identified for the first time in breast cancer in 2003, when Al-Hajj et al[61] reported that a distinct population CD44+CD24-/low ESA+ develops tumors in immunodeficient mice. In pancreatic cancer, the presence of cancer stem cells was reported in 2007 by Shah et al[48] who showed that CD44+CD24+ESA+ cells exhibit high tumorigenic potential.
As often found in many cancers, expression of miRNAs appears to be dysregulated in pancreatic cancer. The miRNA complement of cancer cells appears different than that in normal tissue.
MiRNAs are potent regulators of cell function via their role as translational regulators for the synthesis of key proteins. Most often, several miRNAs exhibit different expression profiles in cancer cells.
MiR-21, miR-155 and miR-17-5p appear upregulated in tumoral cells, and these miRs are often called oncogenic miRNAs[62,63]. Similarly, a series of miRNAs, referred to as tumor suppressor miRs miR-34, miR-15a, miR-16-1 and let-7), are downregulated in cancers[64,65].
Key cell differentiation programs during development are controlled by the members of the let-7 and miR-200 families. In cancer, the loss of let-7 leads to disease progression and de-differentiation. The same let-7 family appears as a regulator of EMT and of stem cell maintenance. The EMT process is regulated by miRNA-dependent mechanisms. In human pancreatic cancer, DCLK1 regulates EMT by a mechanism dependent on miR-200a[66,67].
According to Haselmann et al[65], the inhibition of the maturation of let-7 by nuclear TRAILR2 in pancreatic cancer cell lines increases their proliferation. This result is consistent with high levels of nuclear TRAIL2 in tissue samples from poor outcome patients.
The population of BxPC-3-LN cells (lymph node metastatic pancreatic cells) contains a 5-fold increased population of CD133+/CXCR4+ cells (stem cell-like cells) compared with the parental (non-metastatic) BxPC-3 cells. Remarkably, a different miRNA pattern is exhibited in CSC-like cells compared with the non-CSC-like cells: up-regulated miR-572, miR-206, miR-449a, miR-489 and miR184 were observed in conjunction with downregulated let-7g-3p, let-7i-3p, let-7a-3p, miR-107, miR-128 and miR-141-5p[68].
The miR-200 family members have been identified as key regulators of cell maintenance and EMT. Tumor progression may represent progressive de-differentiation (EMT) towards a cell type having a stem cell-like phenotype. This process appears to be regulated by miRNA-dependent mechanisms. DCLK1 (a putative marker for pancreatic and intestinal cancer stem cells) regulates EMT in human pancreatic cancer cells via a miR-200a-dependent mechanism[69]; DCLK1 also acts as a regulator of let-7a in pancreatic and colorectal cancer cells, supporting the concept that these miRNAs may be novel and relevant targets in solid tumor cancers[63,70]. Sureban et al[23] demonstrated that DCLK1 inhibition results in the up-regulation of miRNAs that negatively regulate some key angiogenic and pluripotency factors. In AsPC1 tumor xenografts, the downregulation of c-MYC and KRAS via let-7a was observed in a mechanism similar to that demonstrated in pancreatic cancer cells.
The repression of two tumor-suppressor miRs, miR-143 and miR-145, is reported in pancreatic cancer as well in other cancers[71]; moreover, experimental restoration of miR 143/145 levels using nano-vector delivery was demonstrated to inhibit pancreatic cancer cell growth[72]. The miR-143/145 cluster cooperates and inhibits the expression of KRAS2 and RREB1, its downstream effector[71]. MiR-145 was demonstrated to inhibit cell proliferation in lung adenocarcinoma by targeting EGFR. In many cancers, including pancreatic cancer, EGFR is upregulated[73], whereas inhibition of EGF signaling inhibits cancer initiation and progression[74]. Furthermore, a suppressive effect of EGFR on miR-143 and miR-145 was demonstrated in models of colon cancer[75]. These findings are indicators of a negative feedback loop between EGFR and miR-143/145, which is similar to KRAS/RREB1-miR-143/145.
The major role of VEGF signaling via its receptors, VEGFR1 and VEGFR2, was demonstrated in tumor vascular growth, angiogenesis, and metastasis, and upregulated angiogenic factors in various cancers (colorectal, breast, renal, liver, and ovarian) have been correlated with poor prognosis. PDAC exhibits endothelial cell proliferation, a mechanisms that increases angiogenesis. Inhibition of VEGF-A, VEGFR1 and VEGFR2 resulted in the inhibition of tumor growth and angiogenesis in mouse models of PDAC. Studies and computational analysis outlined a putative binding site for miR-200 (miR-200a, b and c) in the 3’ UTR of VEGFR1 and VEGFR2[76].
More studies suggest that stem cells convert to cancer stem cells by the deregulation of miRNA expression, which affect several signaling pathways involved in proliferation, apoptosis, and more importantly, renewal and differentiation of stem cells[77,78]. Nanog and Sox2, important regulators of stem cell pluripotency, and the CD44 stem cell surface marker are examples of these miRNAs targets[79].
Using microarray analysis, Jung et al[70] demonstrated that pancreatic cancer stem cells exhibit differential expression of miR-99a, miR-100, miR-125b, miR-192, and miR-429 compared with controls. An in vitro study conducted on the human pancreatic cancer cell lines AsPC-1, AsPC-1-GTR, MiaPaCa-2, and MiaPaCa-2-GTR revealed re-expression of miRNAs (let-7a, let-7b, miR-26a, miR-101, miR-200b, and miR-200c) that are normally lost in pancreatic cancer and especially in pancreatic spheres can revert or destroy CSCs[80]. Another study reports the loss of miR-34 in CD44+CD133+ MiaPaCa2 pancreatic cancer cells, whereas miR-34 restoration led to the inhibition of a side cell population of tumor cell sphere growth and of tumor formation[64].
Wellner et al[71] demonstrated that miR-200c, miR-203 and miR-183 activity can lead to the downregulation of stem cell factors, founding a regulatory feedback loop between miRNAs and CSC in pancreatic cancer.
In this regard, an understanding of miRNAs alterations can lead to the development of better strategies in the treatment of pancreatic cancer patients by the elimination of CSCs.
The identification of dysregulated miR expression and the existence of regulatory loops between miRs and protein regulators of key processes (such as cell growth, angiogenesis, differentiation) suggest the need and potential effectiveness of strategies aiming to restore the “normal phenotype” expression pattern of miRs for cancer treatment. Various approaches have been developed and investigated, such as the delivery of tumor suppressor miRs[81,82], the suppression of expression or action of oncomirs[83,84], targeting the expression of key regulators (such as DCLK1, AMPKα1)[23,85] leading to miR modulation or the simultaneous modulation of multiple miRs, suggesting that using miRs as therapeutic agents or addressing miRNAs as targets represents a potential solution for the therapy of critical cancers.
In pancreatic cancer, surgery is usually accompanied by other complementary treatments such as multi-chemotherapy regimens and radiotherapy. Despite clear progress in the detection and treatment of cancer, current strategies fail to completely remove the tumor and prevent recurrence and metastasis. Existing therapies are toxic and non-specific, being directed towards both normal cells and tumor cells. Most chemotherapeutic regimens are based on gemcitabine but provide a modest improvement in median survival. The response rate was increased by using more than two chemotherapeutic agents[86]. Therapy failure for highly malignant tumors has been explained, at least partially, by the chemo-[87,88] and radioresistant[89] nature of CSCs. Furthermore, studies have demonstrated that gemcitabine regimens, by targeting differentiated cancer cells, lead to a relative enrichment of cancer stem cells[47].
The resistance of CSCs has been explained by several mechanisms: (1) expression of multidrug resistance-linked genes, largely ATP-binding cassette (ABC) drug transporters[90]; (2) activation of Wnt/β-catenin signaling[91]; and (3) activation of Hedgehog pathway[92]. Hence, a series of strategies preferentially target CSCs.
TGFβ-related inhibition abrogated the self-renewal capacity of CSCs and in vivo tumorigenicity and reversed the resistance of orthotopically engrafted cancer stem cells to gemcitabine. The study demonstrated that the tumor response is, however, limited by the stromal hindering of drug delivery. The addition of a stroma-targeting hedgehog pathway inhibitor enhanced the delivery of the Nodal/Activin inhibitor and translated into long-term, progression-free survival[93].
The Hedgehog signaling pathway is usually targeted in experimental designs as an adjuvant to classic chemotherapy. The combined blockade of sonic hedgehog and mTOR signaling together with gemcitabine is capable of eliminating pancreatic CSCs[94]. Inhibition of Smoothened combined with gemcitabine prolonged survival in mice transplanted with pancreatic tumors. Importantly, however, only in mice treated with triple therapy (with mTOR inhibitor rapamycin added) were cancer stem cells virtually completely abrogated, and the authors reported long-term disease stabilization or regression and subsequent long-term survival[95].
Targeting stemness genes (Sox2, Oct4 and c-Myc) through a complex decoy oligonucleotide designed to simultaneously target all three genes was shown to suppresse CSC properties and phenotypes and minimized the tumorigenic capability of the SP cells and the resistance to chemotherapy[42].
Several studies have targeted the Notch pathway using selective γ-secretase inhibitors. In pancreatic cancer xenografts, PF-03084014, a selective γ-secretase inhibitor, alone and in combination with gemcitabine, inhibited the cleavage of the nuclear Notch 1 intracellular domain and Notch targets Hes-1 and Hey-1 and induced tumor regression in 3 of 4 subcutaneously implanted xenograft models. The authors argue that the observed effects are due to PF-03084014 targeting putative aggressvive cancer stem cells[54]. Another potent and selective γ-secretase inhibitor, MRK-003, also led to the downregulation of the nuclear Notch1 intracellular domain, the inhibition of anchorage-independent growth, and a reduction in the number of cells capable of extensive self-renewal. Pretreatment of a pancreatic adenocarcinoma cell line with MRK-003 significantly inhibited the subsequent engraftment in immunocompromised mice, and the mixed regimen MRK-003 and gemcitabine in engrafted mice reduced tumor cell proliferation and induced both apoptosis and intratumoral necrosis[96].
Due to their involvement in cell proliferation, receptor tyrosin-kinases are frequently dysregulated in cancers and have been recently therapeutically targeted by small molecule inhibitors. There are reports of pancreatic cancer trials testing both kinase inhibitors and monoclonal antibodies. Sunitinib targets multiple receptor tyrosine kinases, including stem cell factor receptor (c-KIT) and has been shown to possess antitumor efficacy in in vivo. The combination of gemcitabine with sunitinib could not surpass the effects of single-agent sunitinib[97]. Cabozantinib, a small kinase inhibitor that targets c-Met and VEGFR2, inhibited viability and spheroid formation and induced apoptosis in pancreatic malignant cells with minor effects in non-malignant cells. In primary, CSC-enriched spheroidal cultures, cabozantinib downregulated the CSC markers SOX2, c-Met and CD133 and induced apoptosis[98].
Tumor-necrosis factor family members have also been targeted as possible anticancer therapies through monoclonal antibodies. A combination of tigatuzumab, a fully humanized death receptor 5 agonist monoclonal antibody, with gemcitabine proved to be more efficacious in killing both CSCs and adenocarcinoma cells. The combination therapy produced a remarkable reduction in pancreatic CSCs, tumor remissions, and significant improvements in the time to tumor progression[99].
Cell cycle regulators represent another class of molecules with the potential to be used as targets in anticancer therapies. Thus, inhibiting checkpoint kinase 1 (Chk1), together with gemcitabine was shown to decrease the capacity of PCSCs to initiate tumors. Another interesting finding was that DNA damage mediated by Chk1 was lower in non-stem cells than in stem cells[100].
Given the failure of cytotoxic therapies, new therapy approaches are under investigation. Vaccination therapy aims to increase the patient’s immune response against tumor cells by targeting cancer markers with the aid of specialized antigen-presenting cells such as dendritic cells. Currently, there is a number of vaccines for human pancreatic cancer in clinical trials including the following: (1) whole-cell vaccines; (2) combined dendritic cells with antigen to present to patient leukocytes; (3) peptide and DNA vaccines, iv) Ras peptide vaccine; (4) vaccine against common cancer mutations targetable by CD4/8 T cells; (5) telomerase peptide vaccine; (6) CEA and Mucin 1; and (7) survivin-targeted vaccine[101].
Furthermore, boosting the immune response with additional treatment with dendritic cells (LANEX-DC®) was shown to be highly effective and to extend the median survival times up to 8.9 mo[102].
A rather recent and innovative approach in immunotherapy is personalized peptide vaccination (PPV), in which HLA-matched peptides are selected and administered, based on the pre-existing host immunity before vaccination[103]. PPV is now under investigation for pancreatic adenocarcinoma, and a phase II study for 41 chemotherapy-resistant advanced pancreatic cancer patients has been reported. Vaccine antigens were selected and administered based on the pre-existing IgG responses to 31 different pooled peptides, and no vaccine-related severe adverse events were observed[104].
Salinomycin is a antiprotozoal agent that was recently proven to preferentially kill breast CSCs[105] and was later investigated in other types of malignancies (reviewed in[106]). In an in vitro model of pancreatic adenocarcinoma, salinomycin inhibited the growth of CSCs, and in vivo xenografting studies demonstrated that salinomycin combined with gemcitabine could eliminate the engraftment of human pancreatic cancer more effectively than the individual agents[107]. The mechanisms proposed for the anti-tumor activity of salinomycin include the following: (1) inhibition of Wnt/β-catenin signaling[108]; (2) induction of apoptosis and autophagy via AMPK activation[108]; (3) increased DNA breakage and phosphorylated levels of p53 and H2AX[109]; and (4) cell cycle arrest and apoptosis via downregulation or inactivation of cell cycle-associated oncogenes, such as Stat3, cyclin D1, and Skp2[110]. Adamantyl-substituted retinoid-related molecules (ARRs) inhibit growth and induce apoptosis in the pancreatic stem-like cell population, possibly through decreased IGF-1R and β-catenin expression[111].
Isothiocyanate sulforaphane (SF) was used as sensitizer of pancreatic CSCs to TRAIL (tumor necrosis factor-related apoptosis inducing ligand)-induced apoptosis by quercetin and sorafenib. Combination of SF with a cytotoxic drug efficiently induced apoptosis along with the inhibition of self-renewing potential, ALDH1 activity, clonogenicity, xenograft growth and relapse of GEM-treated tumor cells in nude mice[112].
The flavonoid Quercetin enhances TRAIL-mediated apoptosis, acts as a chemosensitizer for the ABC pump-proteins and can enhance the effects of sulforaphane in inhibiting pancreatic CSC characteristics[113].
Targeted therapeutic delivery is a way to ensure that drugs reach the designated target at the highest concentration within safety limits. Targeted delivery relies on nanoparticles [small metallic or non-metallic molecules, (such as polymeric, carbonic, sillica-for a detalied review please see[114])]. Most nanoparticles accumulate in tumors due to their intense and leaky neovascularization, but some can be retained in the tumors with the use of cancer-specific antigens[115]. In the same manner that nanoparticles are targeted for the bulk tumor, nanoparticles can be targeted for CSCs by CD-133, for example. To increase delivery into the cytosol and prevent early lysosomal degradation, Bostad et al[116] have employed photochemical internalization (PCI), a minimally invasive method for light-controlled, specific delivery of membrane-impermeable macromolecules to increase the cytotoxic effect of an immunotoxin targeting CD133-expressing cancer cells of colon (WiDr and HCT116) and pancreas (BxPC-3) origin.
Pancreatic cancer remains one of the major causes of cancer death with low survival rates due to the metastasis of early-stage tumors and the lack of any effective treatment. Discoveries made in recent years clearly demonstrate that stem cells and EMT-type cells are involved in pancreatic cancer and are responsible for chemoresistance and the metastatic potential of this tumor type. The emergence of cancer stem cells is based on genetic alterations and modifications in signaling pathways that result in the transformation of normal stem cells, progenitors or differentiated cells. Currently, cancer stem cell inhibitors in combination with conventional therapy are being tested in clinical trials and could provide an innovative approach for the treatment of pancreatic cancer.
The authors would like to thank Alina Nita for technical assistance and Irina Radu for technical and linguistic assistance.
P- Reviewer: Cho CH, Song GB, Vincenzo C S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Pandol S, Gukovskaya A, Edderkaoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27 Suppl 2:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis. 2010;28:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 4. | Leenders M, Chuang SC, Dahm CC, Overvad K, Ueland PM, Midttun O, Vollset SE, Tjønneland A, Halkjaer J, Jenab M. Plasma cotinine levels and pancreatic cancer in the EPIC cohort study. Int J Cancer. 2012;131:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Greenhalf W, Malats N, Nilsson M, Bartsch D, Neoptolemos J. International registries of families at high risk of pancreatic cancer. Pancreatology. 2008;8:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008;8:110-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Tanase CP, Neagu M, Albulescu R, Hinescu ME. Advances in pancreatic cancer detection. Adv Clin Chem. 2010;51:145-180. [PubMed] |
| 8. | Dima SO, Tanase C, Albulescu R, Herlea V, Chivu-Economescu M, Purnichescu-Purtan R, Dumitrascu T, Duda DG, Popescu I. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15:817-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Bhat K, Wang F, Ma Q, Li Q, Mallik S, Hsieh TC, Wu E. Advances in biomarker research for pancreatic cancer. Curr Pharm Des. 2012;18:2439-2451. [PubMed] |
| 11. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 681] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 12. | Cruceru ML, Neagu M, Demoulin JB, Constantinescu SN. Therapy targets in glioblastoma and cancer stem cells: lessons from haematopoietic neoplasms. J Cell Mol Med. 2013;17:1218-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Mizuno N, Yatabe Y, Hara K, Hijioka S, Imaoka H, Shimizu Y, Ko SB, Yamao K. Cytoplasmic expression of LGR5 in pancreatic adenocarcinoma. Front Physiol. 2013;4:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Matsuda Y, Kure S, Ishiwata T. Nestin and other putative cancer stem cell markers in pancreatic cancer. Med Mol Morphol. 2012;45:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013;338:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 17. | Rasheed ZA, Kowalski J, Smith BD, Matsui W. Concise review: Emerging concepts in clinical targeting of cancer stem cells. Stem Cells. 2011;29:883-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | La Porta CA. Thoughts about cancer stem cells in solid tumors. World J Stem Cells. 2012;4:17-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 526] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 20. | Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 21. | Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin Cancer Biol. 2010;20:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 23. | Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013;8:e73940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Ma J, Xia J, Miele L, Sarkar FH, Wang Z. Notch Signaling Pathway in Pancreatic Cancer Progression. Pancreat Disord Ther. 2013;3:pii: 1000114. [PubMed] |
| 25. | Goggins M. Markers of pancreatic cancer: working toward early detection. Clin Cancer Res. 2011;17:635-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Castellanos JA, Merchant NB, Nagathihalli NS. Emerging targets in pancreatic cancer: epithelial-mesenchymal transition and cancer stem cells. Onco Targets Ther. 2013;6:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Kong B, Michalski CW, Kleeff J. Tumor initiating cells in pancreatic cancer: A critical view. World J Stem Cells. 2009;1:8-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2430] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 31. | Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol. 2008;14:3903-3907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2141] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 33. | Van den Broeck A, Vankelecom H, Van Delm W, Gremeaux L, Wouters J, Allemeersch J, Govaere O, Roskams T, Topal B. Human pancreatic cancer contains a side population expressing cancer stem cell-associated and prognostic genes. PLoS One. 2013;8:e73968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | van der Voort R, Taher TE, Wielenga VJ, Spaargaren M, Prevo R, Smit L, David G, Hartmann G, Gherardi E, Pals ST. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem. 1999;274:6499-6506. [PubMed] |
| 35. | Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Herreros-Villanueva M, Zubia-Olascoaga A, Bujanda L. c-Met in pancreatic cancer stem cells: therapeutic implications. World J Gastroenterol. 2012;18:5321-5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Duong HQ, Hwang JS, Kim HJ, Kang HJ, Seong YS, Bae I. Aldehyde dehydrogenase 1A1 confers intrinsic and acquired resistance to gemcitabine in human pancreatic adenocarcinoma MIA PaCa-2 cells. Int J Oncol. 2012;41:855-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Sun L, Mathews LA, Cabarcas SM, Zhang X, Yang A, Zhang Y, Young MR, Klarmann KD, Keller JR, Farrar WL. Epigenetic regulation of SOX9 by the NF-κB signaling pathway in pancreatic cancer stem cells. Stem Cells. 2013;31:1454-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Habib M, Saif MW. Pancreatic cancer stem cells: their role in pancreatic cancer patient outcomes and what is future? JOP. 2013;14:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Xu L. Cancer stem cell in the progression and therapy of pancreatic cancer. Front Biosci (Landmark Ed). 2013;18:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, Lu J, Fan X, Zhu S, Wang Y. Knockdown of Oct4 and Nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 2013;340:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Wang X, Liu Q, Hou B, Zhang W, Yan M, Jia H, Li H, Yan D, Zheng F, Ding W. Concomitant targeting of multiple key transcription factors effectively disrupts cancer stem cells enriched in side population of human pancreatic cancer cells. PLoS One. 2013;8:e73942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, de Narvajas AA, Gomez TS, Simeone DM, Bujanda L. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 44. | Amsterdam A, Raanan C, Polin N, Melzer E, Givol D, Schreiber L. Modulation of c-kit expression in pancreatic adenocarcinoma: a novel stem cell marker responsible for the progression of the disease. Acta Histochem. 2014;116:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Van den Broeck A, Gremeaux L, Topal B, Vankelecom H. Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC Cancer. 2012;12:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Rasheed ZA, Matsui W. Biological and clinical relevance of stem cells in pancreatic adenocarcinoma. J Gastroenterol Hepatol. 2012;27 Suppl 2:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629-3637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Jia J, Parikh H, Xiao W, Hoskins JW, Pflicke H, Liu X, Collins I, Zhou W, Wang Z, Powell J. An integrated transcriptome and epigenome analysis identifies a novel candidate gene for pancreatic cancer. BMC Med Genomics. 2013;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15:873-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 52. | Wang Z, Ahmad A, Li Y, Azmi AS, Miele L, Sarkar FH. Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy. Anticancer Res. 2011;31:1105-1113. [PubMed] |
| 53. | Güngör C, Hofmann BT, Wolters-Eisfeld G, Bockhorn M. Pancreatic cancer. Br J Pharmacol. 2014;171:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Rangwala F, Omenetti A, Diehl AM. Cancer stem cells: repair gone awry? J Oncol. 2011;2011:465343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Hao K, Tian XD, Qin CF, Xie XH, Yang YM. Hedgehog signaling pathway regulates human pancreatic cancer cell proliferation and metastasis. Oncol Rep. 2013;29:1124-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43:437-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 59. | Cui J, Jiang W, Wang S, Wang L, Xie K. Role of Wnt/β-catenin signaling in drug resistance of pancreatic cancer. Curr Pharm Des. 2012;18:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 311] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 61. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7730] [Article Influence: 351.4] [Reference Citation Analysis (0)] |
| 62. | Albulescu R, Neagu M, Albulescu L, Tanase C. Tissular and soluble miRNAs for diagnostic and therapy improvement in digestive tract cancers. Expert Rev Mol Diagn. 2011;11:101-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 64. | Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 65. | Haselmann V, Kurz A, Bertsch U, Hübner S, Olempska-Müller M, Fritsch J, Häsler R, Pickl A, Fritsche H, Annewanter F. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology. 2014;146:278-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 66. | Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 67. | Lan CW, Chen MJ, Jan PS, Chen HF, Ho HN. Differentiation of human embryonic stem cells into functional ovarian granulosa-like cells. J Clin Endocrinol Metab. 2013;98:3713-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Luo G, Long J, Cui X, Xiao Z, Liu Z, Shi S, Liu L, Liu C, Xu J, Li M. Highly lymphatic metastatic pancreatic cancer cells possess stem cell-like properties. Int J Oncol. 2013;42:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 70. | Jung DE, Wen J, Oh T, Song SY. Differentially expressed microRNAs in pancreatic cancer stem cells. Pancreas. 2011;40:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1352] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 72. | Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470-1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 73. | Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-Bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin Cancer Res. 2012;18:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 74. | Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol Pharm. 2011;8:2310-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Zhu H, Dougherty U, Robinson V, Mustafi R, Pekow J, Kupfer S, Li YC, Hart J, Goss K, Fichera A. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res. 2011;9:960-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | Choi YC, Yoon S, Jeong Y, Yoon J, Baek K. Regulation of vascular endothelial growth factor signaling by miR-200b. Mol Cells. 2011;32:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | Garg M. MicroRNAs, stem cells and cancer stem cells. World J Stem Cells. 2012;4:62-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Navarro A, Monzo M. MicroRNAs in human embryonic and cancer stem cells. Yonsei Med J. 2010;51:622-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Ahmed A, Ali S, Philip PA, Sarkar FA. The role of cancer stem cells and micrornas in the development and progression of pancreatic cancer. J Stem Cell Res Ther. 2012;2:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila). 2012;5:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 81. | Biray Avcı Ç, Özcan İ, Balcı T, Özer Ö, Gündüz C. Design of polyethylene glycol-polyethylenimine nanocomplexes as non-viral carriers: mir-150 delivery to chronic myeloid leukemia cells. Cell Biol Int. 2013;37:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Cho WC. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin Ther Targets. 2012;16:747-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 84. | Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci USA. 2007;104:16170-16175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 430] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 85. | Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, Liu L, Li X, Niu Y, Deng SC. miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol Cancer Ther. 2013;12:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Belli C, Cereda S, Anand S, Reni M. Neoadjuvant therapy in resectable pancreatic cancer: a critical review. Cancer Treat Rev. 2013;39:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, Nakamura S, Adachi M, Hamamoto Y, Funakoshi S. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32:3847-3853. [PubMed] |
| 88. | Du Z, Qin R, Wei C, Wang M, Shi C, Tian R, Peng C. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. Dig Dis Sci. 2011;56:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 89. | Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther. 2011;89:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 91. | Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 748] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 92. | Huang FT, Zhuan-Sun YX, Zhuang YY, Wei SL, Tang J, Chen WB, Zhang SN. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int J Oncol. 2012;41:1707-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 94. | Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D’Haese JG. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 95. | Hermann PC, Trabulo SM, Sainz B Jr, Balic A, Garcia E, Hahn SA, Vandana M, Sahoo SK, Tunici P, Bakker A. Multimodal Treatment Eliminates Cancer Stem Cells and Leads to Long-Term Survival in Primary Human Pancreatic Cancer Tissue Xenografts. PLoS One. 2013;8:e66371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Mizuma M, Rasheed ZA, Yabuuchi S, Omura N, Campbell NR, de Wilde RF, De Oliveira E, Zhang Q, Puig O, Matsui W. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol Cancer Ther. 2012;11:1999-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. Evaluation of poly-mechanistic antiangiogenic combinations to enhance cytotoxic therapy response in pancreatic cancer. PLoS One. 2012;7:e38477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 99. | Rajeshkumar NV, Rasheed ZA, García-García E, López-Ríos F, Fujiwara K, Matsui WH, Hidalgo M. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9:2582-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Venkatesha VA, Parsels LA, Parsels JD, Zhao L, Zabludoff SD, Simeone DM, Maybaum J, Lawrence TS, Morgan MA. Sensitization of pancreatic cancer stem cells to gemcitabine by Chk1 inhibition. Neoplasia. 2012;14:519-525. [PubMed] |
| 101. | DeVito NC, Saif MW. Advances in immunotherapy for pancreatic cancer: 2013. JOP. 2013;14:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Gansauge F, Poch B, Kleef R, Schwarz M. Effectivity of long antigen exposition dendritic cell therapy (LANEXDC®) in the palliative treatment of pancreatic cancer. Curr Med Chem. 2013;20:4827-4835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Sasada T, Noguchi M, Yamada A, Itoh K. Personalized peptide vaccination: a novel immunotherapeutic approach for advanced cancer. Hum Vaccin Immunother. 2012;8:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Yutani S, Komatsu N, Yoshitomi M, Matsueda S, Yonemoto K, Mine T, Noguchi M, Ishihara Y, Yamada A, Itoh K. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol Rep. 2013;30:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 105. | Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1910] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 106. | Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:950658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 107. | Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen G, Zhang TP, Kang T, Zhao YP. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett. 2011;313:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 108. | Zhu LQ, Zhen YF, Zhang Y, Guo ZX, Dai J, Wang XD. Salinomycin activates AMP-activated protein kinase-dependent autophagy in cultured osteoblastoma cells: a negative regulator against cell apoptosis. PLoS One. 2013;8:e84175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Kim JH, Chae M, Kim WK, Kim YJ, Kang HS, Kim HS, Yoon S. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol. 2011;162:773-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee CH, Kim YN. Salinomycin induces cell death via inactivation of Stat3 and downregulation of Skp2. Cell Death Dis. 2013;4:e693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 111. | Farhana L, Dawson MI, Das JK, Murshed F, Xia Z, Hadden TJ, Hatfield J, Fontana JA. Adamantyl Retinoid-Related Molecules Induce Apoptosis in Pancreatic Cancer Cells by Inhibiting IGF-1R and Wnt/β-Catenin Pathways. J Oncol. 2012;2012:796729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, Büchler MW, Salnikov AV, Herr I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19:188-195. [PubMed] |
| 113. | Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515-528. [PubMed] |
| 114. | Wang LS, Chuang MC, Ho JA. Nanotheranostics--a review of recent publications. Int J Nanomedicine. 2012;7:4679-4695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Kim TH, Lee S, Chen X. Nanotheranostics for personalized medicine. Expert Rev Mol Diagn. 2013;13:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 116. | Bostad M, Berg K, Høgset A, Skarpen E, Stenmark H, Selbo PK. Photochemical internalization (PCI) of immunotoxins targeting CD133 is specific and highly potent at femtomolar levels in cells with cancer stem cell properties. J Control Release. 2013;168:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |