Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10457
Revised: November 4, 2013
Accepted: May 28, 2014
Published online: August 14, 2014
Processing time: 354 Days and 20.9 Hours
AIM: To assess the effect of inhibition of caspase-1 on acute renal injury in rats with severe acute pancreatitis (SAP).
METHODS: Forty-two Sprague-Dawley rats were randomly divided into three groups: healthy controls (HC, n = 6), SAP rats treated with saline (SAP-S, n = 18), or SAP rats treated with a caspase-1/interleukin (IL)-1β-converting-enzyme (ICE) inhibitor (SAP-I-ICE, n = 18). SAP was induced by retrograde infusion of 5% sodium taurocholate into the bile-pancreatic duct. HC rats were subjected to identical treatment and surgical procedures without sodium taurocholate. Rats received an intraperitoneal injection of isotonic saline (SAP-S) or the inhibitor (SAP-ICE-I) at 2 and 12 h after induction of acute pancreatitis. Surviving rats were sacrificed at different time points after SAP induction; all samples were obtained and stored for subsequent analyses. The levels of blood urea nitrogen (BUN) and creatinine (Cr) were measured using automatic methods, and serum IL-1β concentrations were measured by an enzyme-linked immunosorbent assay. Intrarenal expression of IL-1β, IL-18 and caspase-1 mRNAs was detected by RT-PCR. IL-1β protein expression and the pathologic changes in kidney tissues were observed by microscopy after immunohistochemical or hematoxylin and eosin staining, respectively.
RESULTS: The serum levels of BUN and Cr in the SAP-S group were 12.48 ± 2.30 mmol/L and 82.83 ± 13.89 μmol/L at 6 h, 23.53 ± 2.58 mmol/L and 123.67 ± 17.67 μmol/L at 12 h, and 23.60 ± 3.33 mmol/L and 125.33 ± 21.09 μmol/L at 18 h, respectively. All were significantly increased compared to HC rats (P < 0.01 for all). Levels in SAP-ICE-I rats were significantly decreased compared to SAP-S rats both at 12 and 18 h (P < 0.01 for all). Serum IL-1β levels in the SAP-S group were 276.77 ± 44.92 pg/mL at 6 h, 308.99 ± 34.95 pg/mL at 12 h, and 311.60 ± 46.51 pg/mL at 18 h; all significantly higher than those in the HC and SAP-ICE-I groups (P < 0.01 for all). Intrarenal expression of IL-1β mRNA was weak in HC rats, but increased significantly in SAP-S rats (P < 0.01). ICE inhibition significantly decreased the expression of IL-1β and IL-18 mRNAs (P < 0.05 for all vs SAP-S), whereas caspase-1 mRNA expression was not significantly different. Weak IL-1β immunostaining was observed in HC animals, and marked staining was found in the SAP-S group mainly in renal tubular epithelial cells. IL-1β immunostaining was significantly descended in SAP-ICE-I rats compared to SAP-S rats (P < 0.05). Caspase-1 inhibition had no effect on the severity of kidney tissue destruction.
CONCLUSION: The expression of caspase-1-activated cytokines IL-1β and IL-18 plays a pivotal role in acute renal injury in rats with experimental SAP. Caspase-1 inhibition improves renal function effectively.
Core tip: Activation of caspase-1/interleukin (IL)-1β-converting-enzyme (ICE) and the over-production of IL-18 and IL-1β in the kidney play a pivotal role during the process of acute renal injury in severe acute pancreatitis. Furthermore, ICE inhibitors are effective in improving renal function. The mechanisms of ICE inhibition may be associated with descended cytokine mediated injury and decreased renal cell apoptosis. Therefore, the study of the mechanism of acute renal injury in severe acute pancreatitis may help identify measures for the prevention and treatment, as well as supply novel information to further our understanding of systemic inflammatory response syndrome and multiple system organ failure.
- Citation: Zhang XH, Li ML, Wang B, Guo MX, Zhu RM. Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J Gastroenterol 2014; 20(30): 10457-10463
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10457
Many experimental and clinical studies have shown that interleukin (IL)-1β and tumor necrosis factor (TNF)-α play a pivotal role in encouraging tissue destruction and organ failure during the course of severe acute pancreatitis (SAP)[1,2]. Caspase-1, a member of the caspase family of cysteine proteases that is also known as IL-1β-converting-enzyme (ICE), functions by proteolytically cleaving precursors of IL-1β and IL-18, two structurally and functionally homologous proinflammatory cytokines, into their active forms[3]. In addition, ICE also interferes with the process of apoptotic cell death. The inhibition of ICE, and in turn, the inhibition of proper maturation of IL-1β and IL-18, may have significance for the development of SAP and systemic inflammatory response syndrome (SIRS). In this research, a rat model of SAP was used to assess the effect of ICE inhibition on renal function by measuring serum levels of blood urea nitrogen (BUN), creatinine (Cr), and IL-1β. In addition, intrarenal expression of caspase-1, IL-1β and IL-18, as well as pathologic changes in kidney tissues were measured after SAP with or without ICE inhibition.
The Experimental Animal Center of Jinling Hospital in Nanjing provided healthy normal adult male Sprague-Dawley rats weighing 240-250 g. All 42 animals were randomly divided into one of three groups: healthy controls (HC; n = 6), SAP rats treated with saline (SAP-S; n = 18), and SAP rats treated with an ICE inhibitor (SAP-I-ICE; n = 18). The latter two groups were divided into 6, 12, and 18 h time-point groups, each containing six rats. 5% sodium taurocholate was infused retrogradely into the bile-pancreatic duct to induce SAP as previously described[4-8]. The HC group underwent the same operative procedure in the absence of sodium taurocholate. In the SAP-S group, rats received an intraperitoneal injection of isotonic saline at 2 and 12 h after SAP induction. In the SAP-ICE-I group, rats were given an intraperitoneal injection of the ICE inhibitor Ac-Tyr-Val-Ala-Asp-2,6-dimethylbenzoyloxymethylketone of 0.25 mg dissolved in 2 mL phosphate-buffered saline (PBS) first at 2 h and again at 12 h post-SAP induction. Surviving rats were sacrificed at 6, 12 and 18 h, and samples were obtained for subsequent analyses.
An automated HITACHI-7150 analyzer was used to measure serum BUN and Cr concentrations and evaluate the severity of acute renal injury.
Serum IL-1β levels of were measured using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, United States). All samples were tested in duplicate and averaged.
TRIzol reagent (Gibco of Thermo Fisher Scientific, Waltham, MA, United States) was used to extract total RNA from kidney tissue (100 mg total) according to the manufacturer’s protocol. Briefly, kidney tissue was homogenized in 2 mL of TRIzol reagent, and the homogenate was centrifuged at 12000 r/min for 10 min at 4 °C to remove the insoluble material. Homogenized samples were incubated with 0.2 mL chloroform at room temperature for 5 min. Samples were mixed for 15 s, and incubated at room temperature for 3 min, followed by centrifugation at 12000 r/min for 15 min at 4 °C. The upper aqueous phase was transferred to a fresh tube and incubated for 10 min with 0.5 mL of isopropyl alcohol. The tissue sample was again centrifuged at 12000 r/min for 10 min at 4 °C, and the pellet was washed in 1 mL of 75% ethanol. Finally, the tissue sample was vortexed and centrifuged at 7000 r/min for 5 min at 4 °C, briefly air-dried, dissolved in 50 μL of diethylpyrocarbonate-treated water, and stored at -80 °C. Absorbance readings at 260 and 280 nm from an ultraviolet spectrophotometer were used to determine the RNA concentration.
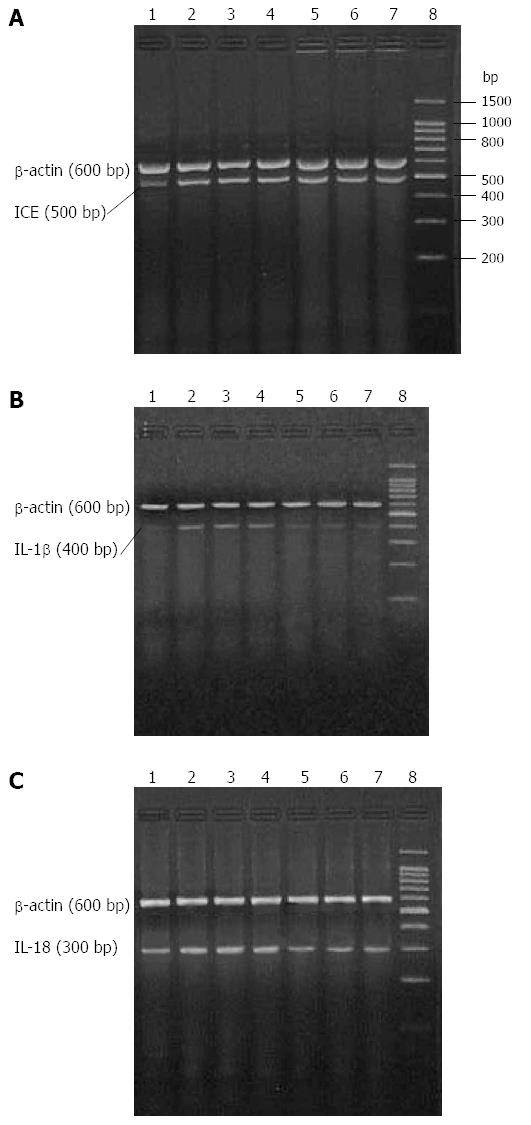
Real-time polymerase chain reaction (RT-PCR) was carried out using a One Step RNA PCR Kit (AMV; Takara Bio Inc., Otsu, Shiga, Japan). Each 50 μL reaction consisted of: 5 μL of 10 × One Step RNA PCR Buffer, 10 μL of MgCl2 (25 mmol/L), 5 μL of dNTPs (10 mmol/L each), 1 μL of RNase Inhibitor (40 U/μL), 1 μL of AMV RTase XL (5U/μL), 1 μL of AMV-Optimized Taq polymerase (5 U/μL), 1 μL of upstream specific primer, 1 μL of downstream specific primer, 1 μL of experimental sample (≤ 1 μg total RNA), and 24 μL of RNase-free dH2O. The sequences of IL-18, IL-1β and β-actin primers (designed with Primer 3 software and synthesized by Sangon Biotechnology Co., Shanghai, China) were as follows (forward and reverse primers): caspase-1, 5′-GTG TTG CAG ATA ATG AGG GC-3′ and 5′-AAG GTC CTG AGG GCA AAG AG-3′ (500 bp product); IL-1β, 5′-TTG GGA TCC ACA CTC TCC AG-3′and 5′-AGA AGC TGT GGC AGC TAC CT-3′ (400 bp product); IL-18, 5′-AGA TAG GGT CAC AGC CAG TC-3′ and 5′-GCT GCA ATA CCA GAA GAA GG-3′ (300 bp product); β-actin, 5′-CAT AGC TCT TCT CCA GGG AG-3′ and 5′-AGG GTG TGA TGG TGG GTA TG-3′ (600 bp product). The following protocol was used for each RT-PCR reaction: 30 min at 50 °C for the reverse transcription reaction followed by enzyme inactivation for 2 min at 94 °C, and 30 cycles of 30 s at 94 °C, 30 s at 51 °C (caspase-1), 53 °C (IL-1β) or 55 °C (IL-18), and 90 s at 72 °C, or 30 s at 55 °C, 30 s at 94 °C, 90 s for 35 cycles at 72 °C (β-actin). All RT-PCR reactions were terminated with an elongation step for 5 min at 72 °C and the PT-PCR products were stored at 4 °C. Reaction products (5 μL) were resolved by 2% agarose electrophoresis and visualized using ethidium bromide and digitally photographed. Band intensity was determined by optical density, and quantified as ratio of individual PCR product/β-actin.
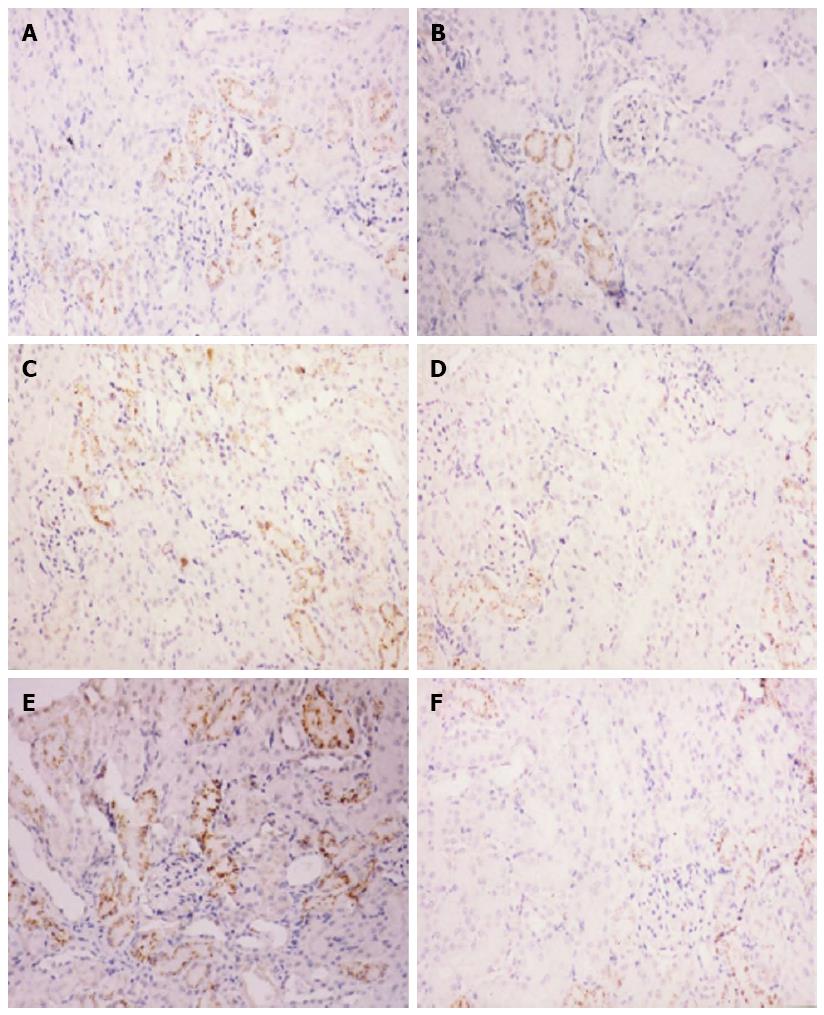
For histopathology, paraffin embedded tissue blocks were cut into 4 μm sections and stained with hematoxylin and eosin according to established protocols. For immunohistochemistry, tissue sections underwent antigen-retrieval by heating for 25 min in 0.01 mol/L sodium citrate (pH 6.0), washed in PBS and pre-incubated with 10% normal goat serum and 10% fetal calf serum in PBS for 20 min. Consecutive sections were then incubated with a rabbit anti-rat IL-1β polyclonal antibody (1:200; Santa Cruz Bio. Inc., Dallas, TX, United States) at 37 °C for 120 min, washed three times in PBS, incubated with a secondary antibody (EnVision System, anti-rabbit HRP; Dako, Glostrup, Denmark) at 37 °C for 40 min, and washed again in PBS. Tissue staining was visualized using a diaminobenzidine substrate-chromogen solution. Slides were counterstained with hematoxylin, dehydrated, and mounted. Two slides per rat were visualized using a BH-2 microscope at 400 × magnification, and five 0.5 cm × 1.0 cm fields were selected randomly from each slide. The average gray value (AGV) was calculated using color image analysis and was inversely associated with IL-1β protein expression.
Analysis of variance (ANOVA) was used for statistical analyses using SPSS 17.0 statistical software. All values are presented as mean ± SD, with a P value < 0.05 considered statistically significant.
Serum levels of BUN and Cr were significantly higher in the SAP-S group compared to the HC group at 6, 12 and 18 h (P < 0.05 for all). Treatment with the ICE inhibitor significantly reduced these levels at 12 and 18 h (P < 0.05 for all vs SAP-S) (Table 1).
| Group | n | BUN (mmol/L) | Cr (mmol/L) | IL-1β(pg/mL) | IL-1βstaining (AGV) |
| HC | 6 | 5.02 ± 0.42 | 41.17 ± 2.48 | 90.13 ± 21.13 | 63.67 ± 6.19 |
| SAP-S | |||||
| 6 h | 6 | 12.48 ± 2.30b | 82.83 ± 13.89b | 276.77 ± 44.92b | 45.00 ± 4.98b |
| 12 h | 6 | 23.53 ± 2.58b | 123.67 ± 17.67b | 308.99 ± 34.95b | 42.33 ± 4.89b |
| 18 h | 6 | 23.60 ± 3.33b | 125.33 ± 21.09b | 311.60 ± 46.51b | 38.33 ± 5.13b |
| SAP-ICE-I | |||||
| 6 h | 6 | 11.17 ± 1.71b | 73.50 ± 11.62b | 151.42 ± 27.26bd | 53.00 ± 6.60bc |
| 12 h | 6 | 18.45 ± 2.66bd | 75.67 ± 14.11bd | 152.47 ± 29.60bd | 49.67 ± 5.39bc |
| 18 h | 6 | 19.23 ± 2.18bd | 77.50 ± 13.90bd | 175.45 ± 29.72bd | 46.50 ± 5.24bc |
Serum IL-1β levels were significantly higher in each SAP experimental group when compared to the HC group at all time points (P < 0.05 for all). In addition, serum IL-1β levels were significantly lower in the SAP-I-ICE group compared to the SAP-S group (P < 0.05 for all) (Table 1).
IL-1β, IL-18 and caspase-1 mRNA levels were detectable in samples from the HC group, though expression of IL-1β mRNA was weak. All transcript levels were significantly higher in samples from the SAP-S group when compared to HC rats (P < 0.05 for all). IL-1β mRNA expression was restored to normal healthy control levels, and IL-18 mRNA expression was significantly descended following ICE inhibition (P < 0.05 for all), whereas the expression of caspase-1 mRNA was not significantly different (Figure 1, Table 2).
| Group | n | Caspase-1 | IL-1β | IL-18 |
| HC | 6 | 0.36 ± 0.03 | 0.13 ± 0.01 | 0.38 ± 0.03 |
| SAP-S | ||||
| 6 h | 6 | 0.45 ± 0.03b | 0.19 ± 0.02b | 0.57 ± 0.04b |
| 12 h | 6 | 0.46 ± 0.04b | 0.20 ± 0.03b | 0.58 ± 0.05b |
| 18 h | 5 | 0.43 ± 0.04b | 0.20 ± 0.03b | 0.55 ± 0.04b |
| SAP-ICE-I | ||||
| 6 h | 6 | 0.45 ± 0.03b | 0.15 ± 0.02d | 0.45 ± 0.04bd |
| 12 h | 6 | 0.44 ± 0.03b | 0.15 ± 0.02d | 0.45 ± 0.04bd |
| 18 h | 5 | 0.45 ± 0.02b | 0.15 ± 0.02d | 0.44 ± 0.04bd |
Immunohistochemical staining demonstrated weak IL-1β expression in normal kidney tissue and strong IL-1β expression in cortical renal tubular epithelial cells from SAP-S kidney tissue. Quantification of the IL-1β immunoreactivity showed that the increase in IL-1β staining with SAP was significant (P < 0.05), and this increase was significantly attenuated with ICE inhibition (P < 0.05 vs SAP-S) (Figure 2, Table 1).
Light microscopy revealed normal kidney tissue morphology in the HC group, whereas morphological changes occurred in the SAP-S and SAP-ICE-I groups at 12 and 18 h, observed as marked capillary congestion in renal glomeruli, swelling, blurry boundaries and scattered necrosis in renal tubular epithelial cells, stenosis or atresia of lumens, visible protein casts, interstitial edema, and inflammatory cell infiltration (Figure 2). Interestingly, caspase-1 inhibition had no effect on the severity of kidney tissue injury as measured by these morphological changes.
Individuals diagnosed with SAP often face complications including multiple system organ failure, and especially respiratory and acute renal failure (ARF), which are the major causes of death[9]. ARF is a frequent and early complication of SAP, often coinciding with a worse prognosis; mortality rates are 71.2% in patients with ARF and 6.8% in those without[10]. Previous studies suggest that the activation of caspase-1/ICE is an important step in the pathophysiologic process of complicated acute pancreatitis, with evidence demonstrating reduced overall severity and mortality following ICE inhibition[11,12]. The mechanism behind this change, however, remains poorly understood.
A number of studies have shown expression of IL-1β mRNA in normal mouse, rat and human kidneys[13,14], whereas others have reported difficulty in detecting expression in normal human kidney and normal glomeruli isolated from rats[15-17]. In this study, expression of IL-1β mRNA and protein was detected, though weak, in the normal rat kidney. The reason for this difference is not clear and could be explained by a difference in species or technique. Tesch et al[18] demonstrated that intrinsic renal cells constitutively express IL-1β and are the major source of the increased IL-1β production seen during rat anti-glomerular basement membrane glomerulonephritis, suggesting that IL-1β production is likely to play an important role in renal injury in experimental glomerulonephritis.
Caspase-1/ICE is a member of cysteine proteases of the caspase family. The proteolytic cleavage of the 31 kD IL-1β precursor into its biologically active 17 kD form is one of the major functions of ICE. Activated lymphocytes and monocytes produce IL-1β, which could cause cardiac dysfunction, circulatory collapse, hypoperfusion, metabolic acidosis, fever, shock and production of acute respiratory distress syndrome. In this research, a highly specific competitive and irreversible inhibitor of ICE was injected into rats 2 h after SAP induction, which is known to alter serum levels of amylase in the pancreas and lungs[11,19], and a remarkable elevation of serum BUN, Cr and IL-1β was observed. In addition, SAP induced an upregulation of intrarenal IL-1β and caspase-1 mRNAs, corresponding to increased IL-1β immunostaining and obvious pathologic changes in kidney tissues. ICE inhibition significantly decreased serum and intrarenal IL-1β expression levels, and improved renal function. These data suggest that ICE inhibition alleviates IL-1β-mediated renal injury in SAP.
In addition to generating large amounts of active IL-1β, the precursor of IL-18 was processed into its bioactive form by ICE[3]. IL-18, which was called interferon (IFN)-γ-inducing factor, is a new pro-inflammatory 18000 kD cytokine, sharing structural and functional similarities with IL-1β. The gene expression and synthesis of IL-1, TNF, and some chemokinesis are induced by IL-18. Also, IL-18 plays a part in the Th-1 response to the stimulation of viral antigen, firstly because natural killer cells and T cells of IFN-γ production could be induced by IL-18. Rau et al[20] reported that IL-18 concentrations in patients with SAP are significantly elevated, and that the development of pancreatic necrosis and organ failure is closely correlated with the IL-18 concentrations in serum. In this research, marked upregulation of expression of IL-18 mRNA in the kidney was observed after induction of SAP, which was significantly decreased with ICE inhibition.
It is confirmed that TNF-α, IL-18 and IL-1β induce the synthesis of each other[3,21,22]. Tomosugi et al[23] demonstrated that TNF and IL-1 can increase the severity of glomerular injury in nephritis. Furthermore, Faubel et al[24] demonstrated that cisplatin-induced ARF is associated with increases in IL-1β, IL-18, and IL-6 and neutrophil infiltration in the kidney. Therefore, we speculate that intrarenal overproduction of IL-18 plays a part during the course of SAP complicated with acute renal injury, and that the alleviation of acute renal injury by ICE inhibition may be due to the decrease in IL-18 expression. Additionally, ICE interferes with the process of apoptotic cell death and triggers TNF-α and Fas-mediated apoptosis. Therefore, the improvement of renal function observed in this study by ICE inhibition may be associated with a decrease in renal cell apoptosis. As the results also revealed no morphological changes in rat kidneys following ICE inhibition, we speculate that these may be associated with extra-pancreatic organ-specific injury and other mechanisms of acute renal injury in SAP.
In conclusion, overproduction of IL-1β and activation of caspase-1/ICE and IL-18 in the kidney play a pivotal role during the course of acute renal injury in SAP, and the ICE inhibition is effective in improving renal function. Mechanisms of the effects of ICE inhibition may share a close relationship with decreased cytokine-mediated injury and with decreased renal cell apoptosis. Therefore, these results supply novel ideas that may help to further understand SIRS and multiple system organ failure, and to explore measures for the prevention and treatment of SAP.
Interleukin (IL)-1β and tumor necrosis factor (TNF)-α play pivotal roles in inducing local tissue destruction and organ failure during the course of severe acute pancreatitis. IL-18 is a pro-inflammatory cytokine, structurally and functionally similar to IL-1β. Caspase-1, termed as IL-1β-converting-enzyme (ICE), functions to proteolytically cleave IL-1β and IL-18 into their active forms and interferes with apoptotic cell death. Therefore, ICE inhibition may play an important role in the treatment of severe acute pancreatitis (SAP) and systemic inflammatory response syndrome (SIRS). In this research, the effects of ICE inhibition on acute renal injury were assessed in an experimental model of SAP by examining pathologic kidney changes and measuring levels of blood urea nitrogen, creatinine, IL-1β, and IL-18.
A number of studies have shown expression of IL-1β mRNA in normal mouse, rat and human kidney, whereas other studies have failed to detect it. Results from this study confirm that expression of IL-1β mRNA is weak in normal rat kidney, and increased with SAP.
The results of this study demonstrate that ICE inhibition alleviates SAP-induced renal injury by regulating the expression of IL-Iβ and IL-18. However, treatment with an ICE inhibitor did not affect gross morphological changes in kidney tissue, suggesting that there are additional extra-pancreatic organ-specific injury mechanisms in SAP.
This study suggests that ICE activity is a novel mechanism in systemic inflammatory response syndrome and multiple system organ failure and that ICE inhibition may be beneficial for the prevention and treatment of SAP.
Caspase-1, termed as IL-1β-converting-enzyme, is a member of the family of cysteine proteases known as caspases.
The authors examined the expression of caspase-1 during the course of acute renal injury in SAP. They show that ICE inhibition attenuates SAP-induced increases in IL-1β and IL-18 mRNAs and alleviates renal injury. The results are interesting and may supply novel ideas to further understand the mechanisms of systemic inflammatory response syndrome and multiple system organ failure, and to explore measures for prevention and treatment of SAP.
P- Reviewer: Pezzilli R S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Papachristou GI. Prediction of severe acute pancreatitis: current knowledge and novel insights. World J Gastroenterol. 2008;14:6273-6275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Luan ZG, Zhang J, Yin XH, Ma XC, Guo RX. Ethyl pyruvate significantly inhibits tumour necrosis factor-α, interleukin-1β and high mobility group box 1 releasing and attenuates sodium taurocholate-induced severe acute pancreatitis associated with acute lung injury. Clin Exp Immunol. 2013;172:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Wang H, Tang AM, Liu D, Li G, Ye L, Li X, Li C, Chen L. Renoprotective activity of sivelestat in severe acute pancreatitis in rats. Exp Ther Med. 2013;6:29-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Zhou MT, Chen BC, Sun HW, Jin YP, Yang FJ, Zhang X, Andersson R, Zhang QY. Continuous regional arterial infusion with fluorouracil and octreotide attenuates severe acute pancreatitis in a canine model. PLoS One. 2012;7:e37347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Nakamura H, Honda H, Tashiro M, Taguchi M, Yoshikawa H, Otsuki M. Increased expression of 19-kD interacting protein-3-like protein and the relationship to apoptosis in the lung of rats with severe acute pancreatitis. Crit Care Med. 2003;31:2527-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Xu P, Zhou XJ, Chen LQ, Chen J, Xie Y, Lv LH, Hou XH. Pioglitazone attenuates the severity of sodium taurocholate-induced severe acute pancreatitis. World J Gastroenterol. 2007;13:1983-1988. [PubMed] |
| 8. | Müller CA, Belyaev O, Burr W, Munding J, McArthur N, Bergmann U, Werner J, Tannapfel A, Uhl W. Effects of FTY720 and rapamycin on inflammation in taurocholate-induced acute pancreatitis in the rat. Pancreas. 2012;41:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Compañy L, Sáez J, Martínez J, Aparicio JR, Laveda R, Griñó P, Pérez-Mateo M. Factors predicting mortality in severe acute pancreatitis. Pancreatology. 2003;3:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Herrera Gutiérrez ME, Seller Pérez G, de La Rubia De Gracia C, Chaparro Sánchez MJ, Nacle López B. [Acute renal failure profile and prognostic value in severe acute pancreatitis]. Med Clin (Barc). 2000;115:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Paszkowski AS, Rau B, Mayer JM, Möller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Ann Surg. 2002;235:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Rau B, Paszkowski A, Lillich S, Baumgart K, Möller P, Beger HG. Differential effects of caspase-1/interleukin-1beta-converting enzyme on acinar cell necrosis and apoptosis in severe acute experimental pancreatitis. Lab Invest. 2001;81:1001-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Boswell JM, Yui MA, Endres S, Burt DW, Kelley VE. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988;141:118-124. [PubMed] |
| 14. | Tovey MG, Content J, Gresser I, Gugenheim J, Blanchard B, Guymarho J, Poupart P, Gigou M, Shaw A, Fiers W. Genes for IFN-beta-2 (IL-6), tumor necrosis factor, and IL-1 are expressed at high levels in the organs of normal individuals. J Immunol. 1988;141:3106-3110. [PubMed] |
| 15. | Noronha IL, Krüger C, Andrassy K, Ritz E, Waldherr R. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int. 1993;43:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Jenkins DA, Wojtacha DR, Swan P, Fleming S, Cumming AD. Intrarenal localization of interleukin-1 beta mRNA in crescentic glomerulonephritis. Nephrol Dial Transplant. 1994;9:1228-1233. [PubMed] |
| 17. | Tam FW, Smith J, Cashman SJ, Wang Y, Thompson EM, Rees AJ. Glomerular expression of interleukin-1 receptor antagonist and interleukin-1 beta genes in antibody-mediated glomerulonephritis. Am J Pathol. 1994;145:126-136. [PubMed] |
| 18. | Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, Atkins RC, Nikolic-Paterson DJ. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Zhang XH, Zhu RM, Xu WA, Wan HJ, Lu H. Therapeutic effects of caspase-1 inhibitors on acute lung injury in experimental severe acute pancreatitis. World J Gastroenterol. 2007;13:623-627. [PubMed] |
| 20. | Rau B, Baumgart K, Paszkowski AS, Mayer JM, Beger HG. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: high correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Antonopoulos C, Cumberbatch M, Mee JB, Dearman RJ, Wei XQ, Liew FY, Kimber I, Groves RW. IL-18 is a key proximal mediator of contact hypersensitivity and allergen-induced Langerhans cell migration in murine epidermis. J Leukoc Biol. 2008;83:361-367. [PubMed] |
| 22. | Meng H, Gong J, Fang L, Song Z, Wu F, Zhou B, Qian M. Effect of interferon-γ on NF-κB and cytokine IL-18 and IL-27 in acute pancreatitis. Bosn J Basic Med Sci. 2013;13:114-118. [PubMed] |
| 23. | Tomosugi NI, Cashman SJ, Hay H, Pusey CD, Evans DJ, Shaw A, Rees AJ. Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol. 1989;142:3083-3090. [PubMed] |
| 24. | Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |